Abstract
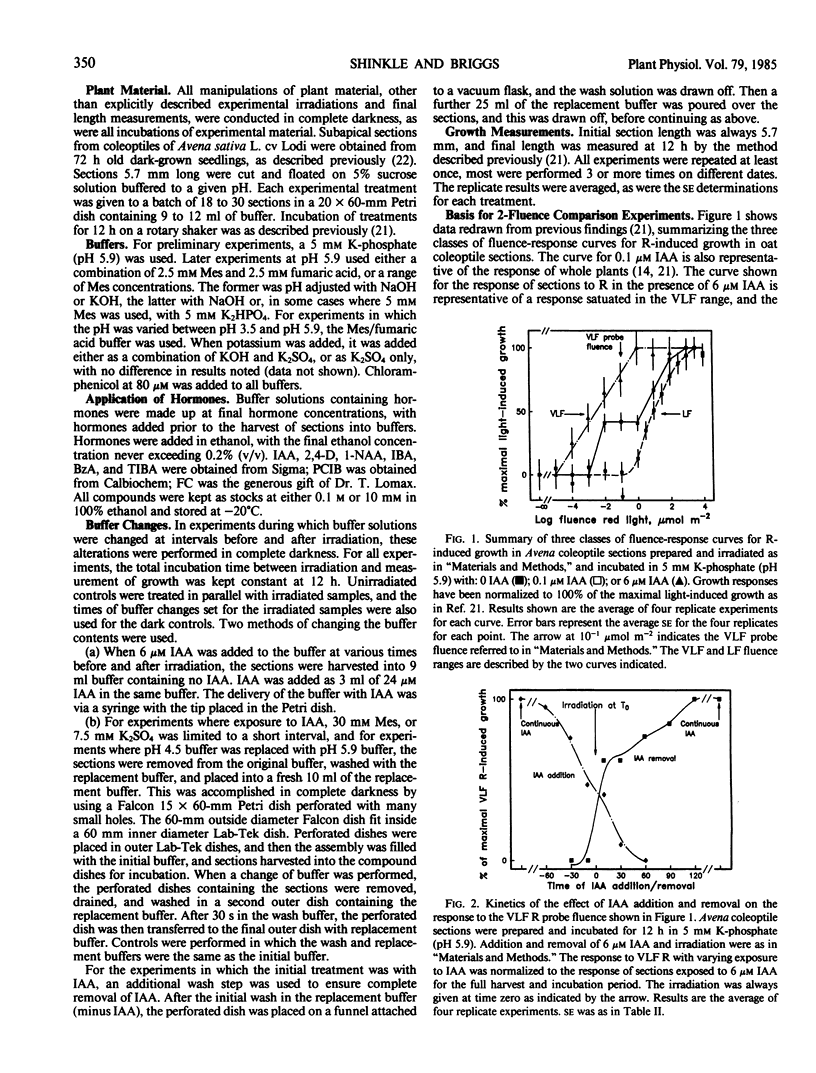
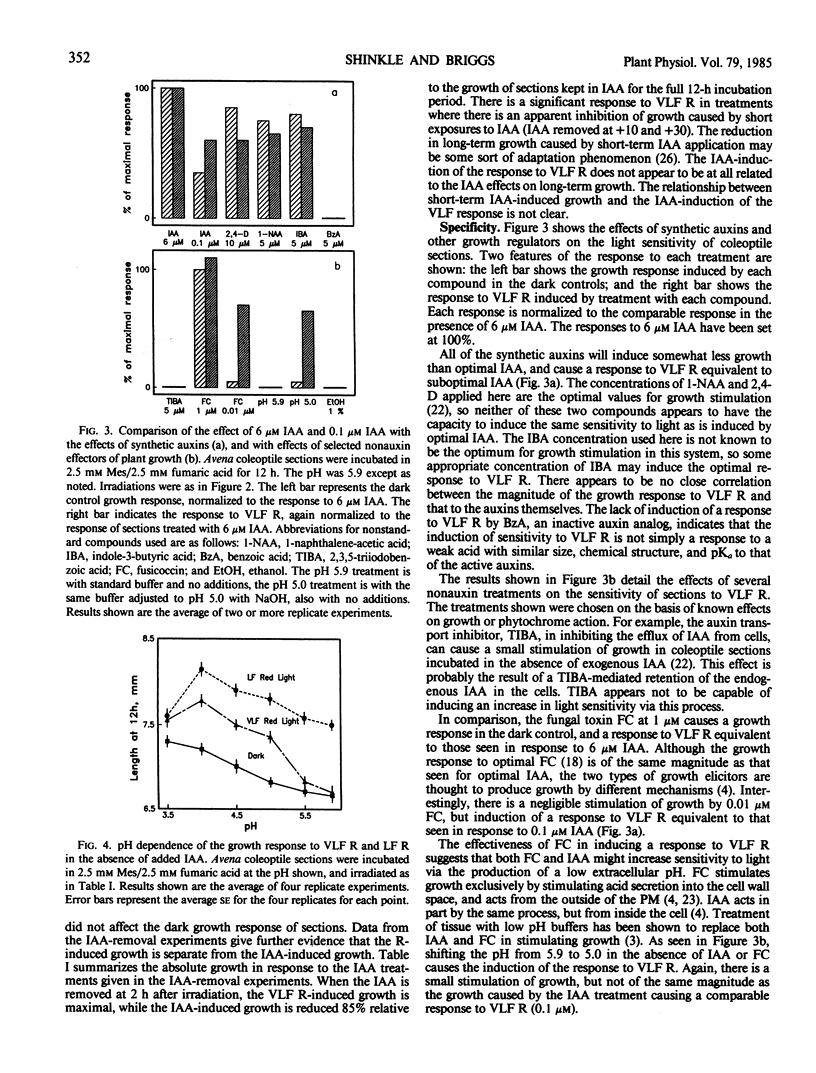
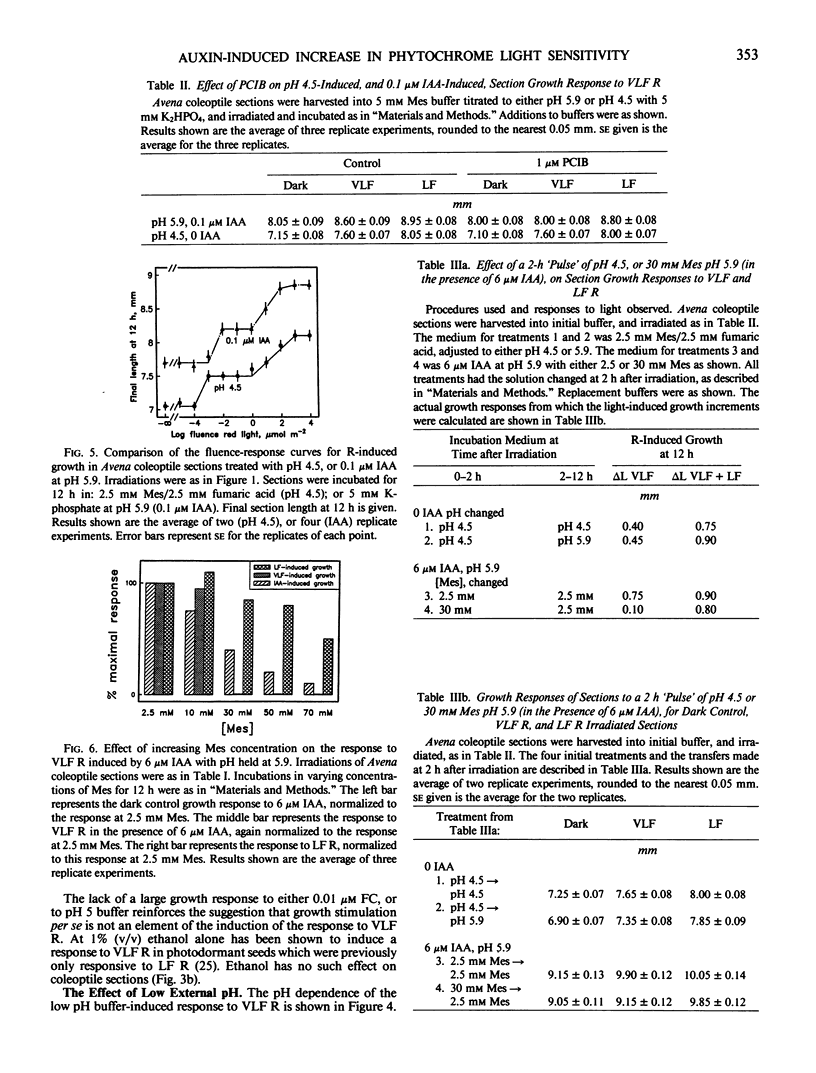
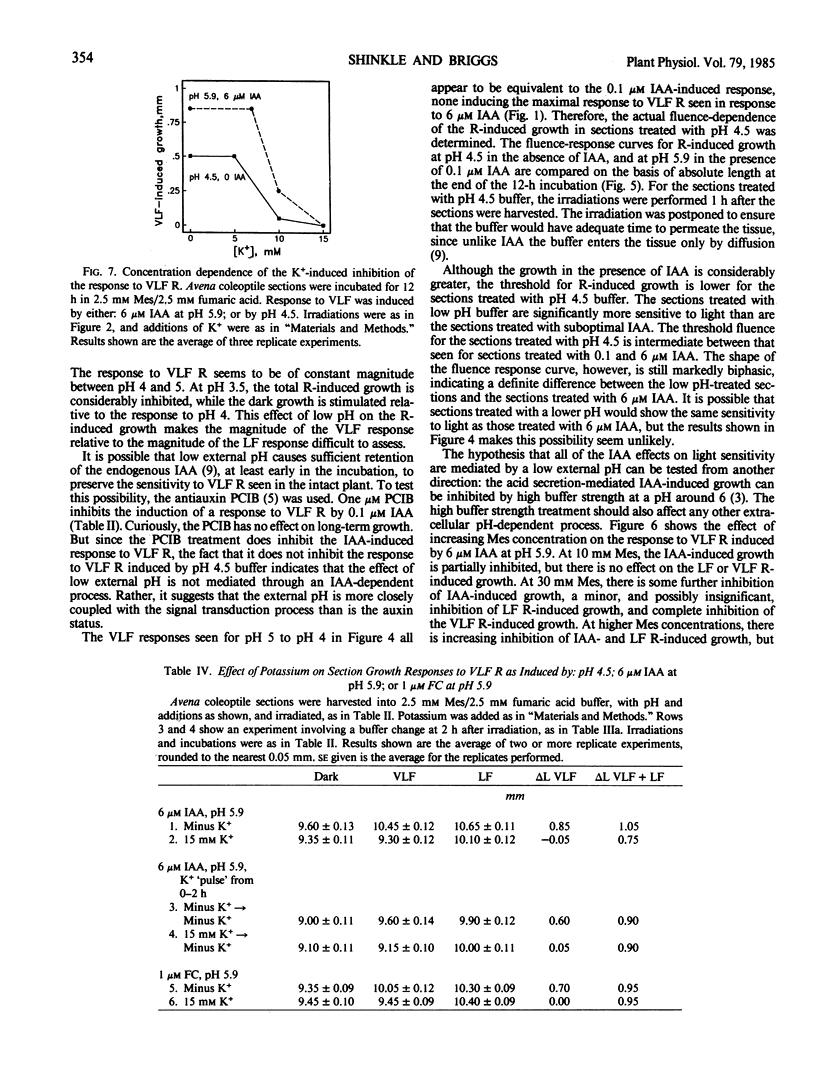
The physiology of the auxin-induced 10,000-fold increase in light sensitivity of a phytochrome-mediated growth response (Shinkle and Briggs, 1984 Proc Natl Acad Sci USA 81: 3742-3746) has been characterized in subapical coleoptile sections from dark-grown oat (Avena sativa L. cv Lodi) seedlings. Six micromolar indole-3-acetic acid (IAA) must be present for 1 hour before to 2 hour after irradiation in order to confer maximal sensitivity to light. The direct effect of IAA on growth can be separated from its effect on light sensitivity. Several classes of synthetic auxins will substitute for IAA in inducing an increase in sensitivity to light, as will both the phytotoxin fusicoccin and treatment of sections with pH 4.5 buffer. The increase in sensitivity to light induced by 6 micromolar IAA is completely inhibited by buffering the sections at pH 5.9 with 30 millimolar 2-(N-morpholino)ethanesulfonic acid. These findings suggest that the capacity to respond to very low fluences of light is regulated by extracellular pH.
