Abstract
Introduction
The genetic determinants of fractional exhalation of nitric oxide (FeNO), a marker of lung inflammation, are understudied in Black individuals. Alpha globin (HBA) restricts nitric oxide signalling in arterial endothelial cells via interactions with nitric oxide synthase; however, its role in regulating the release of NO from respiratory epithelium is less well understood. We hypothesised that an HBA gene deletion, common among Black individuals, would be associated with higher FeNO.
Methods
Healthy Black adults were enrolled at four study sites in North Carolina from 2005 to 2008. FeNO was measured in triplicate using a nitric oxide analyzer. The −3.7 kb HBA gene deletion was genotyped using droplet digital PCR on genomic DNA. The association of FeNO with HBA copy number was evaluated using multivariable linear regression employing a linear effect of HBA copy number and adjusting for age, sex and serum immunoglobulin-E levels. Post-hoc analysis employing a recessive mode of inheritance was performed.
Results
895 individuals were in enrolled in the study and 720 consented for future genetic research; 643 had complete data and were included in this analysis. Median (25th, 75th) FeNO was 20 (13, 31) ppb. HBA genotypes were: 30 (4.7%) -a/-a, 197 (30.6%) -a/aa, 405 (63%) aa/aa and 8 (1.2%) aa/aaa. Subjects were 35% male with median age 20 (19, 22) years. Multivariable linear regression analysis revealed no association between FeNO and HBA copy number (β=−0.005 (95% CI −0.042 to 0.033), p=0.81). In the post-hoc sensitivity analysis, homozygosity for the HBA gene deletion was associated with higher FeNO (β=0.107 (95% CI 0.003 to 0.212); p=0.045).
Conclusion
We found no association between HBA copy number and FeNO using a prespecified additive genetic model. However, a post hoc recessive genetic model found FeNO to be higher among subjects homozygous for the HBA deletion.
Keywords: Exhaled Airway Markers, Airway Epithelium, Clinical Epidemiology, Lung Physiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
The alpha subunit of haemoglobin restricts the release of nitric oxide from vascular endothelial cells, but alpha globin’s role in restricting exhaled nitric oxide is less well understood.
WHAT THIS STUDY ADDS
We examined the association between fractional exhaled nitric oxide and deletion of the alpha globin gene, a frequent genetic polymorphism among people with African or Asian ancestry. We found that healthy Black individuals who were homozygous for the alpha globin gene deletion had higher fractional exhaled nitric oxide, consistent with alpha globin’s role as a nitric oxide restrictor.
How this study might affect research, practice or policy
An alpha globin gene deletion, commonly found among Black individuals, may increase fractional exhaled nitric oxide. Whether this changes the normal range of fractional exhaled nitric oxide for Black individuals or impacts the risk of developing asthma requires further study.
Introduction
Nitric oxide (NO) has numerous biological activities in the lung, serving as a bronchodilator, vasodilator, neurotransmitter and inflammatory mediator. In individuals with asthma, the fractional exhalation of nitric oxide (FeNO) is a validated measure of airway inflammation1 2; however, little is known about genetic factors influencing FeNO, particularly among healthy Black individuals.
Recently, a new paradigm of NO regulation in the vasculature has emerged in which endothelial alpha globin interacts with endothelial NO synthase (eNOS) to limit the release of NO within small arteries.3 Genetic deletion of alpha globin, common among Black Americans, is associated with improved NO-mediated vascular perfusion and with protection from kidney disease.4–6 Alpha globin, beta globin and eNOS are expressed in airway epithelium and while beta globin has been found to interact directly with eNOS to regulate the oxidation of NO, the role of alpha globin in healthy respiratory epithelium remains undefined.7–10 NO is produced by inducible NOS (iNOS) in airway epithelial and inflammatory cells.11 While the interaction of alpha globin with iNOS has not previously been studied in epithelial cells, the structure of iNOS is nearly identical to that of eNOS, including at the sites where eNOS interacts with alpha globin.12 This raised the question of whether alpha globin regulates NO release from the respiratory epithelium. To address this question, we analysed the association between FeNO and HBA copy number in healthy Black adults.
