Abstract
Introduction
Anterior cruciate ligament (ACL) rupture is one of the most common knee injuries in sports, and the gold standard for treating ACL rupture is tendon graft reconstruction. Internal brace technology is being used nowadays for ligament repair; however, more relevant in vivo clinical evidence is required for using internal brace technology in ACL reconstruction (ACLR). We conducted a randomised controlled trial to investigate the clinical efficacy of internal brace technology in ACLR.
Methods and analysis
This randomised, parallel-controlled trial included patients with ACL rupture who underwent inpatient surgery at the Department of Orthopaedics, Xiangya Hospital, Central South University. Random number table method was used to assign the participants to either the test or the control group. The test group underwent ACLR using the internal brace technique, whereas the control group underwent standard ACLR. Uniform postoperative rehabilitation protocol was used for both the groups. Patient-reported outcomes included preoperative baseline and postoperative recovery at 1, 3, 6, 12 and 24 months. The primary outcome was International Knee Documentation Committee function from baseline (ACL rupture) to 6 months postoperatively. Secondary outcomes included (1) other patient outcome reporting metrics, Lysholm knee score, Knee Injury and Osteoarthritis Outcome Score and Visual Analog Scale; (2) the use of Kneelax3 knee stabiliser to assess knee stability; (3) occurrence of adverse events, such as graft refraction or symptomatic instability, postoperative infection and contralateral injury and (4) magnetic resonance images at 12 and 24 months after ACLR.
Ethics and dissemination
This trial was approved by the Medical Ethics Committee of the Xiangya Hospital of Central South University on 26 October 2021. Data will be published in peer-reviewed journals and presented at national and international conferences.
Trial registration number
ChiCTR2200057526.
Keywords: knee, orthopaedic sports trauma, orthopaedic & trauma surgery, clinical trials, adult orthopaedics
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study aims to demonstrate the clinical superiority of the Internal brace compared to conventional anterior cruciate ligament reconstruction (ACLR) through a 2-year follow-up period.
This study will fill an existing gap regarding the efficacy of the internal brace in ACLR by providing robust and high-quality evidence on its role and impact.
The study has a standard and detailed rehabilitation programme to ensure a smooth recovery after ACLR.
This trial was conducted in a single centre, and data lacked generalisability.
Introduction
Anterior cruciate ligament (ACL) rupture is one of the most common knee ligament injuries occurring in young athletes. Mayo-Robson of Leeds performed the first ACL repair in 1895, which was followed by initiation of ACL reconstruction (ACLR) using autologous tissue by Grekow and Hey Groves between 1914 and 1920.1 If ACL ruptures are not actively treated after injury, joint instability and other phenomena often occur, which reduces the quality of life and increases the risk of osteoarthritis.2–4 Over the past few decades, ACL ruptures have been estimated to occur in approximately 30–52 cases per 100 000 person-years.5 ACL injuries reportedly occur in more than 175 000 people annually in the USA, and approximately 100 000 undergo surgery.6–8 With the continuous development of surgical techniques, the current mainstream surgical method involves performing ACLR under arthroscopy.9 A Japanese study reported that the redisruption rate of ACLR grafts after returning to the field was 23% for rugby players under the age of 20 years.10 At the same time, other studies have also shown that the revision rate of ACLR with an allogeneic tendon in adolescents can reach 35%11; however, the effect after revision is not as good as that of primary ACLR.12
Internal brace technology for ligament repair has been promoted since 2010. It uses braided ultrahigh molecular weight polyethene polyester suture tape and knotless bone anchors to reinforce ligament strength, also known as an auxiliary stabilising structure for recovery of motion after ligament repair, which helps prevent secondary damage,13 such as anterior and posterior cruciate ligament (PCL), medial and lateral collateral ligament (LCL) repair of the knee13–15 and ankle and elbow ligament strengthening repair.16 17 Enhanced collateral ligament repair and reconstruction with an internal brace improve limb biomechanics, including greater stiffness and maximum load, while facilitating early rehabilitation in motor and biomechanical environments.12 14 Reinforced ligament repair offers unique advantages over traditional reconstruction techniques, including smaller bores and implants, no risk of disease transmission from the allografts and no risk of tunnel convergence during the procedure.14 18 19 Therefore, ACL reinforcement is an alternative method for synergistically supporting ACL grafts and load sharing, with primary tension on the graft and high-strength suture tape.
In ACLR, the internal brace ligament augmentation technique helps prevent various failure scenarios, including creep and irreversible stretching, traumatic tears and slippage of the tendon-bone interface.20 21 In addition, these failures can be avoided when the graft is small or vulnerable.20 21 Smith in an in vitro trial conducted in 2018 found that ACLR combined with independent suture tape significantly reduced graft elongation and allowed the grafted ligaments to accept higher ultimate disruption loads, thereby reducing the risk of graft rerupture.20 Preliminary short-term studies have shown that the internal brace technique can significantly improve functional recovery after ACLR, thereby enhancing the patient’s quality of life. However, only a few medical institutions use ACLR combined with internal brace technique, and more relevant in vivo clinical evidence is required.
This study was undertaken with an aim to fill this gap by exploring whether internal brace technology can improve the outcomes of patients with ligament injuries and provide a new option for patients with ACL injuries.
