Abstract
Background
The management of advanced oral cavity and oropharyngeal cancers is problematic and has traditionally relied on surgery and radiotherapy, both of which are associated with substantial adverse effects. Radiotherapy has been in use since the 1950s and has traditionally been given as single daily doses. This method of dividing up the total dose, or fractionation, has been modified over the years and a variety of approaches have been developed with the aim of improving survival whilst maintaining acceptable toxicity.
Objectives
To determine which radiotherapy regimens for oral cavity and oropharyngeal cancers result in increased overall survival, disease free survival, progression free survival and locoregional control.
Search methods
The following electronic databases were searched: the Cochrane Oral Health Group's Trials Register (to 28 July 2010), CENTRAL (The Cochrane Library 2010, Issue 3), MEDLINE via OVID (1950 to 28 July 2010) and EMBASE via OVID (1980 to 28 July 2010). There were no restrictions regarding language or date of publication.
Selection criteria
Randomised controlled trials where more than 50% of participants had primary tumours of the oral cavity or oropharynx, and which compared two or more radiotherapy regimens, radiotherapy versus other treatment modality, or the addition of radiotherapy to other treatment modalities.
Data collection and analysis
Data extraction and assessment of risk of bias was undertaken independently by two or more authors. Study authors were contacted for additional information as required. Adverse events data were collected from published trials.
Main results
30 trials involving 6535 participants were included. Seventeen trials compared some form of altered fractionation (hyperfractionation/accelerated) radiotherapy with conventional radiotherapy; three trials compared different altered fractionation regimens; one trial compared timing of radiotherapy, five trials evaluated neutron therapy and four trials evaluated the addition of pre‐operative radiotherapy. Pooling trials of any altered fractionation radiotherapy compared to a conventional schedule showed a statistically significant reduction in total mortality (hazard ratio (HR) 0.86, 95% confidence interval (CI) 0.76 to 0.98). In addition, a statistically significant difference in favour of the altered fractionation was shown for the outcome of locoregional control (HR 0.79, 95% CI 0.70 to 0.89). No statistically significant difference was shown for disease free survival.
No statistically significant difference was shown for any other comparison.
Authors' conclusions
Altered fractionation radiotherapy is associated with an improvement in overall survival and locoregional control in patients with oral cavity and oropharyngeal cancers. More accurate methods of reporting adverse events are needed in order to truly assess the clinical performance of different radiotherapy regimens.
Plain language summary
Interventions for the treatment of oral cavity and oropharyngeal cancer: radiotherapy
Oral cavity (mouth) cancer is usually detected earlier and treated with surgery and radiotherapy. Oropharyngeal (throat) cancer may be at an advanced stage when it is found and is treated with radiotherapy. Both surgery and radiotherapy may be associated with disfigurement and decreased ability to eat, drink and talk. Recent advances show that by altering how the radiotherapy is given to patients, improvements in overall survival can be achieved. The new methods of giving radiotherapy are called accelerated fractionation or hyperfractionation. However, they may be associated with an increase in side effects.
Summary of findings
for the main comparison.
| Altered fractionation compared with conventional radiotherapy for the treatment of oral cavity and oropharyngeal cancer | ||||||
|
Patient or population: people with oral cavity and oropharyngeal cancer Settings: hospital Intervention: altered fractionation Comparison: conventional | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional | Altered fractionation | |||||
| Mortality (follow‐up: 5 years) |
Low risk population | HR 0.86 (0.76 to 0.98) | [3751] (13) | +OOO very low2,3,4 | Analysis conducted on all included studies | |
| 200 per 10001 | 175 per 1000 (156 to 196) | |||||
| Medium risk population | ||||||
| 500 per 1000 | 449 per 1000 (410 to 493) | |||||
| High risk population | ||||||
| 700 per 10001 | 645 per 1000 (599 to 693) | |||||
| Mortality (follow‐up: 5 years) |
Low risk population | HR 0.93 (0.80 to 1.07) | [1511] (5) | +++O moderate2 | Analysis conducted for studies at low risk of bias | |
| 200 per 10001 | 187 per 1000 (163 to 212) |
|||||
| Medium risk population | ||||||
| 500 per 1000 | 475 per 1000 (426 to 524) |
|||||
| High risk population | ||||||
| 700 per 10001 | 674 per 1000 (618 to 724) |
|||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Based on data presented by McGurk 2005
2Studies included patients with other head and neck cancers
3Heterogeneity due to one study
4Assessed as unclear regarding allocation concealment, incomplete outcome data, selective reporting and/or other biases for 8 included trials
Background
Description of the condition
Oral cancers are a significant disease group globally with more than 404,000 new cases worldwide in 2002 (Parkin 2005; Warnakulasuriya 2009). Oral cancers are the sixth most common cancer worldwide, accounting for an estimated 4% of all cancers. The incidence and mortality from oral cancers varies geographically; the highest age standardised rates of oral cancers are reported in parts of Europe (France, Hungary), Botswana and south central Asia (Sri Lanka, Pakistan, Bangladesh and India) (Parkin 2005). There is overwhelming evidence that tobacco use, alcohol consumption and betel quid chewing are the main risk factors in the aetiology of intraoral cancer (La Vecchia 1997; Macfarlane 1995). There is also strong evidence that low socio‐economic status is associated with a higher incidence and poorer survival of oral cancers (Faggiano 1997). There is a higher incidence of oral cancers in men (Freedman 2007) that is generally attributed to a greater exposure to the known risk factors and vast majority of cases occur in men over 50 (Warnakulasuriya 2009) and among low socio‐economic groups (Conway 2008). However, the ratio of males to females diagnosed with oral cancers has declined from approximately 5:1 in the 1960s to less than 2:1 in 2002 (Parkin 2005). Another recent trend is the increasing incidence of oral cavity and oropharyngeal cancers in younger adults in the European Union and the United States (Warnakulasuriya 2009).
The epidemiological data concerning 'oral cancer' obscure the fact that 'oral cancer' includes both oral cavity and oropharyngeal cancers which have clinically different aetiology, are generally diagnosed at different stages and managed in different ways. Patients with oral cavity cancers generally present with early stage disease and the primary treatment is surgery or radiotherapy or both. However, oropharyngeal cancers are likely to be advanced at the time of diagnosis and primary treatment is more likely to be radiation therapy or chemoradiation. It is now recognised that oral infection with human papilloma virus (HPV) is strongly associated with the development of oropharyngeal cancer where HPV infection is found in 40% to 60% of patients (D'Souza 2007), and HPV is thought to be associated with the increased incidence of oropharyngeal cancer (Hammarstedt 2006). The link between oncogenic HPV and oropharyngeal cancer is strong and has been documented in numerous studies, fulfilling the epidemiological criteria for disease causality, especially in the development of oropharyngeal cancer in non‐smokers (Sturgis 2007). The proportion of patients with oropharyngeal cancer who are HPV positive has increased dramatically over recent years (Attner 2010; Ryerson 2008) but it is interesting to note that this group of patients have significantly improved rates of both overall survival and disease free survival (Fakhry 2006; Fakhry 2008; Licitra 2006).
The most common cancer of the oral cavity is the squamous cell carcinoma that arises from the lining of the oral cavity; over 95% of all oral cavity cancers are squamous cell carcinomas. Despite significant technical advances in the treatment of oral cancer, it still has a significant mortality with 128,000 deaths recorded, representing nearly half of the incident cases (48%) (Parkin 2001). Survival following a diagnosis of oral cavity or oropharyngeal cancer remains poor with 5‐year survival around 50% overall, with only limited improvement in the past 3 decades (Warnakulasuriya 2009).
