Abstract
S. typhimurium nit mutants are defective in nitrogen assimilation, despite having normal levels of assimilatory enzymes. Complementation, enzyme assays, and genetic mapping show that nit is nadE. We present evidence that ammonia, not glutamine, is the physiological substrate for eubacterial NAD synthetases and that low activity completely accounts for the mutant phenotype.
Salmonella typhimurium nit mutants cannot grow with low concentrations of ammonia (<1 mM) or alternate nitrogen sources, such as arginine, despite having normal levels of nitrogen-assimilatory enzymes (2). In this paper, we identify the biochemical defect in these mutants and explain their phenotype.
An nit mutant can be complemented by Escherichia coli nadE and has a low level of NAD synthetase.
Plasmid pΔLC3-11 carries DNA from minute 38 of the E. coli chromosome (6), which corresponds to the region of S. typhimurium that contains nit. This plasmid complemented the defect in S. typhimurium SK51 (nit-12), as was indicated by growth of SK51/pΔLC3-11 with arginine as the nitrogen source (Table 1). The plasmid contains astCADBE, nadE, osmY, and three open reading frames for proteins of unknown functions. We subcloned a 1.5-kb HindIII-StuI fragment containing nadE from pΔLC3-11 into pWKS30 (9), and the resulting plasmid also complemented the defect in SK51 (Table 1). As expected for a strain with a defect in nadE, SK51 had significantly less NAD synthetase activity than a wild-type strain (Table 2). Furthermore, TT10746, a previously isolated temperature-sensitive lethal S. typhimurium nadE mutant, could not utilize arginine at the permissive temperature (Table 1); in other words, it was Nit−. We conclude that nit is nadE.
TABLE 1.
Bacterial strains and their phenotypes
| Strain/ plasmid | Genotype of strain/ genotype of plasmid | Arginine utilization | Reference or source |
|---|---|---|---|
| TA1650 | dhuA1 hisJ5601 | + | 2 |
| SK51 | dhuA1 hisJ5601 nit-12 | − | 2 |
| SK51/pΔLC3-11 | dhuA1 hisJ5601 nit-12/nadE+ astCADBE+ | + | This study |
| SK51/pHS5 | dhuA1 hisJ5601 nit-12/nadE+ | + | This study |
| TT10746 | zch-1839::Tn10 nadE381 | − | 3 |
TABLE 2.
Activities of the arginine succinyltransferase pathway enzyme and NAD synthetase in the wild type and an nit strain
| Strain | Sp act ofa:
|
|||
|---|---|---|---|---|
| AstB | AstC | AstE | NAD synthetase | |
| TA1650 (wild type) | 2.0 ± 0.21 | 42 ± 3.5 | 3.3 ± 0.34 | 22 ± 2.9 |
| SK51 (nit-12) | 2.2 ± 0.24 | 40 ± 3.2 | 5.3 ± 0.45 | <3 |
All units are in nanomoles per minute per milligram of protein. The values are the averages of three determinations ± standard errors of the means. Cells for the assays for AstB, AstC, and AstE were grown in minimal medium with 0.4% glucose as the carbon source and 0.2% alanine as the nitrogen source. Of the media that supported growth of SK51, this medium gave the highest levels of arginine succinyltransferase enzymes. Cell growth, extract preparation, and assays for AstB (succinylarginine dihydrolase), AstC (succinylornithine transaminase), and AstE (succinylglutamate desuccinylase) have been described previously (6). Cells for the NAD synthetase assay were grown in minimal medium with 0.4% glucose and 0.2% (NH4)2SO4 at 30°C. Extracts were prepared as described for the other assays, except that the concentration of the phosphate buffer was 10 mM, instead of 50 mM. The assay for NAD synthetase has been described previously (8).
The astCADBE operon specifies enzymes of the arginine succinyltransferase pathway of E. coli, which degrades arginine and other amino acids (6). A defect in this pathway might contribute to the mutant phenotype. However, levels of AstB, AstC, and AstE in SK51/pΔLC3-11 were normal (Table 2).
nadE and nit map to the same locus.