Methods and analysis
Study setting
This randomised parallel-controlled trial will be conducted in accordance with the Consolidated Standards of Reporting Trials statement.22 Patients with ACL rupture who were hospitalised in the Department of Orthopaedics at Xiangya Hospital of Central South University between 1 March 2022 and 28 February 2023 formed our study population. The Medical Ethics Committee of Xiangya Hospital of Central South University approved the ethical application related to this study and filed it (ethical approval number: 202110478). All patients signed an informed consent form prior to the surgery.
Eligibility criteria
The inclusion criteria were: (1) age: 16–45 years; (2) unilateral knee MRI showing unilateral knee ACL fracture; (3) combined meniscal injury that does not interfere with the standard postoperative rehabilitation programme after intraoperative management; (4) combined grade III or lower cartilage injury that does not interfere with the standard postoperative rehabilitation programme; (5) a minimum of 24 months of follow-up; (6) no previous injury to the healthy knee; (7) informed consent provided by the participant and signed relevant documents.
The exclusion criteria were: (1) age of <16 years or >45 years; (2) previous ACLR or bilateral ACL injury; (3) MRI revealing PCL, medial collateral ligament or LCL injury; (4) grade IV cartilage injury or unstable longitudinal meniscus tear requiring repair that interferes with standard rehabilitation protocols after surgical management; (5) not meeting the requirement of 24 follow-up visits; (6) patients with severe underlying medical conditions that make surgery inadvisable, or patients with mental illness, pregnancy during planned trials or other conditions that are not conducive for long-term follow-up.
Participant selection
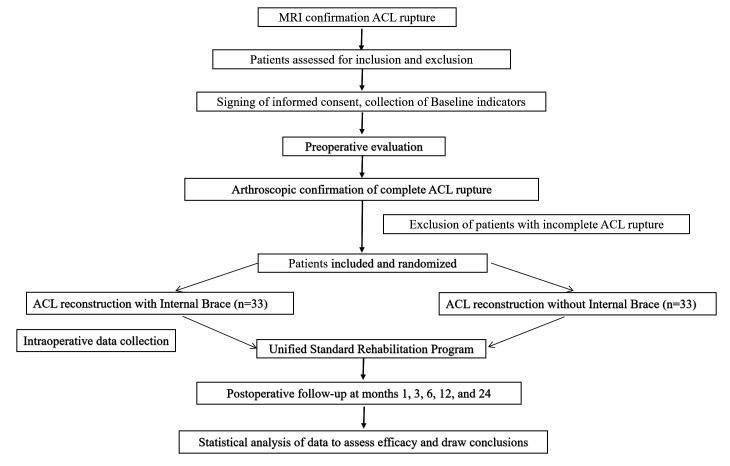
Patients diagnosed with ACL rupture by clinicians through physical examination and MRI were randomly allocated to the trial and control groups after final confirmation of ACL rupture under arthroscopy and agreeing to participate in this study. The trial group underwent ACLR with the internal brace technique, while the control group underwent ACLR without the internal brace technique. Preoperative assessment of the patients was performed before surgery, and a uniform rehabilitation programme was performed after surgery. The inclusion start date of the protocol (V.1.0, March 2022) was March 2022, with an expected cut-off date of March 2023. The follow-up period is 2 years, with the last follow-up expected in March 2025 (the exact end date will be based on the inclusion of the last participant). The specific technical route is shown in figure 1.
Figure 1.
Study flow diagram. ACL, anterior cruciate ligament.
Study sample
International Knee Documentation Committee (IKDC) score consists of three aspects: (1) symptoms, including pain, stiffness, swelling, interlocking/jamming and softening of the legs; (2) motion and daily activities and (3) current knee function. However, knee function prior to knee injury does not count towards the total score, which is the main outcome indicator of this study, based on which the sample size required for this trial was estimated. IKDC scores are continuous measurement data, and this study compares the statistical differences between the means±SD of the two groups. The minimum clinically important difference (MCID) of the IKDC scale was reported to range between 8.8 and 15.6.23–25 We set δ to 10, and the overall sample SD was set to 13 based on the relevant literature.23–25 To satisfy the power of a test (1-β, β means type II error) of 0.8 and the test level α (type I error) of 0.05, the sample size calculated for each group was 27 according to the following formula. Considering a 20% lost visit rate, 33 patients were included in each group with a total of 66 patients included in the study.
Randomisation and concealed grouping
Using a computer-generated random number list, all eligible participants were randomly assigned in a 1:1 ratio to the ACLR endoprosthesis technique group (combined group, n=33) and the standard ACLR group (simple group, n=33), with no restrictions on either group. Randomisation was performed by an investigator who was not involved in the study. Allocation results were sealed in opaque envelopes and maintained by the study coordinator. On the day of surgery, one envelope per patient was given to the surgeon by the study coordinator. The participants and physicians included in the study were informed of the grouping, but neither the rehabilitators who instructed the patients on rehabilitation nor the data collectors who conducted the follow-up visits were aware of the grouping.