Description of the intervention
Surgery has long been the mainstay for the treatment of oral cancer but radiotherapy can be used alone, in combination (adjuvant) with surgery, or in combination with chemotherapy (Garg 2004). Radiotherapy (also referred to as radiation therapy) is a localised treatment and thereby affects cells only in the treated area. Radiotherapy is used alone for small tumours or for patients who cannot have surgery. It may be used before surgery to kill cancer cells and shrink the tumour. It also may be used after surgery to destroy cancer cells that may remain in the area.
Radiotherapy works by damaging the deoxyribonucleic acid (DNA) of rapidly dividing cells so that the usual mechanisms of DNA repair (which are usually less effective in cancer cells compared to normal cells) cannot work and the cells die. However, normal cells that proliferate rapidly will inevitably be affected by therapeutic radiation. Therefore tissues such as hair, salivary glands and the mucosa are commonly affected (CRUK 2009).
Conventional radiotherapy uses high‐energy photons to kill cancer cells. Two types of radiotherapy are commonly used to treat oral and oropharyngeal cancers: teletherapy ‐ where the radiation is produced by a linear accelerator machine (external beam). Patients undergoing this type of therapy have to go to the hospital or clinic daily, usually 5 days a week for several weeks. Alternatively they may receive radiotherapy in the form of brachytherapy (also referred to as implant radiotherapy). Here the radiation comes from a radioactive material placed in seeds, needles, or carried via thin plastic tubes and put directly into the tissue. The patient must stay in hospital for the duration of the implant therapy, typically several days. Some people with oral cancer have both kinds of radiation therapy.
Radiotherapy for the treatment of head and neck cancer has conventionally been given as single daily doses of 1.8 to 2.0 Gy/fraction, 5 days a week to a total dose of 66 to 70 Gy (over 6½ to 7 weeks). This method of dividing up the total dose, or fractionation, has been modified over the years based on the underlying biology of the tumours and normal host tissues and has been recently reviewed by Bernier (Bernier 2005; Bernier 2006). There are two main types of altered fractionation: hyperfractionation and accelerated fractionation. Hyperfractionation uses smaller, multiple daily doses over a similar duration as conventional fractionation to give a higher total dose. Typically twice daily fractions of 1.1 to 1.2 Gy/fraction to a total dose of 74 to 80 Gy are used. Accelerated fractionation uses similar total doses as conventional treatment in a reduced treatment time. Accelerated radiotherapy schedules have been developed recently to overcome tumour cell repopulation during the course of therapy (squamous cell cancers of the head and neck can double the number of cancerous cells in 3 days) (Bourhis 2006). Further variations have been attempted: continuous hyperfractionated accelerated radiotherapy (CHART), intensity modulated radiotherapy (IMRT) and image‐guided radiotherapy (CRUK 2009; Harari 2005).
When used as an adjuvant to surgery, radiotherapy has traditionally been given post‐operatively particularly when there has been incomplete excision or there is extracapsular spread of the tumour out of the cervical lymph nodes. Neoadjuvant radiotherapy (given before surgery) is less common because of the deleterious effects on the tissues making surgery more difficult. Studies have shown improved survival in combination therapy where radiotherapy is given post‐operatively rather than pre‐operatively (Fanucchi 2006).
Tumours can be resistant to radiotherapy for a variety of reasons. Very rapidly proliferating tumour cells can repopulate in between treatments (hence the case for hyperfractionation), tumour cells can be intrinsically resistant to radiation or the tumours may be hypoxic (oxygen is required to enhance the DNA damage of the radiotherapy). In view of this, chemotherapy can be added to enhance the action of radiotherapy.
Why it is important to do this review
The management of advanced oral cavity and oropharyngeal cancers is problematic and has traditionally relied on surgery and radiotherapy, both of which are associated with substantial adverse effects. Although there have been new treatments developed there has been limited improvement in survival over the past 3 decades (Warnakulasuriya 2009). Oropharyngeal cancers have relatively 'silent' symptoms which may not be present during the early stages of the disease, which is a possible explanation for the fact that stage of disease at diagnosis has not altered in the past 40 years despite public education (McGurk 2005). Tumour recurrence and the development of multiple primary tumours are the major causes of treatment failure (Day 1992; Partridge 2000; Woolgar 2003). Surgical treatment may be disfiguring and result in a substantially reduced quality of life as patients are socially isolated, due to difficulties with altered appearance, speech, eating and drinking. Developments in the way in which radiotherapy is delivered aim to improve its efficacy and maintain acceptable levels of toxicity.
This review is undertaken as part of a series of reviews looking at the different treatment modalities of oral cancer (Furness 2010; Oliver 2007; Pavitt 2007). These reviews have been categorised into four intervention groups: surgery, chemotherapy, radiotherapy and immunotherapy. For this radiotherapy review we will aim to answer the broad question 'Does treatment with radiotherapy, in addition to chemotherapy and/or surgery, improve the outcomes for patients with oral cavity and oropharyngeal cancers?'.
Objectives
Primary objective
To determine which radiotherapy regimens for oral cavity and oropharyngeal cancers result in increased overall survival, disease free survival, progression free survival and locoregional control.
Secondary objective
To determine the implication of treatment modalities in terms of morbidity, quality of life, costs, hospital days of treatment, complications and harms.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials comparing radiotherapy to an alternative radiotherapy regimen or other treatment modality, or trials evaluating the addition of radiotherapy to other treatment modalities (including surgery and chemotherapy). Trials with a minimum follow‐up of 6 months will be included. It is anticipated that there will be no studies comparing radiotherapy with placebo (although if there are such studies they will be included).
Types of participants
Patients with oral cancer as defined by the International Classification of Diseases for Oncology (ICD‐O) codes as C01‐C02, C03, C04, C05‐C06 (oral cavity) and cancer of the oropharynx (ICD‐O: C09, C10) will be included but hypopharynx (ICD‐O: C13), nasopharynx (ICD‐O: C11) and larynx (ICD‐O: C32) will be excluded. Cancers of the lip (ICD‐O: C00) will also be excluded (WHO 1992).
Studies of head and neck cancer with cases of oral cancer will be included (so long as at least 50% of participants who have oral cavity or oropharyngeal cancer are included, or data for these cancers alone are available separately).
Cancers will be primary squamous cell carcinomas arising from the oral mucosa. Histological variants of squamous cell carcinomas will be included (adenosquamous, verrucous, basaloid, papillary etc) although they are known to have differing natural history to the majority of conventional squamous cell carcinomas they have a common aetiology, their incidence is low and they are generally managed in the same way. Carcinoma in situ will be included. Epithelial malignancies of the salivary glands, odontogenic tumours, all sarcomas and lymphomas will be excluded as these have a different aetiology and are managed differently.
Types of interventions
Radiotherapy: any mode of administration, dose of fractionation and total dose, number of fractions per day and per week, and duration of radiotherapy will be included.
Comparisons were made between different radiotherapy regimens and radiotherapy versus other treatment modalities including surgery and chemotherapy. The addition of radiotherapy to other treatment modalities were also be evaluated.
The intervention under evaluation must be radiotherapy. Trials where all participants receive the same radiotherapy regimen and are randomised to other treatments were excluded. Trials evaluating the role of chemoradiotherapy compared to radiotherapy alone are covered in the chemotherapy review by Furness 2010.
The treatments received and compared must be the primary treatment for the tumour and patients should not have received any prior intervention other than diagnostic biopsy.
Types of outcome measures
Primary outcome measures
Overall survival/total mortality (disease related mortality will also be studied if possible).
Locoregional control.
Disease free survival.
Progression free survival or time to recurrence.
Secondary outcome measures
Quality of life.
Harms associated with treatment.
Direct and indirect costs to patients and health services.
Patient satisfaction.
