Abstract
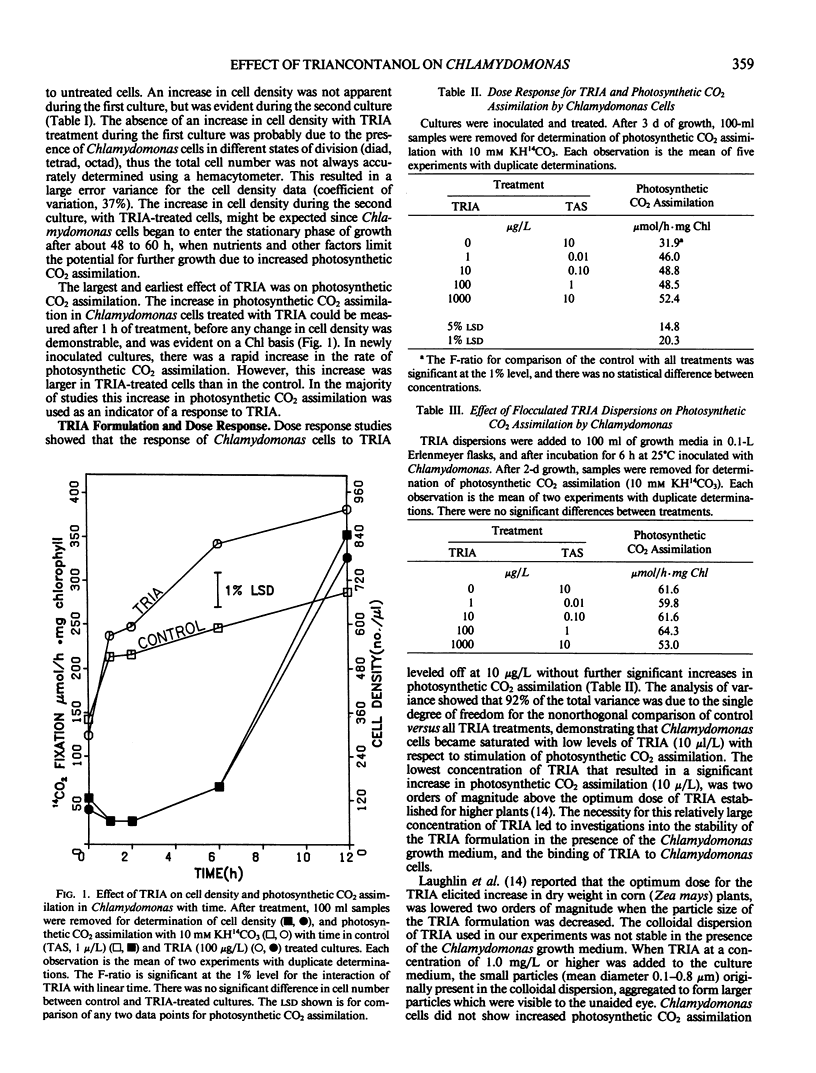
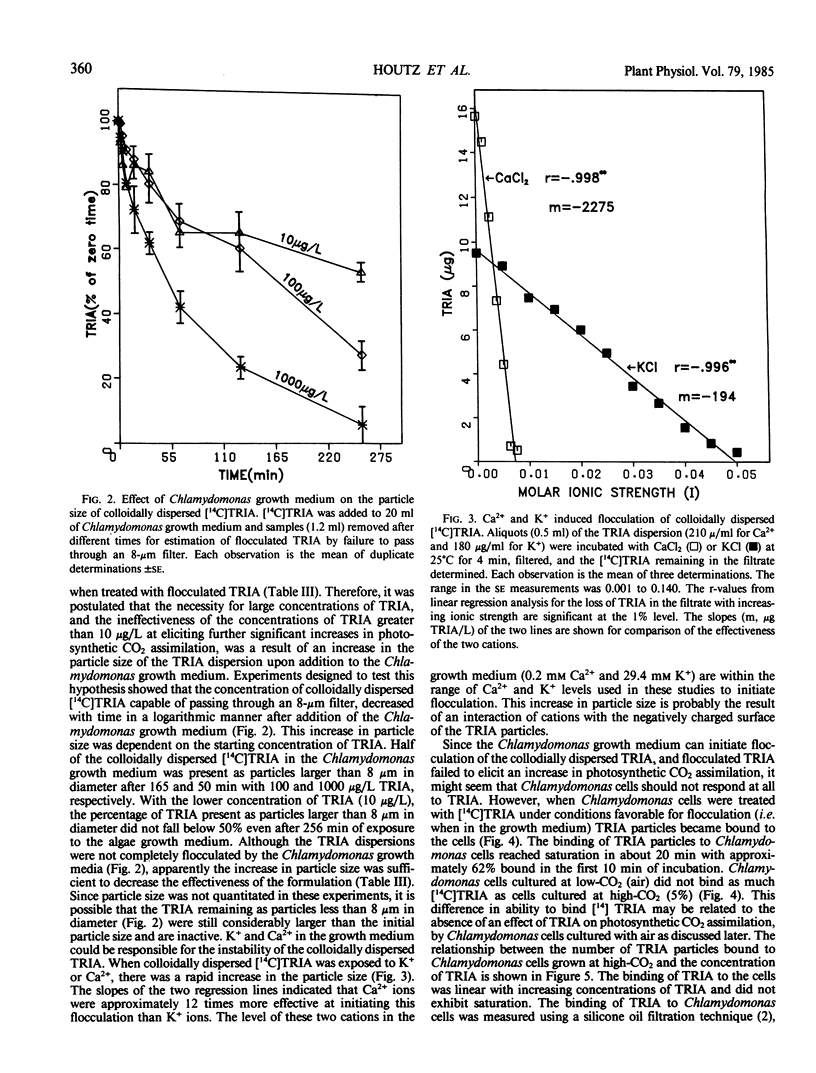
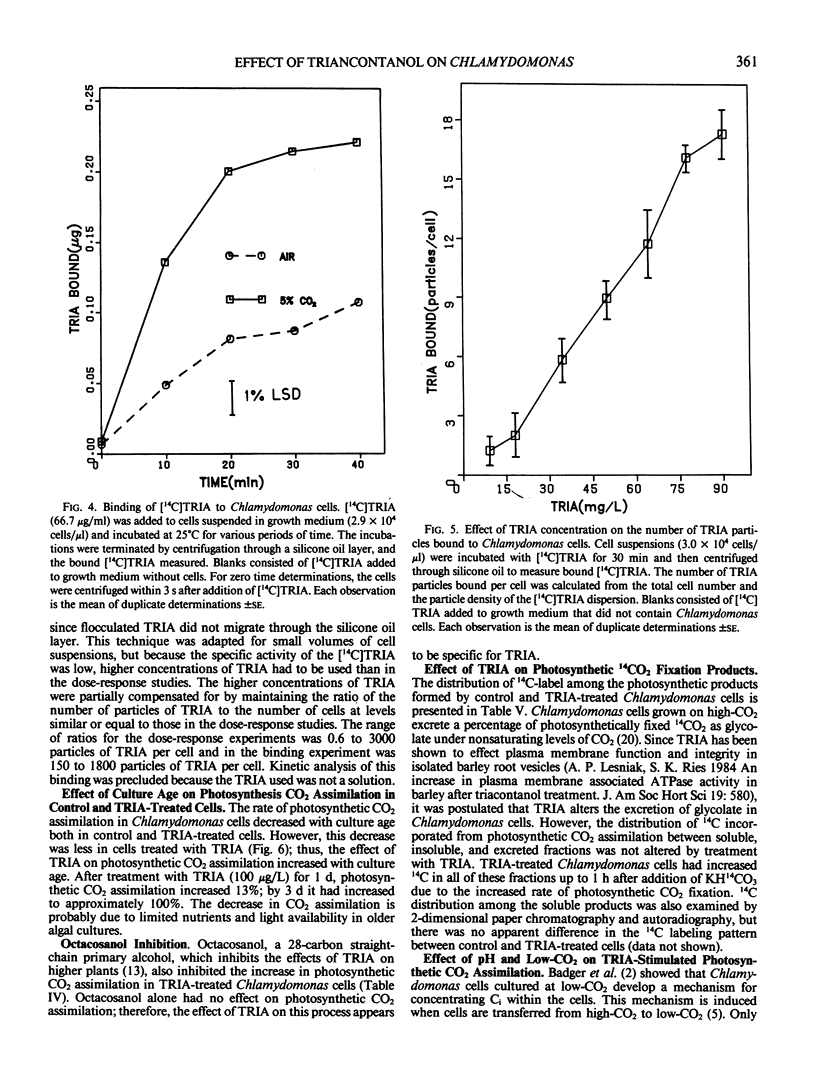
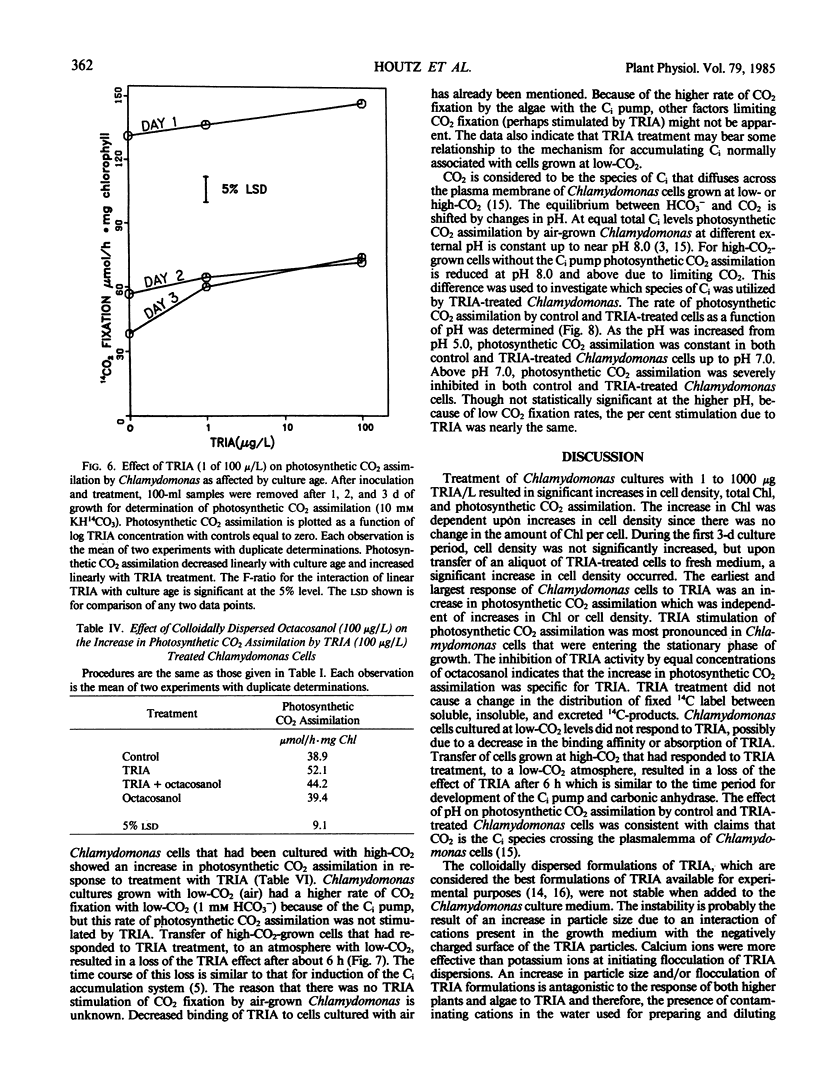
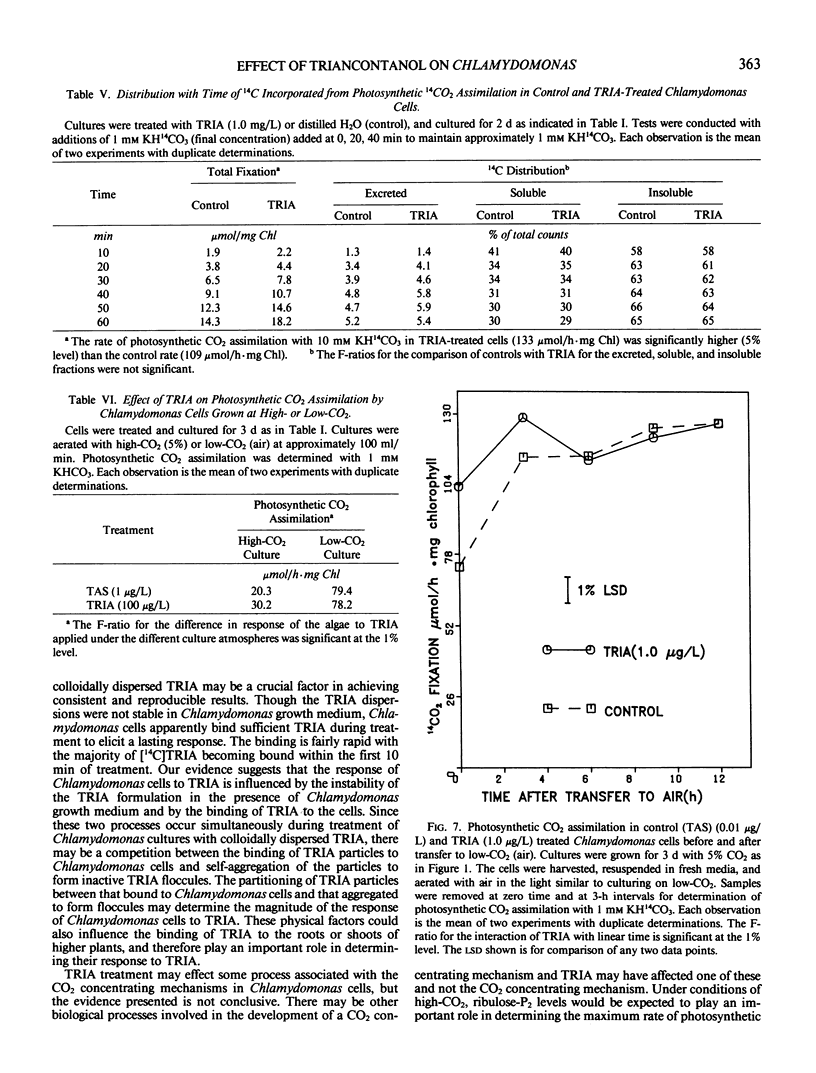
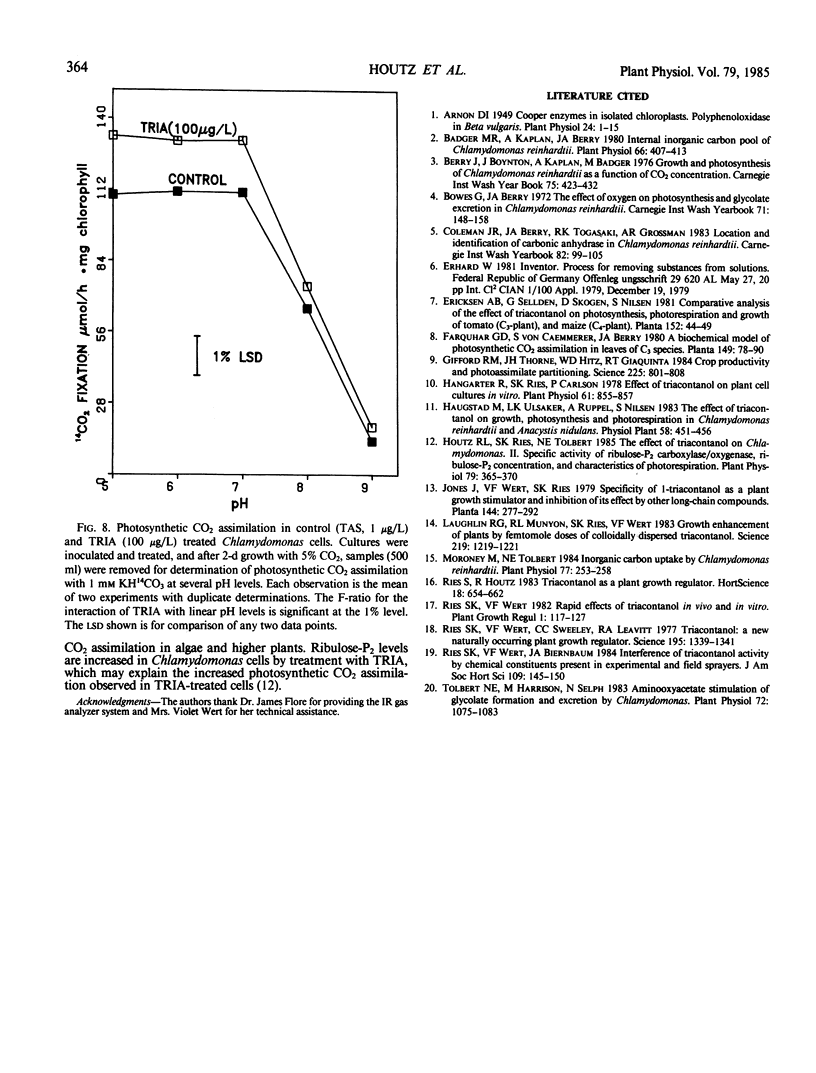
Treatment of Chlamydomonas reinhardtii cells, cultured at 5% CO2, with 1 to 1000 micrograms triacontanol (TRIA) per liter resulted in 21 to 35% increases in cell density, 7 to 31% increases in total chlorophyll, and 20 to 100% increases in photosynthetic CO2 assimilation. The increase in CO2 fixation with TRIA treatment occurred before, and was independent of, increases in total chlorophyll or cell number. Chlamydomonas cells responded to a broad range of TRIA concentrations that