Interventions and surgical techniques
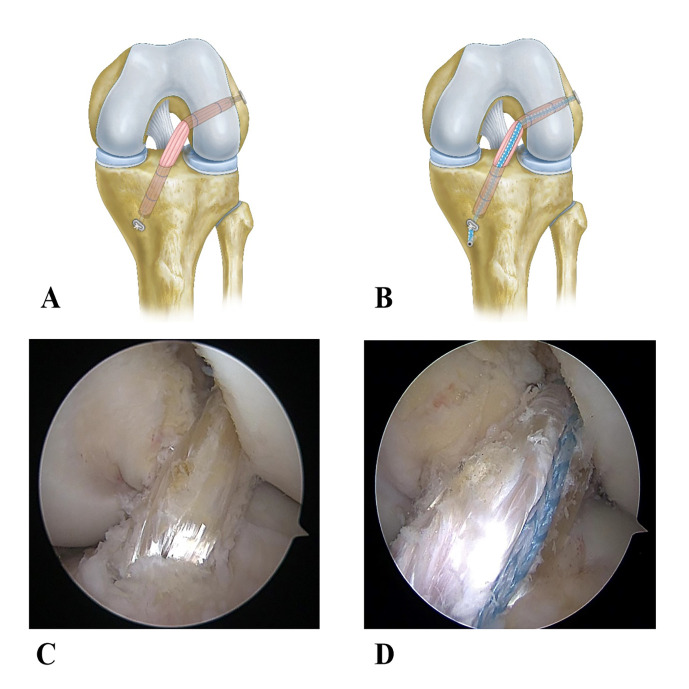
In all patients, all-inside ACLR was performed using autologous semitendinosus tendons with arthroscopy. Semitendinosus tendons were braided with sutures (0 FiberWire Suture, Arthrex) to form four strands of ACL grafts (all between 7.5 mm and 10 mm in diameter, supplemented with semimembranous tendons less than 7.5 mm in diameter). In the ACLR group, the braided graft femoral end and tibial segment were suspended and fixed with a TightRope (ACL TightRope RT Implant, Arthrex). On the other hand, in the ACLR with internal brace group, a separate wire tape (2 mm FiberTape, Arthrex) was added in addition to the components and technique used in the ACLR group. The suture was fixed with a knotless bone anchor (4.75 mm PEEK SwiveLock, Arthrex) distally and a TightRope suspension proximally as in the graft (figure 2).
Figure 2.
(A) and (B) are the models of control and test groups, respectively. (C) and (D) are the pictures of control and test groups after intraoperative arthroscopic ACLR, respectively. ACLR, anterior cruciate ligament reconstruction.
The procedure was as follows: the patient was anaesthetised, a tourniquet was applied to the affected limb, routine surgical disinfection of the knee was performed, a sterile surgical sheet was placed, the semitendinosus tendon was palpated, a straight incision was made medial to the tibial tuberosity, the semitendinosus tendon was removed with a tendon extractor, 10 mL of ropivacaine was injected at the tendon extraction site and the removed semitendinosus tendon was braided into four strands using the GraftLink (Arthrex) technique.26 A conventional knee arthroscopic approach was used on the anteromedial and anterolateral sides of the affected knee to explore the injured structures and remove the remaining portion of the ACL. The knee was flexed to the extreme, and the femoral tract was created at the footprint of the ACL stop at the lateral femoral condyle and the tibial tract at the footprint of the ACL start. The graft and two TightRope titanium plates were pulled into the knee cavity and removed through the femoral and tibial tracts, respectively. The tabs were flipped, the tab rings were tightened, and the titanium plates were fixed. In the ACLR with an internal brace group, the femoral end of the FiberTape wire band was fixed with a TightRope ring, the wire band was tensioned in the extended position and the tibial end was fixed independently with a knotless bone anchor (4.75 mm PEEK SwiveLock, Arthrex).
All patients underwent uniform training and rehabilitation programme after surgery. The entire rehabilitation process was divided into seven phases. The first three phases focused on controlling swelling and restoring the range of motion, which usually required 4 weeks. The detailed rehabilitation plan is presented in table 1. Phases 4–5 focused on restoring quadriceps muscle strength control and balance along with core strength restoration training. Phases 6–7 included gradual resumption of various sport activities, from daily activities to professional activities or contact sports. A detailed rehabilitation plan is presented in tables 2 and 3.
Table 1.
Phases 1–3 of the post-ACLR rehabilitation programme
| Phase | Movement exercises | Gait exercises | Manipulative massage | Rehabilitation goals | Precautions |
| Phase 1 (within 1 week) |
|
|
|
Active mobility of the knee joint reaches 0°–90°. (1) The anterolateral thigh muscles can be tightened better. (2) The affected limb can be fully weight-bearing with the help of braces and crutches. (3) Oedema control is good. (4) Good wound healing. |
|
| Phase 2 (1–2 weeks) |
|
|
|
Active mobility of the knee joint reaches 0°−120°. (1) Straight leg elevation; anterior thigh muscles can be tightened with force. (2) The patient can walk normally using crutches with a nonlocking brace. |
|
| Phase 3 (2–4 weeks) |
|
Exercise with a rope tied around the waist or on a treadmill for forward and backward gait training. |
|
The knee joint moves to an active full angle, aligning with the healthy side leg. (1) Normal gait can be achieved without any walking aid. (2) Self-care in daily life (may have difficulty walking up and down steps). | Fully weight bearing; normal gait can be achieved without a brace or walker at 3 weeks postoperatively. |
ACLR, anterior cruciate ligament reconstruction.