Search methods for identification of studies
Electronic searches
This review is part of a series of Cochrane reviews on the treatment modalities for treating oral cavity and oropharyngeal cancer. The reviews have been broadly divided into four themes concerning surgery, chemotherapy, radiotherapy or immunotherapy/targeted therapies. A search strategy was developed that would encompass three of the four broad themes simultaneously (surgery, chemotherapy, radiotherapy) and further adapted for use in the following databases (date of the most recent searches as indicated):
The Cochrane Oral Health Group's Trials Register (to 28 July 2010) (Appendix 2)
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 3) (Appendix 3)
MEDLINE via OVID (1950 to 28 July 2010) (Appendix 1)
EMBASE via OVID (1980 to 28 July 2010) (Appendix 4).
Current Controlled Trials (www.controlled‐trials.com) was searched for oral cancer or oropharyngeal cancer on 25 January 2010.
Because studies involving oral cancer are often included with those of the head and neck, a broad search was undertaken to include all possible studies. The searches attempted to identify all relevant trials irrespective of language. The reference list of related review articles and articles considered to be potentially relevant were checked for further trials. Authors of identified trials and known specialists in the field were contacted in an attempt to identify any additional published or unpublished trials.
Sensitive search strategies were developed for each database using a combination of free text and MeSH terms; these were based on the search strategy developed for MEDLINE (Appendix 1) but revised appropriately for each database. The search strategy combined the subject search with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity maximising version (2009 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions version 5.0.2 (updated September 2009) (Higgins 2009). The search of EMBASE was linked to the Cochrane Oral Health Group filter for identifying randomised controlled trials in this database (Appendix 4).
Handsearching was done as part of the Cochrane Collaboration's worldwide handsearching programme, see the Cochrane Master List of journals being searched for more information. The reference lists of related reviews and all articles obtained were checked for further trials. Authors of trial reports and specialists in the field known to the review authors were written to concerning further published and unpublished trials.
Data collection and analysis
Selection of studies
The titles and abstracts (when available) of all reports identified through the electronic searches were scanned independently by two review authors. For studies appearing to meet the inclusion criteria, or for which there were insufficient data in the title and abstract to make a clear decision, the full report was obtained. The full reports obtained from all the electronic and other methods of searching were assessed independently by two review authors to establish whether the studies met the inclusion criteria or not. Disagreements were resolved by discussion. Where resolution was not possible, a third review author was consulted. All studies meeting the inclusion criteria underwent a risk of bias assessment and data extraction using a specially designed data extraction form. Studies rejected at this or subsequent stages were recorded in the Characteristics of excluded studies table, and reasons for exclusion recorded.
Data extraction and management
Data were extracted by two review authors independently using specially designed data extraction forms. The data extraction forms were piloted on several papers and modified as required before use. Any disagreements were discussed and a third review author consulted where necessary. However, group discussion was often required following data extraction due to the complexity of the data presented. When necessary authors were contacted for clarification or missing information.
For each trial the following data were recorded:
Year of publication, country of origin and source of study funding
Details of the participants including demographic characteristics and criteria for inclusion and exclusion, proportion with oral cavity and oropharyngeal cancer
Details of the type of intervention, timing and duration
Details of the outcomes reported, including method of assessment, and time intervals.
As the majority of trials were for head and neck cancers the proportion of oral/oropharyngeal cancer patients was recorded (Additional Table 2). Head and neck cancer trials with only combined data (i.e. no outcome data available by primary tumour site) where greater than 50% of participants presented with oral/oropharyngeal cancer were included in this review. However, where separate 'pure' oral/oropharyngeal cancer data were available for a trial, these 'pure' data were extracted and analysed and the combined head and neck data ignored.
1. Percentage of patients with oral cavity (OC) or oropharyngeal (OP) cancer in studies included in this review.
| Trial ID | %OC | %OP | Total % OC/OP |
| Ang 2001* | 49 | 51 | 100 |
| Bergermann 1992 | 100 | 0 | 100 |
| Horiot 1992 | 0 | 100 | 100 |
| Inoue 2001 | 100 | 0 | 100 |
| Marcial 1993 | 0 | 100 | 100 |
| Olmi 2003 | 0 | 100 | 100 |
| Pinto 1991 | 0 | 100 | 100 |
| Sanguineti 2005* | 100 | ||
| Bourhis 2006 | 14 | 77 | 91 |
| Bartelink 2002 | 33 | 47 | 80 |
| Horiot 1997 | 16 | 64 | 80 |
| Griffin 1989 | 27 | 52 | 79 |
| Poulsen 2001 | 11 | 67 | 78 |
| Maor 1986 | 26 | 51 | 77 |
| Maor 1995 | 23 | 51 | 75 |
| Hukku 1991 | 10 | 62 | 72 |
| Dobrowsky 2000 | 30 | 41 | 71 |
| Fu 2000 | 10 | 60 | 71 |
| Lawrence 1974 | 45 | 26 | 71 |
| Terz 1981 | 38 | 29 | 67 |
| Ghoshal 2008 | 0 | 65 | 65 |
| Cox 1990 | 20 | 44 | 64 |
| Weissberg 1983 | 19 | 45 | 64 |
| Fu 1995 | 11 | 51 | 61 |
| MacDougall 1990 | 40 | 21 | 61 |
| Marcial 1987 | 15 | 46 | 61 |
| Griffin 1984 | 25 | 33 | 58 |
| Ketcham 1969 | 56 | unclear | >56 |
| Bjarnason 2009 | 19 | 35 | 54 |
| Skladowski 2006 | 22 | 28 | 50 |
*Data were available from the authors for those participants with cancer of the oral cavity or oropharynx only.
Assessment of risk of bias in included studies
For the studies included in this review assessment of risk of bias was conducted by two review authors using the Cochrane risk of bias assessment tool. Six domains were assessed for each included study: sequence generation, allocation concealment, blinding, completeness of outcome data, risk of selective outcome reporting and risk of other potential sources of bias.
A description of the domains was tabulated for each included trial, along with a judgement of low, high or unclear risk of bias. For example, criteria for risk of bias judgements regarding allocation concealment are given below as described in the Cochrane Handbook for Systematic Reviews of Interventions 5.0.2 (Higgins 2009).
Low risk of bias ‐ adequate concealment of the allocation (e.g. sequentially numbered, sealed, opaque envelopes or centralised or pharmacy‐controlled randomisation).
Unclear risk of bias ‐ unclear about whether the allocation was adequately concealed (e.g. where the method of concealment is not described or not described in sufficient detail to allow a definite judgement).
High risk of bias ‐ inadequate allocation concealment (e.g. open random number lists or quasi‐randomisation such as alternate days, date of birth, or case record number).
A summary assessment of the risk of bias for the primary outcome (across domains) across studies was undertaken (Higgins 2009). Within a study, a summary assessment of low risk of bias was given when there was a low risk of bias for all key domains, unclear risk of bias when there was an unclear risk of bias for one or more key domains, and high risk of bias when there was a high risk of bias for one or more key domains.
Measures of treatment effect
The primary outcome is total mortality expressed as a hazard ratio (it is acknowledged that it is preferable to talk in terms of overall survival, however, statistically the estimate of effect is the hazard ratio of death). If hazard ratios were not quoted in studies, we calculated the log hazard ratio and the standard error (SE) from the available summary statistics or Kaplan‐Meier curves, according to the methods proposed by Parmar et al (Parmar 1998), or these data were requested from authors. A meta‐analysis of individual patient data (IPD) for altered fractionation versus conventional fractionation has previously been published (Bourhis 2006). For trials included in the Bourhis meta‐analysis, the IPD were used instead of data presented in the published reports of the individual trials.
For dichotomous outcomes, the estimates of effect of an intervention were expressed as risk ratios together with 95% confidence intervals. Dichotomous data were only used for primary outcomes where hazard ratios were unavailable or could not be calculated.
Assessment of heterogeneity
Meta‐analyses were conducted only if there were studies of similar comparisons reporting the same outcome measures. The significance of any discrepancies in the estimates of the treatment effects from the different trials was assessed by means of Cochran's test for heterogeneity and the I2 statistic, and any heterogeneity investigated.