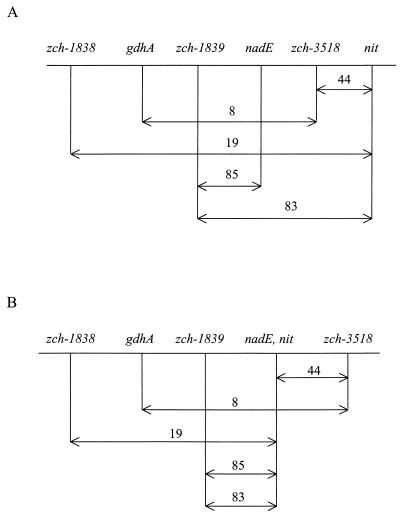
The preceding results were unexpected since nit and nadE, though linked, appeared to map to different loci (Fig. 1A). We suspected a mapping error, since it was proposed that zch-1838 and nit were more closely linked than two markers between them (Fig. 1A, second and third crosses). An order of zch-1838–gdhA–nit–zch-3518 is more consistent with these and other results (Fig. 1B), which places nit near nadE. We tested the revised gene order by determining the linkage between nit and zch-1839 and found that it was identical to that reported between nadE and zch-1839 (Fig. 1B, fourth and fifth crosses). These results confirm that nit is nadE.
FIG. 1.
Cotransduction frequencies in the nadE region of S. typhimurium. (A) Original gene order; (B) corrected gene order. The results for the first three crosses are from the work of Rosenfeld et al. (5), those for the fourth cross are from the work of Hughes et al. (3), and those for the fifth cross are from this study. Only selected results from references 3 and 5 are shown. The nomenclature for the insertions is that of Hughes et al. (3), which differs from that of Rosenfeld et al. (5). The first, third, and fifth crosses in panel B are between nit and another marker; the fourth cross is between nadE and zch-1839.
Evidence that ammonia, not glutamine, is the physiological nitrogen donor for NAD synthetase.
The phenotype of the S. typhimurium nit (nadE) mutant is virtually identical to that reported for a Rhodobacter capsulatus nadE mutant, i.e., the mutants require high concentrations of ammonia for growth (1, 10). Three explanations have been proposed to account for the mutant phenotype. First, if NAD synthetase is glutamine dependent, and a mutation impairs amide transfer, then the mutants would require a high concentration of ammonia, a poor substrate for amidotransferases, in place of glutamine (11). Second, if NAD synthetase is ammonia dependent, then an alteration that diminishes activity or increases the Km for ammonia would result in a requirement for a high concentration of ammonia. Third, NAD synthetase may have an essential regulatory function.
Several lines of evidence favor the hypothesis that NAD synthetase is ammonia dependent. First, if NAD synthetase is glutamine dependent, then diminished amide transfer should not affect ammonia-dependent activity (a property of most amidotransferases). This is easily detected since the purified NAD synthetase, which has virtually no glutamine-dependent activity (8, 10), has an extraordinarily low Km for ammonia (described below). However, the mutations clearly affect ammonia-dependent activity (Table 1). Second, no eubacterial NAD synthetase, including nadE from E. coli (which complements the S. typhimurium mutation), is homologous to any known glutamine-dependent amidotransferase, which implies that NadE lacks a glutamine-binding domain (7, 8, 10). Third, it can be argued that glutamine dependence results from a separate subunit. However, no gene coding for a glutamine-binding subunit is near nadE of E. coli (7), where such a gene is expected (11). Nonetheless, the possibility for such a subunit cannot be completely eliminated. However, such a possibility cannot explain why all nit mutations are in nadE and not in the gene for this hypothetical glutamine-binding subunit. Fourth, the bacterial NAD synthetases have properties that are unprecedented for an amidotransferase. NAD synthetases from S. typhimurium and R. capsulatus crude extracts were twice as active with 1 mM ammonia as with 1 mM glutamine (7, 10). Furthermore, purified E. coli NAD synthetase’s Km for ammonia, 65 μM (8), is lower than that for any known amidotransferase (11). The purified enzyme’s Km for glutamine is 16 mM (8); however, the rate of spontaneous glutamine hydrolysis exceeds that of glutamine-dependent NAD synthesis, which implies that NAD synthetase cannot hydrolyze glutamine (10). Fifth, the only positive evidence suggesting that NAD synthetase is glutamine dependent is its assay from crude extracts of S. typhimurium and R. capsulatus (7, 10). However, such activity is probably the artifactual sum of glutaminase and ammonia-dependent NAD synthetase activities, since glutaminase activity from an R. capsulatus extract is greater than glutamine-dependent NAD synthetase activity (10). This artifact explains why, despite several attempts, glutamine-dependent NAD synthetase activity is lost after the first step of purification; presumably, purification separates the glutaminase activity from NAD synthetase (7, 10).