Table 2.
Phases 4 and 5 of the post-ACLR rehabilitation programme
| Phase | Movement exercises | Gait exercises | Manipulative massage | Rehabilitation goals | Precautions |
| Phase 4 (4–8 weeks) |
|
Go around the obstacles at normal walking speed on different surfaces. | Manual method to loosen surgical scars. |
|
|
| Phase 5 (8–12 weeks) |
|
—— | —— |
|
Patellar tendonitis may occur. |
ACLR, anterior cruciate ligament reconstruction.
Table 3.
Phases 6 and 7 of the post-ACLR rehabilitation programme
| Phase | Movement exercises | Gait exercises | Manipulative massage | Rehabilitation goals | Precautions |
| Phase 6 (12–16 weeks) |
|
—— | —— |
|
Avoid wrong movements or posture. (1) Landing with the knee joint too straight. (2) The knee joint is turned outward or inward when landing. (3) When landing or bouncing, the healthy leg always takes the lead, not the affected leg. |
| Phase 6 (12–16 weeks) |
|
—— | —— | After 20 weeks, they participate in a professional sports programme. | —— |
ACLR, anterior cruciate ligament reconstruction.
Baseline indicators and observations
Baseline and preoperative patient characteristics included sex, age, affected limb (left or right), cause of injury (playing basketball or other sports such as soccer), smoking status (yes or no), time from injury to surgery (fresh injury or old rupture), whether the injury was accompanied by cartilage and meniscal damage, time of surgery and degree of ACL fracture (partial or total).
Study endpoints
Primary outcome/endpoint
IKDC score changes in knee function from preoperative period to 6 months postoperatively was the primary outcome. The IKDC is a commonly used tool to evaluate outcomes after knee surgery, including ACLR.27 The IKDC knee score consists of knee assessment (10 entries) and knee ligament checklist (eight entries), covering joint pain, motor level and daily activity ability, with total score ranging between 0 and 100. The IKDC can be used to assess knee symptoms, function and physical activity. The IKDC assesses symptoms, function and physical activity of the knee. Patients were evaluated using a questionnaire preoperatively and at follow-up visits at 1, 3, 6, 12, and 24 months postoperatively.
Secondary outcomes/endpoints
Lysholm knee score (LKS), Knee Injury and Osteoarthritis Outcome Score (KOOS, including knee symptoms, pain, activities of daily living, sports and recreational activities and quality of life), Visual Analog Scale (VAS, for pain assessment), Lachman test, MRI data and assessment of knee stability using the Kneelax3 knee stability metre.
Kneelax3 (MONITORED REHAB SYSTEMS B.V. Model: KNEELAX3, The Netherlands): the Kneelax3 arthrometer was used for assessment of knee stability preoperatively and 1, 3, 6, 12 and 24 months postoperatively.
LKS: this scoring system consists of eight questions scored on a scale of 0–100, with higher scores representing better functional status of the patient. The main tendency is towards activities of daily living, and patients were assessed using questionnaires before surgery and at 1, 3, 6, 12 and 24 months of postoperative follow-up visits.28
KOOS score: the KOOS consists of five subscales: pain, other knee symptoms, activities of daily living, function in sports and recreation (Sport/Rec) and knee-related quality of life.29 Patients were given 1 week for consideration before answering the questions, and each question had five alternative boxes with scores ranging between 0 and 4. Standard scores were calculated for each subscale (100 for no symptoms and 0 for extreme symptoms), and patients were assessed using questionnaires before surgery and at follow-up visits at 1, 3, 6, 12 and 24 months after surgery.
VAS score: the visual analogue pain scoring method is more sensitive and comparable. In this trial, a 100 mm VAS pain score (including resting-state score, 30 min post-walk score and overall pain level in the past month) was used.30 This protocol only assessed pain at rest, with 0 representing no pain and 100 representing the most severe pain. Patients were evaluated using questionnaires before surgery and at 1, 3, 6, 12 and 24 months of postoperative follow-up.
Lachman test: this assesses ACL function with the patient in supine or prone position and knee flexed at an angle of approximately 30°.31 The examiner uses one hand to immobilise the thigh while the other hand attempts to move the tibia forward. Positive results suggest that patients with ACL injuries be tested preoperatively and at follow-up visits at 1, 3, 6, 12 and 24 months postoperatively.
MRI assessment: MRI was performed at 12 and 24 months postoperatively to assess the patient’s reconstructed ACL.
Patient termination and withdrawal criteria
Patients could withdraw from the trial at any time due to the following reasons:
Surgical failure: (1) occurrence of infection; (2) no secondary rupture of the reconstructed ligament (rupture of the ligament or the internal brace augmentation line or both in the trial group, rupture of the reconstructed ligament in the control group) and (3) knee instability: self-reported knee instability, or Lachman test (+) or Kneelax3 knee arthrometer test revealing a 3 mm difference in comparison with the healthy side.