Data synthesis
Risk ratios were combined for dichotomous data, and hazard ratios for survival data, using a fixed‐effect model, unless there were more than four trials to be combined, when a random‐effects model was used. Hazard ratio data were entered into the meta‐analysis using the inverse variance method.
Subgroup analysis and investigation of heterogeneity
Due to the different natural history and treatment regimens for oral cavity and oropharyngeal cancers we planned to analyse these cancer types separately, if there were sufficient data.
Sensitivity analysis
A sensitivity analysis (to examine the effects of randomisation, allocation concealment, blinded outcome assessment (if appropriate) and quality of follow‐up/completeness of data set) was planned.
Results
Description of studies
Over 5000 research papers were identified through the electronic searching. Screening of the titles and abstracts resulted in the identification of 129 potentially relevant trials for inclusion in the review. Full text copies of these articles were retrieved, where available. Further assessment of the papers resulted in 30 trials (from 68 publications) being included int he review. Forty‐one trials (from 63 publications) were excluded, the reasons for which are presented in Characteristics of excluded studies.
Of the 30 trials included in the review, 19 were multicentred, with the number of centres ranging from 2 to 26. Fourteen trials were undertaken in the US (one linked with centres in Canada and one linked to the UK), four in centres across Europe, two in Italy, two in Germany, two in India, one across Australia and NewZealand and one solely in the UK, France, Japan, Brazil, and Poland.
Participants were recruited over periods ranging from 1 year to 10 years, with the earliest recruitment commencing in 1969 (Lawrence 1974; Terz 1981).
Fifteen of the included trials reported the cancer stage of recruited participants. Five of the trials recruited those with stages II‐IV, nine included patients with stages III‐IV and one trial recruited those with stages I‐IV. Tumour extent (TNM) was reported in 25 of the included trials, 13 of which included patients with T1 to T4 tumours. The remaining 12 included T2 to T4 or T3 to T4.
Of the 30 included trials, only two included recruited participants with oral cavity cancer only and a further four included only those with oropharyngeal cancer. The authors of two trials provided us with separate data (see Additional Table 2 for details) and one trial recruited only those with cancer of either the oral cavity or oropharynx. In the remaining included trials at least 50% of participants had either oral cavity or oropharyngeal cancer.
Trials were grouped into five main categories.
Altered fractionation
Hyperfractionated versus conventional (Fu 2000; Horiot 1992; Pinto 1991).
Hyperfractionated/accelerated versus conventional (Bourhis 2006; Dobrowsky 2000; Marcial 1987; Poulsen 2001).
Hyperfractionated/accelerated/split versus conventional (Bartelink 2002; Fu 2000; Horiot 1997; Olmi 2003).
Accelerated versus conventional (Skladowski 2006; Weissberg 1983).
Accelerated/boost versus conventional (Ang 2001; Fu 2000; Ghoshal 2008; Sanguineti 2005).
Accelerated/split versus conventional (Marcial 1993).
Hyperfractionated/accelerated split course radiotherapy versus accelerated boost (Fu 1995).
Split course versus accelerated (Hukku 1991).
Variable total dose/duration (Cox 1990).
Note: Conventional radiotherapy was defined as 66‐77 Gy in 2 Gy fractions, for 5 days a week.
Neutron therapy
Mixed beam versus photon (Griffin 1989).
Neutron versus photon (Griffin 1984; MacDougall 1990; Maor 1986; Maor 1995).
Pre‐operative radiotherapy
Pre‐operative radiotherapy versus surgery alone (Ketcham 1969; Lawrence 1974; Terz 1981).
Pre‐operative and post‐operative radiotherapy versus post‐operative radiotherapy alone (Bergermann 1992).
Timing of radiotherapy regimen
Morning radiotherapy versus afternoon radiotherapy (Bjarnason 2009).
Other
Low dose rate interstitial radiotherapy versus high dose rate interstitial radiotherapy (Inoue 2001).
Risk of bias in included studies
A summary of the risk of bias assessment is presented in Figure 1.
1.
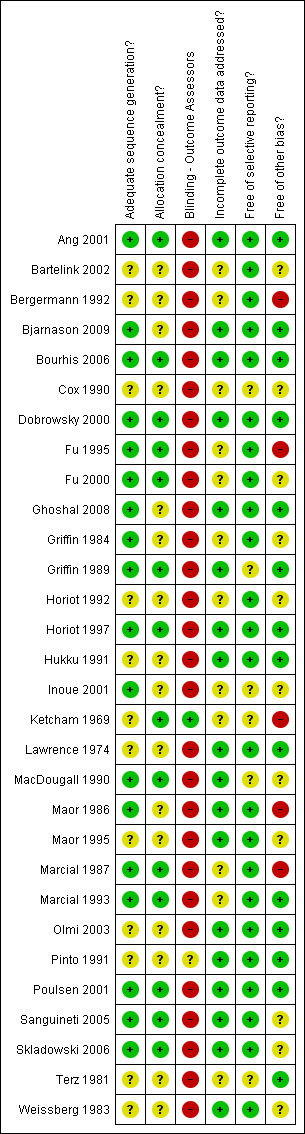
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Eighteen of the included trials were assessed as having adequate sequence generation. In the remaining 12 trials, the sequence generation was considered to be unclear. Fourteen trials were assessed as having adequate allocation concealment, the remaining trials providing insufficient information on this item.
Blinding
In most trials of radiotherapy, blinding of participants and clinicians would be difficult. A decision was made to assess those not explicitly reporting on blinding of outcome assessors as having no blinding. It was felt that for objective outcomes (such as total mortality) the lack of blinding was unlikely to result in bias. However, for more subjective outcomes, lack of blinding was considered to represent a potential risk of bias. Only one trial reported blind outcome assessment (Ketcham 1969).
Incomplete outcome data
Twelve of the included trials were assessed as being at an unclear risk of bias with regard to incomplete outcome data. All other trials were assessed as low risk with regard to this item, due to no missing outcome data, balanced missing outcome data across groups, missing outcome data unlikely to be related to true outcome, or unlikely to have clinical impact on estimate of effect.
Selective reporting
Majority of the included trials (24/30) were assessed as being free of selective reporting bias, reporting on expected, clinically important outcomes. Six trials were assessed as being at unclear risk of bias for this item due to reasons such as lack of information to determine if subgroup analyses were preplanned.
Other potential sources of bias
Five trials were assessed as being at high risk of bias with regard to other potential sources of bias (Bergermann 1992; Fu 1995; Ketcham 1969; Maor 1986; Marcial 1987). Fourteen were assessed as being at low risk of bias with regard to other potential sources of bias and 11 trials assessed as unclear risk of bias.
The overall assessment of risk of bias in the included trials is described within the section Effects of interventions.
Effects of interventions
See: Table 1
Altered fractionation
Within this section trials have been grouped to ensure they are similar in terms of dose/fraction (Gy), fractions/week, total dose (Gy) and duration of radiotherapy. The following definitions have been used.
Conventional ‐ single daily doses of 1.8 to 2.0 Gy/fraction, 5 days a week to a total dose of 66 to 70 Gy (typically over 6½ to 7 weeks).
Hyperfractionated ‐ total dose is divided into small doses, with more than 1 fraction/day.
Accelerated ‐ total dose given over a shorter period of time (< 6 weeks).
Hyperfractionated versus conventional radiotherapy
Three trials were included in this comparison (Fu 2000; Horiot 1992; Pinto 1991), with a total of 966 randomised participants. All three trials were considered to be at unclear risk of bias with regard to total mortality. For less objective outcomes, two trials were considered to be at high risk of bias (Fu 2000; Horiot 1992) and one trial at unclear risk of bias (Pinto 1991). Two trials included patients with primary tumours of the oropharynx only (Horiot 1992; Pinto 1991). Fu 2000 included patients with head and neck tumours, of which 10% were located in the oral cavity and 60% in the oropharynx. The radiotherapy regimens evaluated in the three trials are presented in the table below.