were at least one order of magnitude above the optimum concentration established for higher plants. The necessity for larger concentrations of TRIA may be due to destabilizing effects of Ca2+ and K+ present in the Chlamydomonas growth medium. These ions caused flocculation of the colloidally dispersed TRIA in apparent competition with binding of [14C]TRIA to Chlamydomonas cells. Octacosanol inhibited the effect of TRIA on photosynthetic CO2 assimilation. TRIA treatment did not alter the distribution of 14C-label among photosynthetic products. The effect of TRIA on photosynthetic CO2 assimilation increased with time after treatment up to 3 days. Chlamydomonas cells that had been grown at low-CO2 (air) did not respond to TRIA, and transfer of high-CO2 (5%) grown cells that had responded to TRIA to a low-CO2 atmosphere resulted in a loss of the effect of TRIA. The effect of pH on photosynthetic CO2 assimilation indicated that CO2 is probably the species of inorganic carbon utilized by control and TRIA-treated Chlamydomonas cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R., Kaplan A., Berry J. A. Internal Inorganic Carbon Pool of Chlamydomonas reinhardtii: EVIDENCE FOR A CARBON DIOXIDE-CONCENTRATING MECHANISM. Plant Physiol. 1980 Sep;66(3):407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford R. M., Thorne J. H., Hitz W. D., Giaquinta R. T. Crop productivity and photoassimilate partitioning. Science. 1984 Aug 24;225(4664):801–808. doi: 10.1126/science.225.4664.801. [DOI] [PubMed] [Google Scholar]