Patient withdrawal from the trial: all participants had the right to withdraw at any time during the study period. A participant was withdrawn from the trial if any of the following occurred during the trial: (1) withdrawal of informed consent by the participant; (2) a person who, in the opinion of the investigator, was no longer suitable for continuation of the clinical trial; (3) a woman who became pregnant during the clinical trial; (4) death of the participant or (5) the participant was lost to follow-up.
Trial termination: the clinical trial institution and investigator found that the risks to patients by continuing the clinical trial exceeded the possible benefits; (2) the ethics committee found that the rights of the participants could not be protected; (3) the sponsor requested termination of the trial for various reasons and (4) the national administrative authority requested termination.
The timing and reasons for withdrawal from the trial were recorded in detail in the case report form. Follow-up care was no longer provided for participants who voluntarily withdrew from the treatment as well as for those for whom follow-up data were not being collected. However, participants who withdrew from the trial due to adverse events or because of surgical failure were to be followed up until the adverse events stabilised or resolved or until the investigator deemed that further follow-up was no longer necessary.
Data management
The trial used an electronic data collection (EDC) system for data management. The investigator or investigator-authorised research staff completed the electronic Case Report Form through the EDC system in an accurate, timely, complete and standardised manner, based on the original information from the participants. Questionnaire checking, data cleaning and summarisation were performed promptly after each follow-up visit. A follow-up survey is proposed to adopt the electronic questionnaire system and on-site questionnaire survey. The on-site survey results will be saved in time and organised in the database later. The investigator or investigator’s authorised researcher will enter the data into the EDC according to their respective accounts. The data administrator verifies the reliability, completeness and accuracy of the data in the EDC. If any questionable data are found, a challenge can be issued in the system, and the investigator or the investigator’s delegated researcher will verify, correct or answer the query. When all the data have been entered into the database and all queries have been resolved, the database will be locked by the data administrator. If a problem is found after the database is locked and there is a need to correct it, the process of unlocking and relocking the data will be followed. After the database is locked, the data manager will submit the data to the analyst for statistical analysis as scheduled.
Statistical analysis
Statistical design: this was a randomised controlled clinical trial.
-
Principles of statistical analysis: all statistical analyses were performed using SAS V.9.4 or later, R V.3.3.2 or later, or pSPSS V.24 (IBM Corporate Headquarters, Armonk, New York). All statistical tests were two-sided tests, and p values ≤0.05 were considered statistically significant for the differences tested (unless otherwise specified).
Summary statistics for continuous variables: including the mean, SD, median, minimum, maximum, lower quartile (Q1) and upper quartile (Q3); summary statistics for categorical variables, including the number of cases and percentages of each category.
Between-group comparisons of demographic baseline characteristics: group comparisons for continuous variables will be made using independent samples t-test (χ2, normal distribution) or Wilcoxon rank-sum test depending on data distribution and χ2 test or exact probability method for categorical variables (if χ2 test is not applicable).
Completion and demographic analyses: baseline analyses were based on full analysis set (FAS). The enrolment and completion status of the trials were summarised and the reasons for non-completion are described in a detailed table. The participants’ demographic characteristics were described and compared with measure comparability between the two groups. Validity reporting data were accepted only if the baseline was balanced between the groups; otherwise, the validity data were corrected before reporting.
Patient-reported outcome validity evaluation indices: outcome validity evaluation will be based on the FAS and the per-protocol set. The statistical description and inference of the data will be based on the characteristics of the data, the selection of applicable descriptive indicators, and hypothesis testing methods.
Primary patient-reported outcome: comparison of IKDC knee scores at 6 months (±2 weeks) postoperatively in the trial and control groups. We will use a linear regression model for analysis to correct for possible confounding factors such as age, sex and cause of injury. Preoperative baseline IKDC scores will be used as predictors when conducting the analysis, and IKDC scores at 6 months (±2 weeks) will be used as indicators of post-treatment outcomes.
Secondary patient-reported outcomes
Since IKDC scores were measured multiple times during the follow-up period, we will use a linear mixed model to compare the changes over time between the trial and control groups.
The anterior tibial translation distance is measured using the Kneelax3 knee stability metre, and comparisons of the anterior tibial translation distance between the test and control groups at the same postoperative time points with the same force will be performed using either an independent samples t-test (χ2, normally distributed) or a Wilcoxon rank-sum test. A linear mixed model will also be used to compare and analyse the evolution of these consecutive results over time in both groups.
A comparative analysis of failure and infection rates in the control group versus the test group during the main 6 months will be performed using the χ2 test or t-test.
Linear regression of continuous outcomes from baseline to 24 months, such as LKS, KOOS score (including knee symptoms, pain, activities of daily living, sports and recreational activities, quality of life), Lachman test and pain VAS protocols, will be used to compare and analyse the evolution of these continuous outcomes over time in the two groups using linear mixed models.
The morphology and signal intensity of the postoperative ACL will be assessed using MRI, and different treatments will be compared at different time points using χ2 test.
Analysis of safety indicators
Safety evaluation will be based on a safety-set (SS) analysis dataset. The internationally accepted Medical Dictionary for Regulatory Activities (MedDRA) term set classification was used for adverse event coding. The types of adverse events, frequency, severity and relationship with internal brace enhancement line generation and surgery were summarised by group. A detailed list of the various adverse events is provided, with special notations for participants who discontinued the trial because of adverse events and for those who experienced serious adverse events. The proportions of patients who developed complications between treatments were compared using the χ2 test.