Summary of radiotherapy regimens: hyperfractionated versus conventional radiotherapy
| Hyperfractionated | Conventional | ||||||||
| Dose/fraction (Gy) | Fractions/week | Total dose (Gy) | Total duration (weeks) | Dose/fraction | Fractions/week | Total dose (Gy) | Total duration (weeks) | ||
| Fu 2000 | 1.2 | 10 | 81.6 | 7 | 2 | 5 | 70 | 7 | |
| Horiot 1992 | 1.15 | 10 | 80.5 | 7 | 1.75‐2 | 5 | 70 | 7‐8 | |
| Pinto 1991 | 1.1 | 10 | 70.4 | 6.4 | 2 | 5 | 66 | 6.5 | |
For all three trials individual patient data (IPD) were available from Bourhis 2006 for total mortality and locoregional control. A statistically significant difference was shown in favour of the hyperfractionated radiotherapy for both total mortality (hazard ratio (HR) 0.78, 95% confidence interval (CI) 0.68 to 0.90) and locoregional control (HR 0.74, 95% CI 0.62 to 0.89) (Analysis 1.1; Analysis 1.2).
1.1. Analysis.
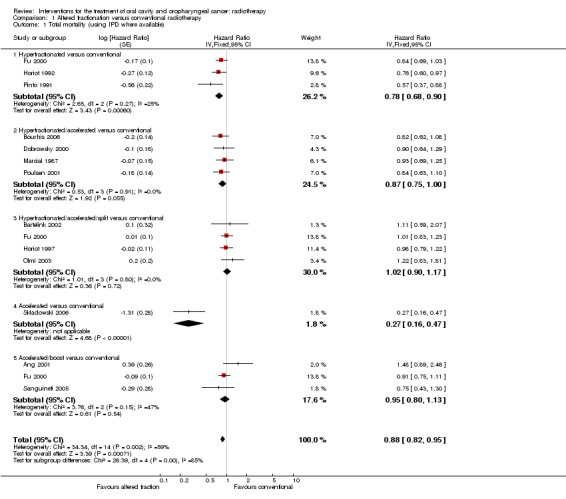
Comparison 1 Altered fractionation versus conventional radiotherapy, Outcome 1 Total mortality (using IPD where available).
1.2. Analysis.
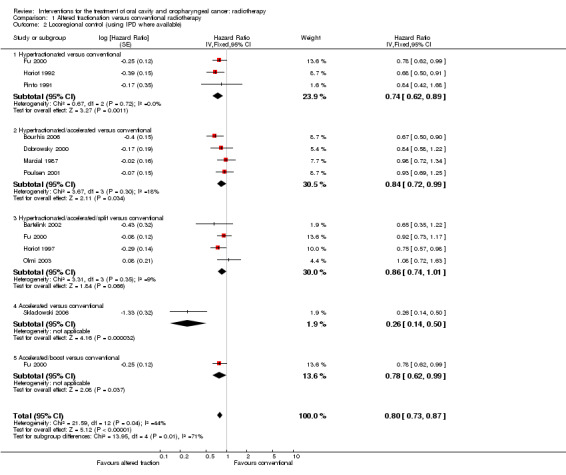
Comparison 1 Altered fractionation versus conventional radiotherapy, Outcome 2 Locoregional control (using IPD where available).
Disease free survival data were available for one of the trials (Fu 2000) and showed no statistically significant difference between hyperfractionated and conventional radiotherapy (Analysis 1.3).
1.3. Analysis.
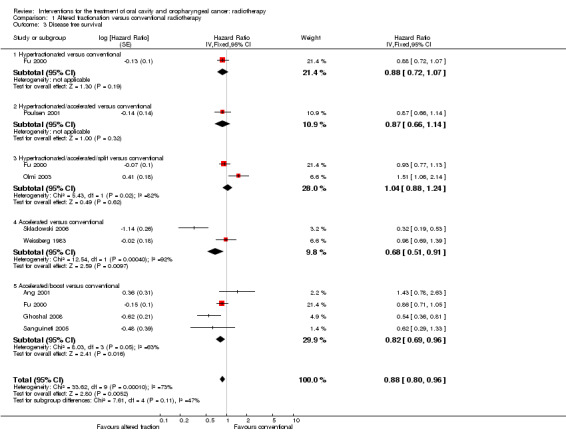
Comparison 1 Altered fractionation versus conventional radiotherapy, Outcome 3 Disease free survival.
Hyperfractionated/accelerated versus conventional
Four trials compared a hyperfractionated/accelerated radiotherapy regimen with conventional radiotherapy (Bourhis 2006; Dobrowsky 2000; Marcial 1987; Poulsen 2001), with a total of 1071 randomised participants. Three trials were considered to be at low risk of bias with regard to assessment of total mortality (Bourhis 2006; Dobrowsky 2000; Poulsen 2001) and high risk of bias for other outcomes. Marcial 1987 was considered to be at high risk of bias across all outcomes.
All trials recruited patients with tumours of the head and neck. The percentage of participants with cancer of the oral cavity or oropharynx ranged from 61% (Marcial 1987) to 91% (Bourhis 2006). The radiotherapy regimens are presented in the table below. Both arms in the trial by Poulsen 2001 received a reduced total dose in comparison to the other three trials.
Summary of radiotherapy regimens: hyperfractionated/accelerated versus conventional radiotherapy
| Hyperfractionated/accelerated | Conventional | ||||||||
| Dose/fraction (Gy) | Fractions/week | Total dose (Gy) | Total duration (weeks) | Dose/fraction | Fractions/week | Total dose (Gy) | Total duration (weeks) | ||
| Bourhis 2006 | 2 | 10 | 62‐64 | 3 | 2 | 5 | 70 | 7 | |
| Dobrowsky 2000 | 2.5 on day 1 then 1.65 | 14 | 55.3 | 2.4 | 2 | 5 | 70 | 7 | |
| Marcial 1987 | 1.2 | 10 | 60 | 5 | 1.8‐2 | 5 | 66‐73.8 | 7‐8 | |
| Poulsen 2001 | 1.8 | 14 | 39.6 | 2.3 | 2 | 5 | 50 | 5 | |
IPD for total mortality and locoregional control were available for all four trials (Bourhis 2006). The pooled HR for total mortality was not statistically significant (HR 0.87, 95% CI 0.75 to 1.00) but the pooled estimate for locoregional control just attained statistical significance (HR 0.84, 95% CI 0.72 to 0.99) (Analysis 1.1; Analysis 1.2).
Only one of the four trials presented data for disease free survival (Poulsen 2001). No statistically significant difference between treatment groups was shown (Analysis 1.3).
Hyperfractionated/accelerated/split versus conventional
Four trials were included in this comparison (Bartelink 2002; Fu 2000; Horiot 1997; Olmi 2003), including a total of 1299 randomised participants.
Only one trial was considered to be at low risk of bias with regard to the assessment of total mortality (Horiot 1997); the remaining trials were considered to be at unclear risk of bias. With regard to more subjective outcomes, all trials were considered to be at either high or unclear risk of bias.
One of these trials recruited participants with cancer of the oropharynx only (Olmi 2003). The remaining three trials recruited participants with head and neck cancer with the percentage of those with cancer of the oral cavity or oropharynx ranging from 71% (Fu 2000) to 80% (Bartelink 2002; Horiot 1997).
The radiotherapy regimens for the five trials are presented in the table below.