Methods
Healthy Black individuals aged 18–40 years were enrolled in a multicentre, cross-sectional cohort at four university sites near Durham, North Carolina from 2005 to 2008.13 All participants provided oral and written informed consent. Age, sex, race and ethnicity were self-reported. Only non-Hispanic African American participants were enrolled. Participants were asked to confirm they were healthy (ie, no chronic illnesses or chronic use of any medication except oral contraceptives); had no history of asthma, allergic rhinitis, hay fever, or atopic dermatitis; and were non-smokers. Blood samples were obtained. HBA copy number was measured by droplet digital PCR on genomic DNA. Total serum immunoglobulin-E (IgE) was measured using the Pharmacia CAP system. FeNO was measured in triplicate with a Sievers 280i Nitric Oxide Analyzer (GE Analytical Instruments, Boulder, Colorado) according to American Thoracic Society recommendations.1 A 50 mL/s flow rate was established against resistance to maintain 5 cmH2O oropharyngeal pressure.13 Additional exclusion criteria for this analysis were: not consenting to future research and serum cotinine level > 25 ng/mL signifying active tobacco use.
Statistical methods
For continuous measures, medians and 25th and 75th percentiles were calculated by HBA genotype. Group differences were assessed by Kruskal-Wallis test. Categorical variables were calculated as percentages and differences were assessed by Fisher’s exact test. IgE and FeNO were log transformed due to skewness. The association of HBA genotype with FeNO was evaluated using multivariable linear regression employing a linear effect of HBA gene copy number with adjustment for age, sex and total serum IgE levels. Body mass index was previously found not to be associated with FeNO in this cohort and was not included in the model.13 Two post-hoc sensitivity analyses were performed: one evaluated HBA copy number as a categorical variable and one evaluated a recessive mode of inheritance in which the homozygous deletion genotype (-a/-a) was compared against all other genotypes (-a/aa, aa/aa, and aa/aaa).
Patient and public involvement
Patients or members of the public were not included in the conceptual design of the original study completed in 2008. We plan to discuss our research findings with members of the public who may be enrolled in other studies of genetic globin variants. In addition, we recognise the importance of involving patients and the public in the conceptual design of future studies.
Results
Of 895 original study participants, 720 consented for future research and had DNA available for genotyping. Sixty-four participants were excluded due to high cotinine levels and 13 were excluded due to indeterminate HBA genotype. The remaining 643 participants were 35% male and had a median (25th, 75th) age of 20 (19, 22) years, serum IgE level of 58.3 (22, 160) kU/L and FeNO value of 20 (13, 31) ppb (table 1).
Table 1.
Participant characteristics grouped by alpha globin genotype
| HBA genotype | ||||||
| All participants | -a/-a | -a/aa | aa/aa | aa/aaa | P value† | |
| No. participants* | 643 | 30 (4.7%) | 197 (30.6%) | 408 (63%) | 8 (1.2%) | |
| Male sex, no. (%) | 222 (35) | 12 (40) | 66 (34) | 142 (35) | 4 (50) | 0.678 |
| Age, years | 20 (19, 22) | 20 (19, 22) | 20 (19, 22) | 20 (19, 22) | 19 (19, 24) | 0.833 |
| Mean FeNO‡,ppb | 20 (13, 31) | 25 (18, 39) | 20 (12, 27) | 20 (13, 32) | 37 (9, 52) | 0.107 |
| Total IgE, IU/mL | 58 (22, 160) | 28 (16, 96) | 66 (25, 176) | 57 (21, 158) | 36 (24, 46) | 0.125 |
| Body mass index, kg/m2 | 27 (24, 32) | 28 (26, 33) | 27 (23, 33) | 26 (23, 32) | 27 (25, 34) | 0.778 |
| Height, inches | 168 (162, 175) | 170 (161, 177) | 168 (163, 173) | 168 (162, 175) | 168 (163, 176) | 0.961 |
| Weight, kilograms | 77 (65, 92) | 81 (69, 93) | 77 (67, 93) | 76 (65, 91) | 85 (74, 101) | 0.716 |
| Systolic blood pressure, mm Hg | 117 (109, 124) | 117 (108, 130) | 117 (110, 123) | 117 (109, 125) | 127 (126, 133) | 0.242 |
| Diastolic blood pressure, mm Hg | 68 (63, 74) | 68 (63, 73) | 67 (62, 74) | 68 (63, 75) | 79 (70, 85) | 0.703 |
| Mean arterial pressure, mm Hg | 84 (79, 90) | 85 (80, 88) | 82 (79, 89) | 84 (80, 91) | 99 (91, 100) | 0.352 |
Values are median (25th, 75th percentile) except where otherwise indicated.
*P values calculated for differences between groups by Kruskal-Wallis non-parametric analysis of variance and for categorical variables p values were calculated as percentages within each category and differences were assessed by Fischer’s exact test.