Patient and public involvement
Patients and/or the public were not involved in the study design, conduct, reporting, or dissemination plans.
Discussion
This prospective, randomised controlled trial aimed to investigate the clinical efficacy of ACLR with and without the internal brace technique. In this 2-year follow-up study, the subjective, objective and functional outcomes of patients who underwent ACLR with the internal brace technique or ACLR alone were compared. This study hypothesised that ACLR with internal brace technique would be more stable than ACLR alone in the early postoperative period, with a lower incidence of secondary injury, reduced duration and extent of pain and earlier return to preinjury activity levels. However, there may be no significant difference between the two groups in terms of patient-reported outcome indicators with increase in recovery time.
ACL rupture is a common knee injury among athletes and has been extensively studied. Studies from as early as the late 20th century have shown that ACLR is superior to ACL repair.32 33 The ACL is an important structure for maintaining knee stability, preventing anterior tibial displacement and limiting intratibial rotation,34 making ACLR the gold standard treatment for patients recovering motion or performing rotational activities after ACL rupture in the knee.35 The recovery of knee function after surgery depends on the ability of the ACL graft to withstand appropriate loads during rehabilitation and after returning to sports. Tendon grafts implanted in the human knee joint survive in the intra-articular environment and gradually in the ligaments. The graft is fragile and vulnerable to reinjury during the preremodelling phase before ligamentisation,36 so a carefully designed rehabilitation programme should be developed to prevent reinjury during the rehabilitation period. Several studies have found a higher probability of rerupture or secondary revision in athletes and adolescents or for ACLR using either autologous or allogeneic tendons.10 37 Therefore, surgical approachs that increases the structural strength of the graft and protects it during the early stages of graft ligamentization are important.
The biomechanical properties of the intraarticular reconstructed ligaments reportedly improved 8 weeks postoperatively when the FiberTape suture was applied in a rabbit model. Additionally, during this period, FiberTape did not adversely affect bone tunnel healing or cause a long-term increase in indicators of inflammation.12 In a dog model, no severe inflammation, immune response, bone erosion or premature osteoarthritis development was observed 6 months postoperatively.38 The results of these studies support the biocompatibility and safety of intra-articular suture tape for ACLR enhancement.
A study on load sharing after ACL graft enhancement using suture tape reported that load sharing began at 200 N and 300 N for 7 mm and 9 mm grafts, respectively. The final peak load (400 N) was shared by 31% (7 mm graft) and 20% (9 mm graft) when using suture tape.39 Suture tape ligament augmentation may protect biological grafts from excessive peak loading and elongation. After ACLR in the early recovery phase, suture band augmentation reportedly increases ACL graft stiffness by 104% and the ultimate breaking load by 57%, reducing the graft failure rate in clinical situations.
A recent systematic review by Christopher et al concluded from biomechanical, animal and clinical studies that suture tape augmentation in ACLR increased biomechanical stability.40 Therefore, in this randomised controlled trial, we will conduct a prospective observational comparison and long-term follow-up to elucidate the clinical efficacy of internal bracing in ACLR.
Ethics and dissemination
The trial was approved by the Medical Ethics Committee of Xiangya Hospital of Central South University on 26 October 2021 (ethics number ‘202110478’) and prospectively registered in the China Clinical Trials Registry on 14 March 2022 (registration number: ChiCTR2200057526). All participants signed an informed consent form before participating in this trial, and we will protect the patients from any invasion of their privacy. All investigators will keep the study results confidential until after the data are made public and will release no data related to the database without approval from the principal investigator. We will publish our findings and data in peer-reviewed journals and present them at national and international conferences.
Supplementary Material
Acknowledgments
We would like to extend our gratitude to all the medical and nursing staff of the Department of Bone and Joint Surgery of Xiangya Hospital of Central South University for their unfailing care and great help to our patients. We also thank the patients for their support and trust in us. We also thank Djandan Tadum Arthur Vithran, who contributed greatly to revising and improving the language of the manuscript.
Footnotes
Contributors: WfX and YL established the study design; WL conceived the design and wrote the draft manuscript; DL, ZC and LP were involved in data acquisition, analysis, and interpretation; and WqX, HJ and XL designed the rehabilitation protocol. All the authors have read and approved the final version of the manuscript.