Summary of radiotherapy regimens: hyperfractionated/accelerated/split versus conventional radiotherapy
| Hyperfractionated/accelerated/split | Conventional | ||||||||
| Dose/fraction (Gy) | Fractions/week | Total dose (Gy) | Total duration (weeks) | Dose/fraction | Fractions/week | Total dose (Gy) | Total duration (weeks) | ||
| Bartelink 2002 | 1.6 | 15 | 72 | weeks 1, 4 and 7 | 2 | 5 | 70 | 7 | |
| Fu 2000 | 1.6 | 10 | 67.2 | 2.5 weeks, 2 weeks rest then 1.5 weeks | 2 | 5 | 70 | 7 | |
| Horiot 1997 | 1.6 | 21 (14 after split) | 72 | 1.1 weeks, 2 weeks rest then 2.4 weeks | 2 | 5 | 70 | 7 | |
| Olmi 2003 | 1.6 | 10 | 64‐67.2 | 2 weeks, 2 weeks rest then 3 weeks | 2 | 5 | 66‐70 | 6.5‐7 | |
Total mortality and locoregional control data were available for the calculation of HR in all four the trials; IPD were available for Fu 2000, Horiot 1997 and Olmi 2003 and HR data were calculated from a Kaplan‐Meier graph for Bartelink 2002. No statistically significant difference was shown between the two radiotherapy schedules with regard to either total mortality (HR 1.02, 95% CI 0.90 to 1.17) or locoregional control (HR 0.86, 95% CI 0.74 to 1.01) (Analysis 1.1; Analysis 1.2).
Only two of the four trials presented data on disease free survival (Fu 2000; Olmi 2003). The radiotherapy schedules were similar in both trials. Fu 2000 recruited 542 participants with head and neck cancers (10% oral cavity, 60% oropharynx) to the two radiotherapy regimens, and Olmi 2003 recruited 192 participants with cancer of the oropharynx. The results with regard to disease free survival are contradictory, with substantial statistical heterogeneity (P = 0.02, I2 = 82%) (Analysis 1.3).
Accelerated versus conventional
Two trials compared an accelerated regimen (with no split, boost or hyperfractionation) with conventional radiotherapy (Skladowski 2006; Weissberg 1983), including a total of 164 randomised participants.
Both trials were considered to be at unclear risk of bias for the assessment of total mortality and high risk of bias for subjective outcomes.
Both trials recruited participants with head and neck cancer; 64% of those recruited by Weissberg 1983 and 50% of those recruited by Skladowski 2006 had tumours of the oral cavity or oropharynx.
The radiotherapy regimens for the two trials are presented in the table below, and differ substantially. The accelerated course used by Skladowski 2006 was of longer duration with a higher total dose than Weissberg 1983. Also, Skladowski 2006 gave a lower dose/fraction for 7 fractions/week (rather than 5 fractions/week). The conventional radiotherapy schedules were similar.
Summary of radiotherapy regimens: accelerated versus conventional radiotherapy
| Accelerated | Conventional | ||||||||
| Dose/fraction (Gy) | Fractions/week | Total dose (Gy) | Total duration (weeks) | Dose/fraction | Fractions/week | Total dose (Gy) | Total duration (weeks) | ||
| Weissberg 1983 | 4 | 5 | 40‐48 | 2‐3 | 2 | 5 | 60‐70 | 6‐7 | |
| Skladowski 2006 | 2 | 7 | 66+/‐2 (T2) 70+/‐2 (T3‐4) |
4.7‐5.1 | 2 | 5 | 66+/‐2 (T2) 70+/‐2 (T3‐4) |
6.7‐7.1 | |
Only one trial reported data for total mortality and locoregional control (Skladowski 2006), for which IPD were also available (Bourhis 2006). A statistically significant difference in favour of the altered fractionation was shown (Analysis 1.1; Analysis 1.2). Both trials reported on disease free survival, however, given the substantial clinical and statistical heterogeneity between the trials' results (P = 0.0004, I2 = 92%) a pooled analysis is not reported here (Analysis 1.3).
Accelerated/boost versus conventional
Four trials compared an accelerated regimen incorporating a radiotherapy boost with conventional radiotherapy (Ang 2001; Fu 2000; Ghoshal 2008; Sanguineti 2005), with a total of 1203 randomised participants.
One trial was assessed as being at low risk of bias with regard to total mortality (Ang 2001) and three were assessed as being at unclear risk of bias with regard to total mortality (Fu 2000; Ghoshal 2008; Sanguineti 2005). For subjective outcomes, all four trials were considered to be at high risk of bias.
For two of the trials data were available from the authors for those participants with cancer of the oral cavity or oropharynx only (Ang 2001; Sanguineti 2005). In the trials by Fu 2000 and Ghoshal 2008 71% and 65% of recruited participants had cancer of the oral cavity or oropharynx.
The radiotherapy regimens for the four trials are presented in the table below and are similar across trials. However, it should be noted that in the trials by Ang 2001 and Sanguineti 2005, radiotherapy was given post‐operatively.
Summary of radiotherapy regimens: accelerated/boost versus conventional radiotherapy
| Accelerated/boost | Conventional | ||||||||
| Dose/fraction (Gy) | Fractions/week | Total dose (Gy) | Total duration (weeks) | Dose/fraction | Fractions/week | Total dose (Gy) | Total duration (weeks) | ||
| Ang 2001* | 1.8 1.8 (boost) |
5 10 (boost) |
63 | 5 (boost last 21 days of radiotherapy) |
1.8 | 5 | 63 | 7 | |
| Fu 2000 | 1.8 1.5 (boost) |
5 7 (boost) |
70.5 | 6 (boost last 11 days of radiotherapy) |
2 | 5 | 70 | 7 | |
| Ghoshal 2008 | 1.8 1.5 (boost) |
5 5 (boost) |
67.5 | 5 (boost last 21 days of radiotherapy) |
2 | 5 | 66 | 6.5 | |
| Sanguineti 2005* | 2 1.4 (first week boost) 1.6 (fifth week boost) |
5 5 (boost) |
64 | 5 (boost during first and fifth week of radiotherapy) |
2 | 5 | 50‐60 | 5‐6 | |
* post‐operative radiotherapy
Three of the trials provided data for the calculation of HR for total mortality; IPD were available for one trial (Fu 2000, data presented in Bourhis 2006) and data were provided by the authors for two trials (Ang 2001; Sanguineti 2005). No statistically significant difference in total mortality between treatment schedules was shown (HR 0.95, 95% CI 0.80 to 1.13) (Analysis 1.1).
IPD on locoregional control was available for one trial (Fu 2000, data from Bourhis 2006 ). A statistically significant difference in favour of the accelerated schedule with boost was shown in this single study (Analysis 1.2).
All four trials provided data on disease free survival, and the pooled estimate showed a statistically significant difference on favour of the accelerated/boost schedule. However, it should be noted that there is statistical heterogeneity (P = 0.05, I2 = 63%) (Analysis 1.3).
Accelerated/split versus conventional
One trial, recruiting 147 participants, compared split course, accelerated radiotherapy with conventional radiotherapy (Marcial 1993). The trial was assessed as being at unclear risk of bias for the outcome of total mortality and high risk of bias for more subjective outcomes. The trial recruited participants with cancer of the oropharynx only.
Summary of radiotherapy regimens: accelerated/split versus conventional radiotherapy
| Accelerated/split | Conventional | ||||||||
| Dose/fraction (Gy) | Fractions/week | Total dose (Gy) | Total duration (weeks) | Dose/fraction | Fractions/week | Total dose (Gy) | Total duration (weeks) | ||
| Marcial 1993 | 3 | 5 | 60 | 2 weeks, 3 weeks rest then 2 weeks | 2‐2.2 | 5 | 60‐66 | 6 | |
Dichotomous data were available for the calculation of 5 year risk ratios for total mortality (Additional Table 3). No statistically significant difference was shown between schedules for any of these outcomes.