Between 10 and 15 millimolar K+ will inhibit the induction of the increased sensitivity to light, independent of the mechanism of induction. The effect of K+ appears to be specific to the process by which the sections respond to very low levels of light.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Foster R. J., McRae D. H., Bonner J. Auxin-Antiauxin Interaction at High Auxin Concentrations. Plant Physiol. 1955 Jul;30(4):323–327. doi: 10.1104/pp.30.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorton H. L., Briggs W. R. Phytochrome Responses to End-of-Day Irradiations in Light-grown Corn Grown in the Presence and Absence of Sandoz 9789. Plant Physiol. 1980 Dec;66(6):1024–1026. doi: 10.1104/pp.66.6.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins W. G., Hillman W. S. Relationships between phytochrome state and photosensitive growth of Avena coleoptile segments. Plant Physiol. 1966 Apr;41(4):593–598. doi: 10.1104/pp.41.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson V. R., Weintraub R. L. Interactions of microtubule disorganizers, plant hormones, and red light in wheat coleoptile segment growth. Plant Physiol. 1975 Jun;55(6):1062–1066. doi: 10.1104/pp.55.6.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandoli D. F., Briggs W. R. Phytochrome control of two low-irradiance responses in etiolated oat seedlings. Plant Physiol. 1981 Apr;67(4):733–739. doi: 10.1104/pp.67.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Escherichia coli adenylate cyclase complex: regulation by the proton electrochemical gradient. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1099–1103. doi: 10.1073/pnas.76.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein B. Responses of Avena coleoptiles to suboptimal fusicoccin: kinetics and comparisons with indoleacetic Acid. Plant Physiol. 1981 Sep;68(3):543–547. doi: 10.1104/pp.68.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaer J. A., Mandoli D. F., Briggs W. R. Phytochrome-mediated cellular photomorphogenesis. Plant Physiol. 1983 Jul;72(3):706–712. doi: 10.1104/pp.72.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkle J. R., Briggs W. R. Auxin concentration/growth relationship for Avena coleoptile sections from seedlings grown in complete darkness. Plant Physiol. 1984 Feb;74(2):335–339. doi: 10.1104/pp.74.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkle J. R., Briggs W. R. Indole-3-acetic acid sensitization of phytochrome-controlled growth of coleoptile sections. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3742–3746. doi: 10.1073/pnas.81.12.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout R. G., Cleland R. E. Partial characterization of fusicoccin binding to receptor sites on oat root membranes. Plant Physiol. 1980 Sep;66(3):353–359. doi: 10.1104/pp.66.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhoef L. N., Quail P. H., Briggs W. R. Red Light-inhibited Mesocotyl Elongation in Maize Seedlings: II. Kinetic and Spectral Studies. Plant Physiol. 1979 Jun;63(6):1062–1067. doi: 10.1104/pp.63.6.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesper M. J., Evans M. L. Time-dependent Changes in the Auxin Sensitivity of Coleoptile Segments: Apparent Sensory Adaptation. Plant Physiol. 1978 Feb;61(2):204–208. doi: 10.1104/pp.61.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]