Funding: This work was supported by National Clinical Research Center for Geriatric Disorders (Xiangya Hospital, Grant No.2021KFJJ02 and 2021LNJJ05), National Clinical Research Center for Orthopedics, Sports Medicine and Rehabilitation (2021-NCRC-CXJJ-PY-40), and National Natural Science Foundation of Hunan Province (2021JJ31105, 2023JJ30949).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Robson AW. VI. ruptured crucial ligaments and their repair by operation. Ann Surg 1903;37:716–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson BE, Culvenor AG, Barton CJ, et al. Worsening knee osteoarthritis features on magnetic resonance imaging 1 to 5 years after anterior cruciate ligament reconstruction. Am J Sports Med 2018;46:2873–83. 10.1177/0363546518789685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suter LG, Smith SR, Katz JN, et al. Projecting lifetime risk of symptomatic knee osteoarthritis and total knee replacement in individuals sustaining a complete anterior cruciate ligament tear in early adulthood. Arthritis Care Res (Hoboken) 2017;69:201–8. 10.1002/acr.22940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filbay SR, Culvenor AG, Ackerman IN, et al. Quality of life in anterior cruciate ligament-deficient individuals: a systematic review and meta-analysis. Br J Sports Med 2015;49:1033–41. 10.1136/bjsports-2015-094864 [DOI] [PubMed] [Google Scholar]
- 5.Moses B, Orchard J, Orchard J. Systematic review: annual incidence of ACL injury and surgery in various populations. Res Sports Med 2012;20:157–79. 10.1080/15438627.2012.680633 [DOI] [PubMed] [Google Scholar]
- 6.Griffin LY, Albohm MJ, Arendt EA, et al. Understanding and preventing noncontact anterior Cruciate ligament injuries: a review of the hunt valley II meeting. Am J Sports Med 2006;34:1512–32. 10.1177/0363546506286866 [DOI] [PubMed] [Google Scholar]
- 7.de Sa D, Shanmugaraj A, Weidman M, et al. All-inside anterior cruciate ligament reconstruction-A systematic review of techniques, outcomes, and complications. J Knee Surg 2018;31:895–904. 10.1055/s-0038-1627446 [DOI] [PubMed] [Google Scholar]
- 8.Di Benedetto P, Di Benedetto E, Fiocchi A, et al. Causes of failure of anterior cruciate ligament reconstruction and revision surgical strategies. Knee Surg Relat Res 2016;28:319–24. 10.5792/ksrr.16.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaishya R, Agarwal AK, Ingole S, et al. Current trends in anterior cruciate ligament reconstruction: a review. Cureus 2015;7:e378. 10.7759/cureus.378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takazawa Y, Ikeda H, Saita Y, et al. Return to play of rugby players after anterior cruciate ligament reconstruction using hamstring autograft: return to sports and graft failure according to age. Arthroscopy 2017;33:181–9. 10.1016/j.arthro.2016.06.009 [DOI] [PubMed] [Google Scholar]
- 11.Ellis HB, Matheny LM, Briggs KK, et al. Outcomes and revision rate after bone-patellar tendon-bone allograft versus autograft anterior cruciate ligament reconstruction in patients aged 18 years or younger with closed Physes. Arthroscopy 2012;28:1819–25. 10.1016/j.arthro.2012.06.016 [DOI] [PubMed] [Google Scholar]
- 12.Soreide E, Denbeigh JM, Lewallen EA, et al. In vivo assessment of high-molecular-weight polyethylene core Suture tape for intra-articular ligament reconstruction: an animal study. Bone Joint J 2019;101-B:1238–47. 10.1302/0301-620X.101B10.BJJ-2018-1282.R2 [DOI] [PubMed] [Google Scholar]
- 13.Lubowitz JH, MacKay G, Gilmer B. Knee medial collateral ligament and posteromedial corner anatomic repair with internal bracing. Arthrosc Tech 2014;3:e505–8. 10.1016/j.eats.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilmer BB, Crall T, DeLong J, et al. Biomechanical analysis of internal bracing for treatment of medial knee injuries. Orthopedics 2016;39:e532–7. 10.3928/01477447-20160427-13 [DOI] [PubMed] [Google Scholar]
- 15.Heusdens CHW, Hopper GP, Dossche L, et al. Anterior cruciate ligament repair with independent suture tape reinforcement: a case series with 2-year follow-up. Knee Surg Sports Traumatol Arthrosc 2019;27:60–7. 10.1007/s00167-018-5239-1 [DOI] [PubMed] [Google Scholar]
- 16.Nishimura A, Nakazora S, Senga Y, et al. Arthroscopic internal brace augmentation with arthroscopic modified broström operation for chronic ankle instability. Arthrosc Tech 2021;10:e995–1000. 10.1016/j.eats.2020.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilk KE, Arrigo CA, Bagwell MS, et al. Repair of the ulnar collateral ligament of the elbow: rehabilitation following internal brace surgery. J Orthop Sports Phys Ther 2019;49:253–61. 10.2519/jospt.2019.8215 [DOI] [PubMed] [Google Scholar]
- 18.van Eck CF, Limpisvasti O, ElAttrache NS. Is there a role for internal bracing and repair of the anterior cruciate ligament? A systematic literature review. Am J Sports Med 2018;46:2291–8. 10.1177/0363546517717956 [DOI] [PubMed] [Google Scholar]
- 19.Cook JL, Smith P, Stannard JP, et al. A canine arthroscopic anterior cruciate ligament reconstruction model for study of synthetic augmentation of tendon allografts. J Knee Surg 2017;30:704–11. 10.1055/s-0036-1597618 [DOI] [PubMed] [Google Scholar]