2. Results from comparisons where there are data from a single study only.
| Total mortality | Locoregional control | Disease free survival | ||
| Accelerated/split versus conventional | ||||
| Marcial 1993 | 5 years RR 1.17 (95% CI 0.57 to 2.43) |
|||
| Split course versus accelerated | ||||
| Hukku 1991 | 2 years RR 1.00 (95% CI 0.82 to 1.22) |
2 years RR 1.33 (95% CI 0.67 to 2.67) |
2 years RR 0.83 (95% CI 0.29 to 2.42) |
|
| Variable total dose/duration of radiotherapy | ||||
| Cox 1990 | High versus standard HR 1.08 (95% CI 0.69 to 1.70) |
High versus standard HR 0.78 (95% CI 0.55 to 1.11) |
||
| Low versus standard HR 1.38 (95% CI 0.83 to 2.29) |
Low versus standard HR 1.13 (95% CI 0.78 to 1.64) |
|||
| Morning versus afternoon radiotherapy | ||||
| Bjarnason 2009 | 2 years RR 1.09 (95% CI 0.74 to 1.59) |
HR 0.92 (95% CI 0.60 to 1.42) | ||
| Mixed beam versus photon | ||||
| Griffin 1989 | HR 1.12 (95% CI 0.92 to 1.36) | HR 1.04 (95% CI 0.84 to 1.29) | ||
| Neutron versus photon | ||||
| MacDougall 1990 | 5 years RR 1.15 (95% CI 0.95 to 1.40) |
5 years RR 0.97 (95% CI 0.69 to 1.36) |
5 years RR 0.63 (95% CI 0.36 to 1.09) |
|
| Pre‐operative radiotherapy versus surgery alone | ||||
| Ketcham 1969 | Timing unclear RR 0.63 (95% CI 0.28 to 1.46) |
|||
| Terz 1981 | HR 0.83 (95% CI 0.64 to 1.07) | |||
| Pre‐operative and post‐operative radiotherapy versus post‐operative radiotherapy alone | ||||
| Bergermann 1992 | HR 0.67 (95% CI 0.35 to 1.28) | HR 1.13 (95% CI 0.78 to 1.64) | HR 1.13 (95% CI 0.78 to 1.64) | |
| Low dose rate interstitial radiotherapy versus high dose rate interstitial radiotherapy | ||||
| Inoue 2001 | HR 1.00 (95% CI 0.16 to 6.44) | |||
CI = confidence interval; HR = hazard ratio; RR = risk ratio.
Summary: any altered fractionation radiotherapy versus conventional radiotherapy
When the 13 trials providing data on any altered fractionation radiotherapy regimen compared to a conventional schedule were pooled using a random‐effects model, a statistically significant reduction in total mortality was shown (HR 0.86, 95% CI 0.76 to 0.98) (Figure 2). It should be noted that there was statistically significant heterogeneity between the trials for total mortality (P = 0.002, I2 = 59%).
2.
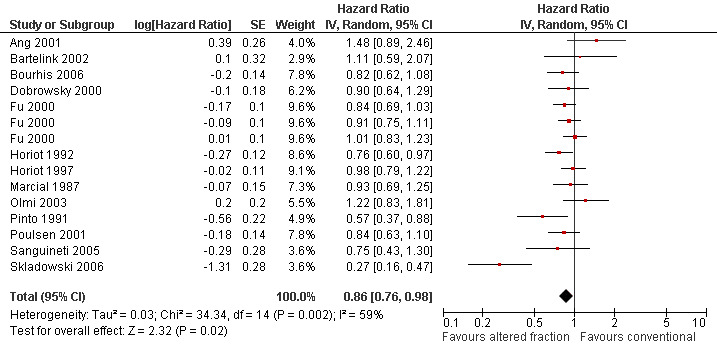
Forest plot of comparison: Summary analyses for altered fractionation versus conventional radiotherapy, outcome: Total mortality.
Pooling of 11 trials providing data on locoregional control and comparing any altered schedule with conventional also showed a statistically significant difference in favour of the altered fractionation (HR 0.79, 95% CI 0.70 to 0.89) (Figure 3). No statistically significant difference was shown between altered fractionation and conventional radiotherapy when the eight trials providing data on disease free survival were combined (HR 0.85, 95% CI 0.70 to 1.03) (Figure 4).
3.
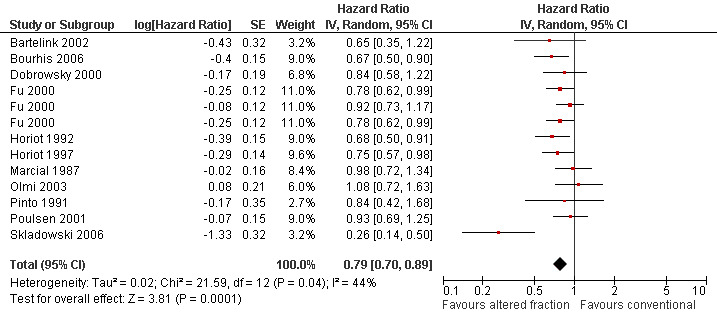
Forest plot of comparison: Summary analyses for altered fractionation versus conventional radiotherapy, outcome: Locoregional control.
4.
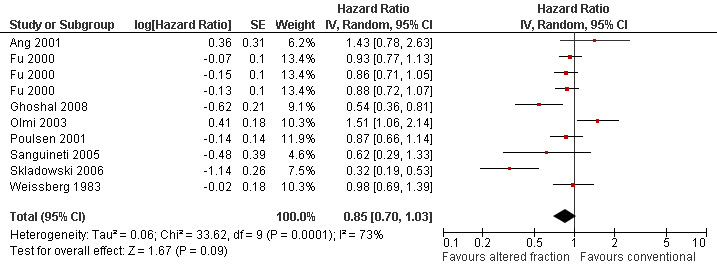
Forest plot of comparison: Summary analyses for altered fractionation versus conventional radiotherapy, outcome: Disease free survival.
A sensitivity analysis was undertaken to determine the effect of excluding trials assessed as being at high or unclear risk of bias (Additional Table 4). When the five trials assessed as being at low risk of bias were pooled using a random‐effects model, no statistically significant difference in total mortality was shown between altered fractionation radiotherapy regimens compared to conventional schedules (HR 0.93, 95% CI 0.80 to 1.07). The findings of the sensitivity analyses for locoregional control and disease free survival were HR 0.79, 95% CI 0.68 to 0.91 (random‐effects model) and HR 0.95, 95% CI 0.0.74 to 1.22 (fixed‐effect model) respectively.
3. Sensitivity analyses: altered fractionation versus conventional radiotherapy.
| All trials | Trials assessed as being at low risk of bias | |
| Total mortality | HR 0.86 (95% CI 0.76 to 0.98) (13 trials) |
HR 0.93 (95% CI 0.80 to 1.07) (5 trials) |
| Locoregional control | HR 0.79 (95% CI 0.70 to 0.89) (11 trials) |
HR 0.79 (95% CI 0.68 to 0.91) (4 trials) |
| Disease free survival | HR 0.85 (95% CI 0.70 to 1.03) (8 trials) |
HR 0.95 (95% CI 0.0.74 to 1.22)* (2 trials) |
*Fixed‐effect model due to limited number of trials. CI = confidence interval; HR = hazard ratio.