In summary, the available evidence strongly suggests that ammonia, not glutamine, is the physiological nitrogen donor for NAD synthetases from E. coli, S. typhimurium, and R. capsulatus. The careful study of Willison and Tissot supports this conclusion (10). If eubacterial NAD synthetases require ammonia, then NAD synthetase is only the second known essential ammonia-dependent enzyme; glutamine synthetase is the other (4).
The nitrogen utilization defect of nadE (nit) mutants.
To explain the mutant phenotype, we propose that a high concentration of ammonia is required for sufficient activity of the residual NAD synthetase. When ammonia becomes limiting, i.e., during nitrogen-limited growth, the NAD synthetase activity presumably becomes insufficient. This explains why impaired glutamine synthetase activity, which will increase available ammonia, suppresses the Nit− phenotype (2). Based on the presence of certain motifs found in regulatory proteins, the possibility that NAD synthetase possesses an essential regulatory function was considered (10). While this possibility cannot be rigorously excluded, such a function is unnecessary to account for all aspects of the mutant phenotype.
Concluding remarks.
In summary, we have shown that nit is nadE, ammonia is probably the preferred substrate for NAD synthetase, and low NAD synthetase activity can account for the nitrogen utilization defect. It is not obvious why several (perhaps all) eubacterial NAD synthetases appear to be ammonia dependent whereas the eukaryotic enzymes are glutamine dependent. Perhaps differences in mechanisms of nitrogen acquisition, or the availability of nitrogen reserves in eukaryotes (which may provide a reliable source of glutamine), ultimately account for this difference in substrate specificity.
Acknowledgments
We thank Sydney Kustu and John Roth for strains.
This work was supported by National Institute of General Medical Sciences grant GM47965.
REFERENCES
- 1.Allibert P, Willison J C, Vignais P M. Complementation of nitrogen-regulatory (ntr-like) mutations in Rhodobacter capsulatus by an Escherichia coli gene: cloning and sequencing of the gene and characterization of the gene product. J Bacteriol. 1987;169:260–271. doi: 10.1128/jb.169.1.260-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broach J, Neumann C, Kustu S. Mutant strains (nit) of Salmonella typhimurium with a pleiotrophic defect in nitrogen metabolism. J Bacteriol. 1976;128:86–98. doi: 10.1128/jb.128.1.86-98.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes K T, Olivera B M, Roth J R. Structural gene for NAD synthetase in Salmonella typhimurium. J Bacteriol. 1988;170:2113–2120. doi: 10.1128/jb.170.5.2113-2120.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reitzer L J. Ammonia assimilation and the biosynthesis of glutamine, glutamate, aspartate, asparagine, l-alanine, and d-alanine. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 391–407. [Google Scholar]
- 5.Rosenfeld S A, Dendinger S M, Murphy C H, Brenchly J E. Genetic characterization of the glutamate dehydrogenase gene (gdh) of Salmonella typhimurium. J Bacteriol. 1982;150:796–803. doi: 10.1128/jb.150.2.795-803.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider B L, Kiupakis A K, Reitzer L J. Arginine catabolism and the arginine succinyltransferase pathway in Escherichia coli. J Bacteriol. 1998;180:4278–4286. doi: 10.1128/jb.180.16.4278-4286.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider, B. L., and L. J. Reitzer. Unpublished results.
- 8.Spencer R L, Preiss J. Biosynthesis of diphosphopyridine nucleotide. J Biol Chem. 1967;242:385–392. [PubMed] [Google Scholar]
- 9.Wang R F, Kushner S R. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991;100:195–199. [PubMed] [Google Scholar]
- 10.Willison J C, Tissot G. The Escherichia coli efg gene and the Rhodobacter capsulatus adgA gene code for NH3-dependent NAD synthetase. J Bacteriol. 1994;176:3400–3402. doi: 10.1128/jb.176.11.3400-3402.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zalkin H. The amidotransferases. Adv Enzymol. 1993;66:203–309. doi: 10.1002/9780470123126.ch5. [DOI] [PubMed] [Google Scholar]