Abstract
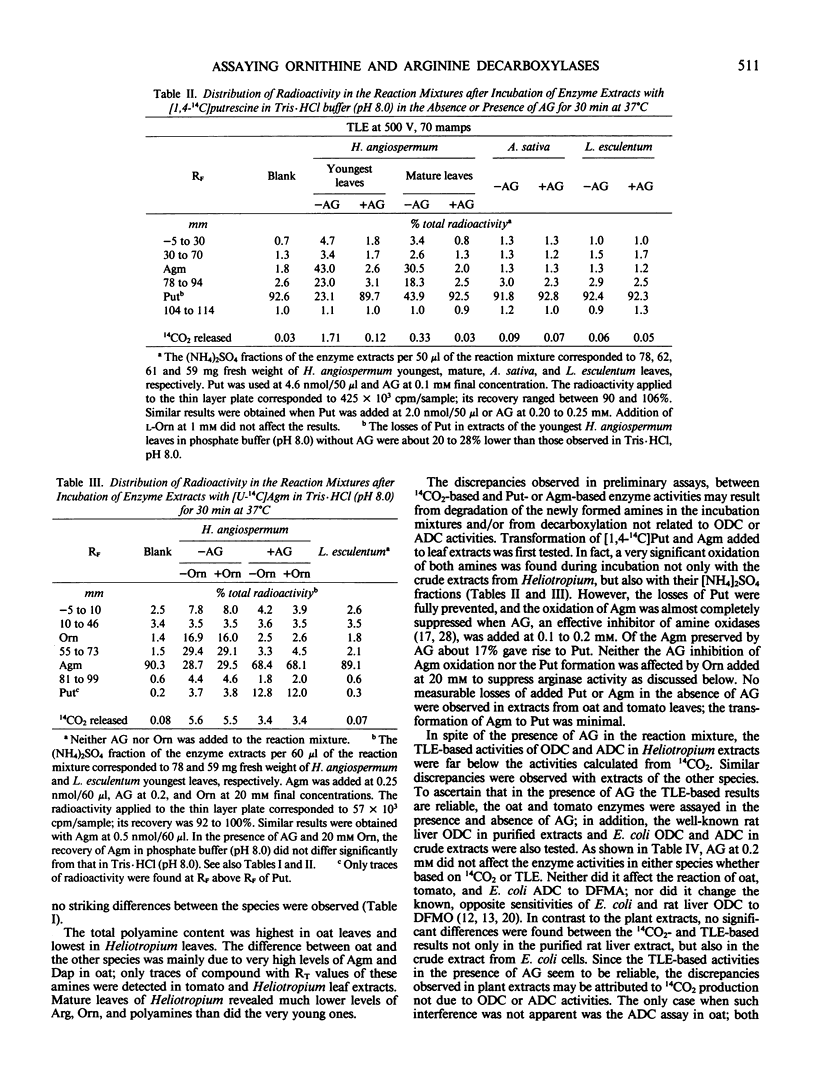
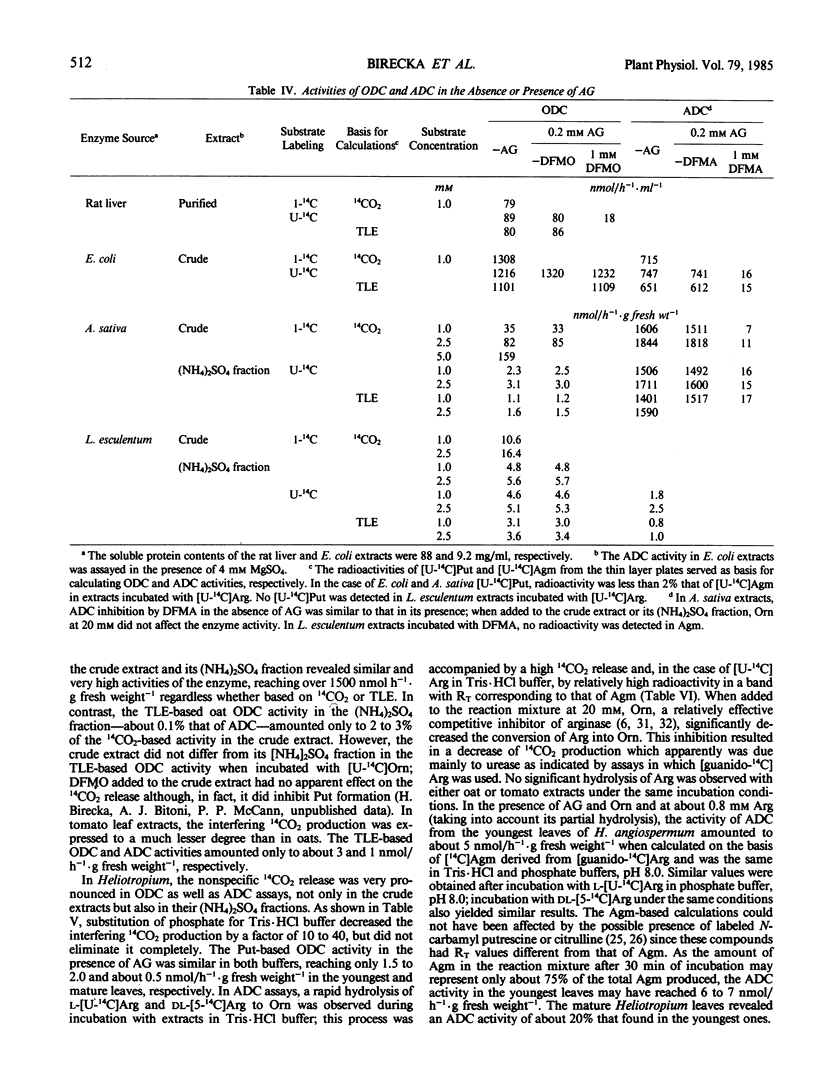
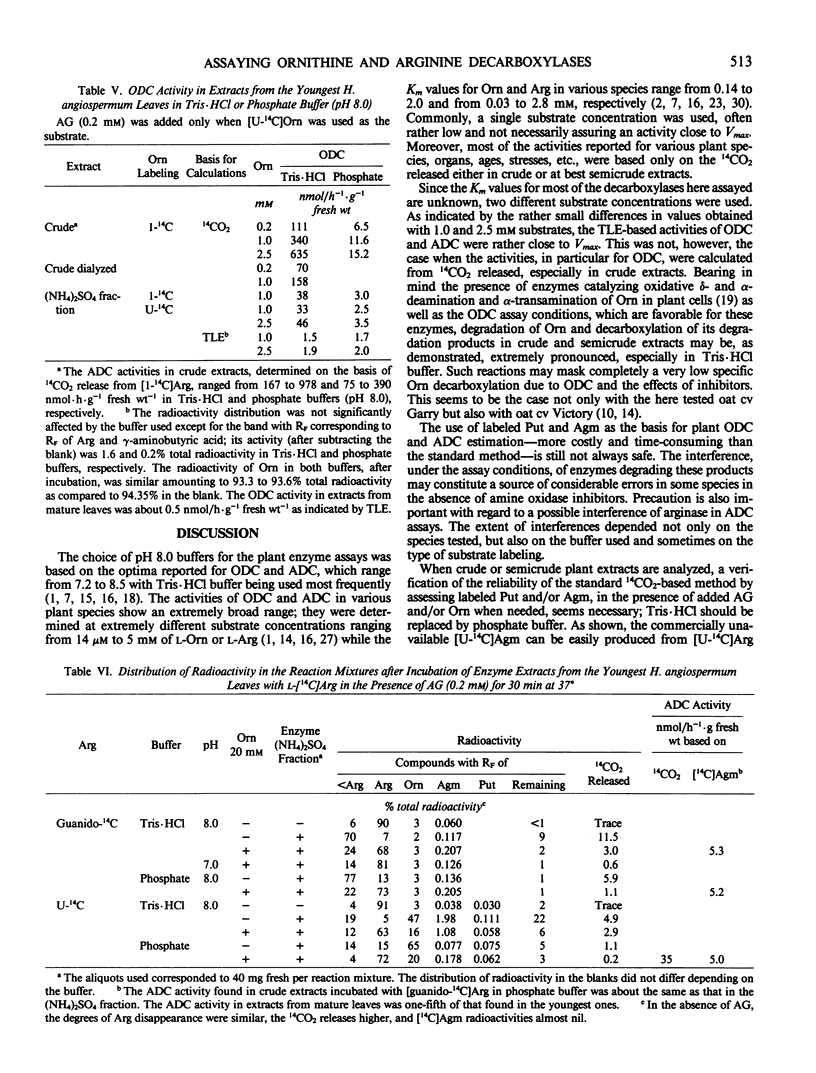
A release of 14CO2 not related to ornithine decarboxylase activity was found in crude leaf extracts from Lycopersicon esculentum, Avena sativa, and especially from the pyrrolizidine alkaloid-bearing Heliotropium angiospermum when incubated with [1-14C]- or [U-14C]ornithine. The total 14CO2 produced was about 5- to 100-fold higher than that due to ornithine decarboxylase activities calculated from labeled putrescine (Put) found by thin-layer electrophoresis in the incubation mixtures. Partial purification with (NH4)2SO4 did not eliminate completely the interfering decarboxylation. When incubated with labeled arginine, a very significant 14CO2 release not related to arginine decarboxylase activity was observed only in extracts from H. angiospermum leaves, especially in Tris·HCl buffer. Under the assay conditions, these extracts exhibited oxidative degradation of added Put and agmatine (Agm) and also revealed a high arginase activity. Amino-guanidine at 0.1 to 0.2 millimolar prevented Put degradation and greatly decreased oxidative degradation of Agm; ornithine at 15 to 20 millimolar significantly inhibited arginase activity. A verification of the reliability of the standard 14CO2-based method by assessing labeled Put and/or Agm—formed in the presence of added aminoguanidine and/or ornithine when needed—is recommended especially when crude or semicrude plant extracts are assayed.