- 20.Bachmaier S, Smith PA, Bley J, et al. Independent suture tape reinforcement of small and standard diameter grafts for anterior cruciate ligament reconstruction: a biomechanical full construct model. Arthroscopy 2018;34:490–9. 10.1016/j.arthro.2017.10.037 [DOI] [PubMed] [Google Scholar]
- 21.Bedi A. “Editorial commentary: buckle up surgeons: "safety belt" reinforcement of knee anterior cruciate ligament reconstruction grafts”. Arthroscopy 2018;34:500–1. 10.1016/j.arthro.2017.12.009 [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg 2012;10:28–55. 10.1016/j.ijsu.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 23.Collins NJ, Misra D, Felson DT, et al. Measures of knee function: International knee documentation committee (IKDC) subjective knee evaluation form, knee injury and osteoarthritis outcome score (KOOS), knee injury and osteoarthritis outcome score physical function short form (KOOS-PS). Arthritis Care Res (Hoboken) 2011;63 Suppl 11:S208–228. 10.1002/acr.20632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irrgang JJ, Anderson AF, Boland AL, et al. Responsiveness of the international knee documentation committee subjective knee form. Am J Sports Med 2006;34:1567–73. 10.1177/0363546506288855 [DOI] [PubMed] [Google Scholar]
- 25.Li Y-L, Ning G-Z, Wu Q, et al. Single-bundle or double-bundle for anterior cruciate ligament reconstruction: a meta-analysis. Knee 2014;21:28–37. 10.1016/j.knee.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 26.Lubowitz JH. All-inside anterior cruciate ligament graft link: graft preparation technique. Arthrosc Tech 2012;1:e165–8. 10.1016/j.eats.2012.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greco NJ, Anderson AF, Mann BJ, et al. Responsiveness of the international knee documentation committee subjective knee form in comparison to the Western Ontario and Mcmaster universities osteoarthritis index, modified cincinnati knee rating system, and short form 36 in patients with focal articular cartilage defects. Am J Sports Med 2010;38:891–902. 10.1177/0363546509354163 [DOI] [PubMed] [Google Scholar]
- 28.Briggs KK, Lysholm J, Tegner Y, et al. The reliability, validity, and responsiveness of the lysholm score and tegner activity scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med 2009;37:890–7. 10.1177/0363546508330143 [DOI] [PubMed] [Google Scholar]
- 29.Collins NJ, Prinsen CAC, Christensen R, et al. Knee injury and osteoarthritis outcome score (KOOS): systematic review and meta-analysis of measurement properties. Osteoarthritis Cartilage 2016;24:1317–29.:S1063-4584(16)01071-2. 10.1016/j.joca.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 30.Wewers ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health 1990;13:227–36. 10.1002/nur.4770130405 [DOI] [PubMed] [Google Scholar]
- 31.Coffey R, Bordoni B. Treasure Island (FL): StatPearls Publishing Copyright © 2022. StatPearls Publishing LLC, 2022. [Google Scholar]
- 32.Engebretsen L, Benum P, Fasting O, et al. A prospective, randomized study of three surgical techniques for treatment of acute ruptures of the anterior cruciate ligament. Am J Sports Med 1990;18:585–90. 10.1177/036354659001800605 [DOI] [PubMed] [Google Scholar]
- 33.Grøntvedt T, Engebretsen L, Benum P, et al. A prospective, randomized study of three operations for acute rupture of the anterior cruciate ligament. five-year follow-up of one hundred and thirty-one patients. J Bone Joint Surg Am 1996;78:159–68. 10.2106/00004623-199602000-00001 [DOI] [PubMed] [Google Scholar]
- 34.Noyes FR. The function of the human anterior cruciate ligament and analysis of single- and double-bundle graft reconstructions. Sports Health 2009;1:66–75. 10.1177/1941738108326980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ardern CL, Webster KE, Taylor NF, et al. Return to sport following anterior cruciate ligament reconstruction surgery: a systematic review and meta-analysis of the state of play. Br J Sports Med 2011;45:596–606. 10.1136/bjsm.2010.076364 [DOI] [PubMed] [Google Scholar]
- 36.Claes S, Verdonk P, Forsyth R, et al. “The "ligamentization" process in anterior cruciate ligament reconstruction: what happens to the human graft? A systematic review of the literature”. Am J Sports Med 2011;39:2476–83. 10.1177/0363546511402662 [DOI] [PubMed] [Google Scholar]
- 37.Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am 2012;94:531–6. 10.2106/JBJS.K.00733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith PA, Bozynski CC, Kuroki K, et al. Intra-articular biocompatibility of multistranded, long-chain polyethylene suture tape in a canine ACL model. J Knee Surg 2019;32:525–31. 10.1055/s-0038-1655765 [DOI] [PubMed] [Google Scholar]
- 39.Bachmaier S, Smith PA, Argintar EH, et al. Independent suture augmentation with all-inside anterior cruciate ligament reconstruction reduces peak loads on soft-tissue graft. A biomechanical full-construct study. Arthroscopy 2022;38:88–98. 10.1016/j.arthro.2021.09.032 [DOI] [PubMed] [Google Scholar]
- 40.E A Mackenzie C, Huntington LS, Tulloch S. Suture tape augmentation of anterior cruciate ligament reconstruction increases biomechanical stability: a scoping review of biomechanical, animal, and clinical studies. Arthroscopy 2022;38:2073–89. 10.1016/j.arthro.2021.12.036 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.