- Hangarter R., Ries S. K. Effect of triacontanol on plant cell cultures in vitro. Plant Physiol. 1978 May;61(5):855–857. doi: 10.1104/pp.61.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtz R. L., Ries S. K., Tolbert N. E. Effect of Triacontanol on Chlamydomonas: II. Specific Activity of Ribulose-Bisphosphate Carboxylase/Oxygenase, Ribulose-Bisphosphate Concentration, and Characteristics of Photorespiration. Plant Physiol. 1985 Oct;79(2):365–370. doi: 10.1104/pp.79.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin R. G., Munyon R. L., Ries S. K., Wert V. F. Growth enhancement of plants by femtomole doses of colloidally dispersed triacontanol. Science. 1983 Mar 11;219(4589):1219–1221. doi: 10.1126/science.219.4589.1219. [DOI] [PubMed] [Google Scholar]
- Moroney J. V., Tolbert N. E. Inorganic Carbon Uptake by Chlamydomonas reinhardtii. Plant Physiol. 1985 Feb;77(2):253–258. doi: 10.1104/pp.77.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries S. K., Wert V., Sweeley C. C., Leavitt R. A. Triacontanol: a new naturally occurring plant growth regulator. Science. 1977 Mar 25;195(4284):1339–1341. doi: 10.1126/science.195.4284.1339. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E., Harrison M., Selph N. Aminooxyacetate stimulation of glycolate formation and excretion by chlamydomonas. Plant Physiol. 1983 Aug;72(4):1075–1083. doi: 10.1104/pp.72.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]