When based on Put and/or Agm formed at 1.0 to 2.5 millimolar of substrate, the activities of ornithine decarboxylase and arginine decarboxylase in the youngest leaves of the tested species ranged between 1.1 and 3.6 and 1 and 1600 nanomoles per hour per gram fresh weight, respectively. The enzyme activities are discussed in relation to the biosynthesis of pyrrolizidine alkaloids.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman A., Friedman R., Levin N. Arginine and ornithine decarboxylases, the polyamine biosynthetic enzymes of mung bean seedlings. Plant Physiol. 1982 Apr;69(4):876–879. doi: 10.1104/pp.69.4.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitonti A. J., McCann P. P., Sjoerdsma A. Restriction of bacterial growth by inhibition of polyamine biosynthesis by using monofluoromethylornithine, difluoromethylarginine and dicyclohexylammonium sulphate. Biochem J. 1982 Nov 15;208(2):435–441. doi: 10.1042/bj2080435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal N., Acoria M., Rodríguez J. P., Fernández M., Martínez J. Evidence for cooperative effects in human liver arginase. Biochim Biophys Acta. 1982 Feb 4;701(1):146–148. doi: 10.1016/0167-4838(82)90324-7. [DOI] [PubMed] [Google Scholar]
- Cohen E., Arad S. M., Heimer Y. M., Mizrahi Y. Participation of ornithine decarboxylase in early stages of tomato fruit development. Plant Physiol. 1982 Aug;70(2):540–543. doi: 10.1104/pp.70.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E., Heimer Y. M., Mizrahi Y. Ornithine decarboxylase and arginine decarboxylase activities in meristematic tissues of tomato and potato plants. Plant Physiol. 1982 Aug;70(2):544–546. doi: 10.1104/pp.70.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores H. E., Galston A. W. Polyamines and plant stress: activation of putrescine biosynthesis by osmotic shock. Science. 1982 Sep 24;217(4566):1259–1261. doi: 10.1126/science.217.4566.1259. [DOI] [PubMed] [Google Scholar]
- Heimer Y. M., Mizrahi Y. Characterization of ornithine decarboxylase of tobacco cells and tomato ovaries. Biochem J. 1982 Feb 1;201(2):373–376. doi: 10.1042/bj2010373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio A., McCann P. P., Bey P. DL-alpha-(Difluoromethyl)arginine: a potent enzyme-activated irreversible inhibitor of bacterial decarboxylases. Biochemistry. 1981 May 26;20(11):3163–3168. doi: 10.1021/bi00514a027. [DOI] [PubMed] [Google Scholar]
- Kallio A., McCann P. P. Difluoromethylornithine irreversibly inactivates ornithine decarboxylase of Pseudomonas aeruginosa, but does not inhibit the enzymes of Escherichia coli. Biochem J. 1981 Oct 15;200(1):69–75. doi: 10.1042/bj2000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur-Sawhney R., Shih L. M., Flores H. E., Galston A. W. Relation of polyamine synthesis and titer to aging and senescence in oat leaves. Plant Physiol. 1982 Feb;69(2):405–410. doi: 10.1104/pp.69.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. A. Spectrophotometric method for the estimation of arginine decarboxylase. Anal Biochem. 1979 Jan 15;92(2):331–337. doi: 10.1016/0003-2697(79)90666-3. [DOI] [PubMed] [Google Scholar]
- Srivenugopal K. S., Adiga P. R. Enzymic conversion of agmatine to putrescine in Lathyrus sativus seedlings. Purification and properties of a multifunctional enzyme (putrescine synthase). J Biol Chem. 1981 Sep 25;256(18):9532–9541. [PubMed] [Google Scholar]
- Villanueva V. R., Giret M. Human platelet arginase. Mol Cell Biochem. 1980 Dec 10;33(1-2):97–100. doi: 10.1007/BF00224574. [DOI] [PubMed] [Google Scholar]
- Wagner J., Danzin C., Mamont P. Reversed-phase ion-pair liquid chromatographic procedure for the simultaneous analysis of S-adenosylmethionine, its metabolites and the natural polyamines. J Chromatogr. 1982 Feb 12;227(2):349–368. doi: 10.1016/s0378-4347(00)80389-8. [DOI] [PubMed] [Google Scholar]
- Winer L., Vinkler C., Apelbaum A. Partial purification and characterization of arginine decarboxylase from avocado fruit, a thermostable enzyme. Plant Physiol. 1984 Sep;76(1):233–237. doi: 10.1104/pp.76.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]