†Total number of participants (n=643). Missing data are as follows: sex (n=2, <1%); age (n=13, 2%); mean FeNO (n=4, <1%); total IgE (n=25, 3.8%}; body mass index and weight (n=4, <1%); systolic blood pressure, diastolic blood pressure and mean arterial pressure (n=292, 45.4%).
‡Mean FeNO levels measured according to ATS recommendations (reported here as median (25th, 75th percentile) of the mean recorded FeNO).
FeNO, fractional exhaled nitric oxide; IgE, Immunoglobulin E; IU, international unit; MAP, mean arterial pressure; mL, millilitre; mm Hg, millimetres of mercury; No, number; ppb, parts per billion.
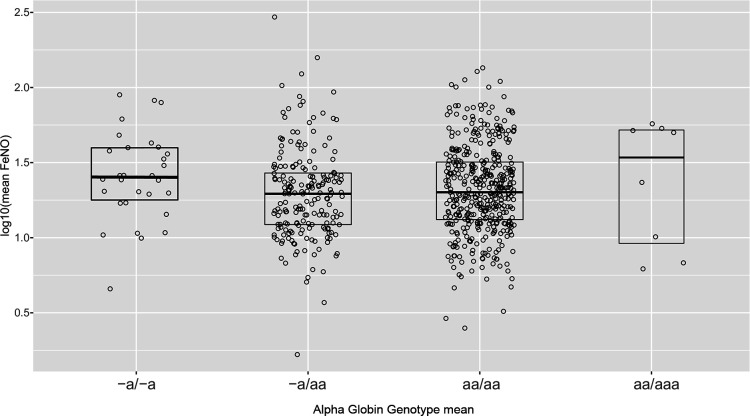
HBA deletion was common with 30 (4.7%) -a/-a, 197 (30.6%) -a/aa, 405 (63%) aa/aa and 8 (1.2%) aa/aaa genotypes. Median (25th, 75th) FeNO was 25 (18, 39) ppm in the -a/-a group, 20 (12, 27) ppm in the -a/aa group, 20 (13, 32) ppm in the aa/aa group, and 37 (9, 52) ppm in the aa/aaa group (table 1 and figure 1). In an unadjusted linear regression analysis using the prespecified additive genetic model, the coefficient for HBA copy number with FeNO was 0.001 (95% CI −0.039 to 0.040; p=0.978). After adjustment for sex, age and serum IgE, the coefficient for HBA copy number with FeNO was −0.005 (95% CI −0.042 to 0.033; p=0.811; table 2). In post hoc sensitivity analyses, the adjusted association between the homozygous genotype -a/-a and FeNO was 0.099 (95% CI −0.007 to 0.206; p=0.066) when analysed as a categorical variable and 0.107 (95% CI 0.003 to 0.212; p=0.045; table 2) when analysed using a recessive mode of inheritance.
Figure 1.
Fractional exhaled nitric oxide levels in 643 Black individuals grouped by alpha globin genotype. Bold lines represent 50th percentile and upper and lower box lines represent 25th and 75th percentiles for each genotype.
Table 2.
Multivariable regression analysis of HBA genotype and fractional exhaled nitric oxide*
| Multivariable linear regression model employing HBA genotype as an integer gene copy number | |||
| Beta coefficient | 95% CI | P value | |
| HBA copy number, per copy | −0.005 | (−0.042 to 0.033) | 0.811 |
| Age, per year | −0.001 | (−0.006 to 0.003) | 0.587 |
| Male sex | 0.122 | (0.074 to 0.170) | < 0.001 |
| Log10 IgE | 0.137 | (0.098 to 0.176) | < 0.001 |
| Post hoc multivariable linear regression model employing HBA genotype with a recessive mode of inheritance | |||
| Beta coefficient | 95% CI | P value | |
|
HBA genotype, -a/-a |
0.107 | (0.003 to 0.212) | 0.045 |
| Age, per year | −0.001 | (−0.006 to 0.003) | 0.541 |
| Male sex | 0.120 | (0.071 to 0.166) | < 0.001 |
| Log10 IgE | 0.140 | (0.102 to 0.181) | <0.001 |
*Out of n=643 participants contributing data to the multivariable regression models n=44 participants were excluded due to missing data and n=599 participants were included in the multivariable regression models. Missing data by variable are as follows: age (n=13, 2%); sex (n=2, <1%); total IgE (n=25, 3.8%); mean FeNO (n=4, <1%).
.HBA, alpha globin gene; IgE, immunoglobulin E; Log10, log base 10; No., number.
Discussion
Alpha and beta globin have recently emerged as regulators of NO signalling in the vascular endothelium and respiratory epithelium, respectively3 10; however, there are few studies evaluating the impact of globin gene variants on NO signalling in vivo. In this study, we characterised a common HBA deletion in healthy Black individuals and examined the relationship between HBA copy number and FeNO. We found no association between HBA genotype and FeNO using a prespecified additive genetic model; however, a post hoc analysis using a recessive mode of inheritance identified homozygosity for the HBA gene deletion to be associated with higher FeNO levels. This latter finding is consistent with the proposed mechanism that alpha globin limits the release of NO and suggests that lower alpha globin expression allows greater release of NO from respiratory epithelium in healthy individuals without asthma. More work is needed to understand the role of epithelial alpha globin in the setting of inflammatory lung disease and to determine whether alpha globin interacts with iNOS, which is structurally similar to eNOS, and is expressed under allergic or inflammatory conditions.12 14
Study strengths included the large cohort size, representation of an understudied minority population, high frequency of the HBA gene deletion and a well-defined quantitative outcome measure. Adjustment for serum IgE, which is associated with FeNO, was a strength of this study; however, the absence of evaluation for subclinical IgE sensitisation was a limitation. Other limitations included post hoc testing of different genetic inheritance models, performing FeNO measurement at a single flow rate that does not distinguish alveolar from bronchial NO sources,15 and absence of data on other factors that influence FeNO such as recent upper respiratory tract infection, eosinophilic cationic protein, eosinophil count, neutrophil count and other inflammatory markers such as periostin.
The genetic epidemiological approach we used relies on variation in the alpha globin gene, which has previously been associated with vascular NO signalling and vascular disease risk, but has not previously been studied in relation to exhaled NO. Our approach did not distinguish between iNOS or eNOS as the source of NO. Our observation of a significant association with alpha thalassaemia trait (two alpha globin deletions) and increased FeNO may be consistent with prior epidemiological studies that noted a positive association with thalassaemia and asthma in Taiwanese children.16 Future studies are needed to better understand the mechanisms by which globins regulate NO signalling in the human respiratory epithelium and whether genetic variation in epithelial globin expression influences asthma risk across different ethnic groups, including those with African ancestry and southeast Asian ancestry.
Conclusions
HBA copy number was not associated with FeNO among healthy Black adults when analysed using a prespecified additive genetic model. However, FeNO was found to be higher among subjects who were homozygous for the HBA deletion in a post hoc analysis employing a recessive mode of inheritance. This relationship between HBA copy number and exhaled NO and merits further study in other populations where alpha globin is polymorphic. The mechanistic link between alpha globin and NO synthase warrants further characterisation to better understand the impact of human genetic variation in alpha globin on respiratory function and health.
Acknowledgments
Several coauthors have new current affiliations, Jarrett Jackson is at the Vanderbilt University Medical Center, Nashville, TN, United States; Carlos Carhuas is at the Comprehensive Sickle Cell Disease Program, Children’s National Medicine Center, Washington, DC, United States; and Jessica Nino De Rivera is at the Columbia University Vagelos College of Physicians and Surgeons, New York, NY, United States
Footnotes
Twitter: @AParkerRuhl
Contributors: APR and HCA are both responsible for the full content of this article as guarantors. Substantial contributions to the conception or design of the work (APR, HCA, LQ and JBW); or the acquisition, analysis, or interpretation of data for the work (APR, HCA, JMJ, CJC, JGNdR, MPF, LQ); drafting the work (APR) or revising it critically for important intellectual content and final approval of the version to be published (all).
Funding: The original study was supported by the Sandler Program for Asthma Research; ES011185 from the National Institute of Environmental Health Sciences; and MO1-RR-30 from the National Center for Research Resources, Clinical Research Centers Program, National Institutesof Health. The current analysis was supported in part by the Divisions of Intramural Research, National Institute of Allergy and Infectious Diseases project AI001150 (APR, JMJ, CMC, JGN, MPF, HCA), National Heart, Lung, and Blood Institute (NHLBI) project HL006196 (APR, HCA). This work was also funded in part by NHLBI grants NIH R01-HL107590 and R01HL153641 (LGQ) and the Durham VA Medical Center Research Service (JBW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or NHLBI.
Disclaimer: The content of this publication does not necessarily reflect the view or policy of the Department of Health and Human Services, nor does mention of trade names, commercial products or organisations imply endorsement by the government. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the US government.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. Deidentified data and statistical code from this article will be available to researchers. Data can be obtained upon reasonable request by contacting the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The protocol was approved by the Duke University Institutional Review Board (#Pro00004947). Participants gave informed consent to participate in the study before taking part.
References
- 1.Khatri SB, Iaccarino JM, Barochia A, et al. Use of fractional exhaled nitric oxide to guide the treatment of asthma: an official American Thoracic society clinical practice guideline. Am J Respir Crit Care Med 2021;204:e97–109. 10.1164/rccm.202109-2093ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeppegaard M, Veidal S, Sverrild A, et al. Validation of ATS clinical practice guideline cut-points for Feno in asthma. Respir Med 2018;144:22–9. 10.1016/j.rmed.2018.09.014 [DOI] [PubMed] [Google Scholar]
- 3.Straub AC, Lohman AW, Billaud M, et al. Endothelial cell expression of Haemoglobin a regulates nitric oxide signalling. Nature 2012;491:473–7. 10.1038/nature11626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denton CC, Shah P, Suriany S, et al. Loss of alpha-Globin genes in human subjects is associated with improved nitric oxide-mediated vascular perfusion. Am J Hematol 2021;96:277–81. 10.1002/ajh.26058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romana M, Reminy K, Moeckesch B, et al. Loss of alpha Globin genes is associated with improved Microvascular function in patients with sickle cell anemia. Am J Hematol 2021;96:E165–8. 10.1002/ajh.26126 [DOI] [PubMed] [Google Scholar]
- 6.Ruhl AP, Jeffries N, Yang Y, et al. Alpha Globin gene copy number is associated with prevalent chronic kidney disease and incident end-stage kidney disease among Black Americans. J Am Soc Nephrol 2022;33:213–24. 10.1681/ASN.2021050653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhaskaran M, Chen H, Chen Z, et al. Hemoglobin is expressed in alveolar epithelial type II cells. Biochem Biophys Res Commun 2005;333:1348–52. 10.1016/j.bbrc.2005.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newton DA, Rao KMK, Dluhy RA, et al. Hemoglobin is expressed by alveolar epithelial cells. J Biol Chem 2006;281:5668–76. 10.1074/jbc.M509314200 [DOI] [PubMed] [Google Scholar]
- 9.Grek CL, Newton DA, Spyropoulos DD, et al. Hypoxia up-regulates expression of hemoglobin in alveolar epithelial cells. Am J Respir Cell Mol Biol 2011;44:439–47. 10.1165/rcmb.2009-0307OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marozkina N, Smith L, Zhao Y, et al. Somatic cell hemoglobin modulates nitrogen oxide metabolism in the human airway epithelium. Sci Rep 2021;11:15498. 10.1038/s41598-021-94782-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricciardolo FLM, Sterk PJ, Gaston B, et al. Nitric oxide in health and disease of the respiratory system. Physiol Rev 2004;84:731–65. 10.1152/physrev.00034.2003 [DOI] [PubMed] [Google Scholar]
- 12.Fischmann TO, Hruza A, Niu XD, et al. Structural characterization of nitric oxide synthase Isoforms reveals striking active-site conservation. Nat Struct Biol 1999;6:233–42. 10.1038/6675 [DOI] [PubMed] [Google Scholar]
- 13.Levesque MC, Hauswirth DW, Mervin-Blake S, et al. Determinants of exhaled nitric oxide levels in healthy, Nonsmoking African American adults. J Allergy Clin Immunol 2008;121:396–402. 10.1016/j.jaci.2007.09.031 [DOI] [PubMed] [Google Scholar]
- 14.Roos AB, Mori M, Grönneberg R, et al. Elevated exhaled nitric oxide in Allergen-provoked asthma is associated with airway epithelial iNOS. PLoS One 2014;9:e90018. 10.1371/journal.pone.0090018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linn WS, Rappaport EB, Eckel SP, et al. Multiple-flow exhaled nitric oxide, allergy, and asthma in a population of older children. Pediatr Pulmonol 2013;48:885–96. 10.1002/ppul.22708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piel FB, Weatherall DJ. The Α-Thalassemias. N Engl J Med 2014;371:1908–16. 10.1056/NEJMra1404415 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request. Deidentified data and statistical code from this article will be available to researchers. Data can be obtained upon reasonable request by contacting the corresponding author.