Summary of altered fractionation regimens versus conventional radiotherapy with data from more than one trial
| Total mortality | |
| Hyperfractionated versus conventional radiotherapy | HR 0.78 (95% CI 0.68 to 0.90) (3 trials) |
| Hyperfractionated/accelerated versus conventional radiotherapy | HR 0.87 (95% CI 0.75 to 1.00) (4 trials) |
| Hyperfractionated/accelerated/split versus conventional radiotherapy | HR 1.02, (95% CI 0.90 to 1.17) (4 trials) |
| Accelerated/boost versus conventional radiotherapy | HR 0.95 (95% CI 0.80 to 1.13) (3 trials) |
| Any altered fractionation versus conventional radiotherapy (all trials) | HR 0.86 (95% CI 0.76 to 0.98) (13 trials) |
| Any altered fractionation versus conventional radiotherapy (low risk of bias trials) | HR 0.93 (95% CI 0.80 to 1.07) (5 trials) |
Hyperfractionated/accelerated split course radiotherapy versus accelerated radiotherapy with concomitant boost
One small trial (including 75 randomised participants), designed as a feasibility study, compared an accelerated split course with an accelerated schedule with boost (Fu 1995). The trial was assessed as being at high risk of bias for all outcomes. The trial participants had head and neck cancer; 61% had cancer of the oral cavity or oropharynx. The authors report no significant difference between the schedules in terms of total mortality, locoregional control or disease free survival, however, data are not presented in a way that allow for the calculation of HR.
Summary of radiotherapy regimens: hyperfractionated/accelerated split course versus accelerated boost
| Hyperfractionated/accelerated split course | Accelerated boost | ||||||||
| Dose/fraction (Gy) | Fractions/week | Total dose (Gy) | Total duration (weeks) | Dose/fraction | Fractions/week | Total dose (Gy) | Total duration (weeks) | ||
| Fu 1995 | 1.6 | 10 | 67.2 | 2.5 weeks, 2 weeks rest then 1.5 weeks | 1.8 1.5 (boost) |
5 7 (boost) |
70.5 | 6 (boost last 11 days of radiotherapy) |
|
Split course versus accelerated
A single trial of head and neck cancer patients (110 randomised participants; 72% oral cavity or oropharynx) compared a split course with an accelerated course of radiotherapy (Hukku 1991). The trial was assessed to be at unclear risk of bias for total mortality and high risk of bias for locoregional control and disease free survival. Dichotomous data were available for the calculation of 2‐year risk ratios for total mortality, locoregional control and disease free survival (Additional Table 3). No statistically significant difference was shown between schedules for any of these outcomes.
Summary of radiotherapy regimens: split course versus accelerated
| Split course | Accelerated | ||||||||
| Dose/fraction (Gy) | Fractions/week | Total dose (Gy) | Total duration (weeks) | Dose/fraction | Fractions/week | Total dose (Gy) | Total duration (weeks) | ||
| Hukku 1991 | 2.3 (2.5 after split) | 5 | 59.5 | 3 weeks, 2 weeks rest then 2 weeks | 4 | 5 | 44 | 2.1 | |
Variable total dose/duration
One trial compared three different total doses, delivered over varying times (Cox 1990). A total dose of 72 Gy was considered to be the conventional dose. Total doses of 76.8 Gy (over 6.4 weeks) and 67.2 Gy (over 5.6 weeks) were compared with the conventional dose. The trial was considered to be at unclear risk of bias for total mortality and high risk of bias for subjective outcomes. Data were available for the calculation of HR for total mortality and locoregional control (Additional Table 3). No statistically significant difference was shown between either of the altered doses and the conventional dose with regard to total mortality or locoregional control.
Summary of radiotherapy regimens: altered dose versus conventional dose
Neutron therapy
Mixed beam versus photon
One trial (327 randomised participants) compared mixed beam radiotherapy with conventional photon radiotherapy (Griffin 1989). The trial was assessed as being at unclear risk of bias with regard to total mortality and high risk of bias for the assessment of locoregional control. A total of 79% of those recruited had cancer of the oral cavity or oropharynx. No statistically significant difference was shown between the two schedules for either total mortality (Analysis 3.1) or locoregional control (Additional Table 3).
3.1. Analysis.
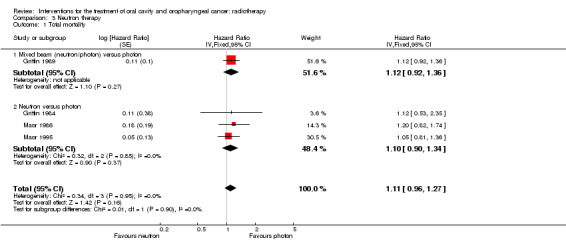
Comparison 3 Neutron therapy, Outcome 1 Total mortality.
Neutron versus photon
Four trials compared neutron radiotherapy with conventional photon radiotherapy, including a total of 531 participants (Griffin 1984; MacDougall 1990; Maor 1986; Maor 1995). All trials were considered to be at unclear or high risk of bias for the outcome of total mortality, and high risk of bias for subjective outcomes.
All four trials included participants with head and neck cancer, the percentage of those with cancer of the oral cavity or oropharynx varied from 58% (Griffin 1984) to 77% (Maor 1986).
Three of the four trials provided data that allowed for the calculation of a HR for total mortality (Griffin 1984; Maor 1986; Maor 1995). No statistically significant difference was shown between neutron or photon radiotherapy (HR 1.10, 95% CI 0.90 to 1.34) (Analysis 3.1). MacDougall 1990 provided dichotomous data allowing calculation of 5‐year risk ratios for total mortality. Again, no statistically significant difference was shown between the two groups (Additional Table 3).
Only one trial provided useable data for the outcome of locoregional control and disease free survival (MacDougall 1990). 5‐year risk ratios were calculated; no statistically significant differences were shown for either outcome (Additional Table 3).
Pre‐operative radiotherapy
Pre‐operative radiotherapy versus surgery alone
Three trials were included in this comparison, including over 470 randomised participants (Ketcham 1969; Lawrence 1974; Terz 1981). All three trials were considered to be at unclear or high risk of bias for all outcomes. All included participants with head and neck cancer, the percentage of those with cancer of the oral cavity or oropharynx varied from 56% (Ketcham 1969) to 77% (Lawrence 1974).
Only one trial provided data on total mortality in a useable format (Terz 1981). No statistically significant difference was shown between the two groups (Additional Table 3). Ketcham 1969 provides dichotomous data on locoregional control which showed no statistically significant difference between groups, however, the timing of the assessment of this outcome is unclear (Additional Table 3).
Pre‐operative and post‐operative radiotherapy versus post‐operative radiotherapy alone
One trial was included in this comparison (Bergermann 1992). The trial, including patients with cancer of the oral cavity alone, was judged to be at high risk of bias across all outcomes. Data were available for the calculation of HRs for total mortality, locoregional control and disease free survival. No statistically significant difference was seen for any outcome (Additional Table 3).
Timing of radiotherapy
Morning radiotherapy versus afternoon radiotherapy
One trial recruited 216 participants to either morning or afternoon radiotherapy (Bjarnason 2009). Four different schedules were used, either 50 Gy in 25 fractions, or 60 Gy in 25‐30 fractions or 66 Gy in 33 fractions, or 70 Gy in 25 fractions and randomisation was stratified on planned total dose. The trial was assessed as being at unclear risk of bias for objective outcomes and high risk of bias for all other outcomes. The primary aim of the trial was to assess associated toxicity with the different radiotherapy regimens. No statistically significant differences were shown in terms of overall survival or locoregional control (Additional Table 3). Morning radiotherapy was associated with significantly less weight loss after 5 months but no statistically significant difference in quality of life scores (data not reported).
Other
Low dose rate interstitial radiotherapy versus high dose rate interstitial radiotherapy
One trial randomised 59 patients with early mobile tongue cancer (Inoue 2001) to receive either low or high dose rate interstitial radiotherapy. The trial was assessed as being at unclear risk of bias for total mortality and high risk of bias for all subjective outcomes. No statistically significant difference was shown for locoregional control between treatment groups (Additional Table 3).
Discussion
Summary of main results
The main comparison within this review was altered fractionation radiotherapy and conventional radiotherapy. Pooling of all studies of altered fractionation versus conventional radiotherapy showed a statistically significant difference in favour of the altered fractionation for the outcomes of total mortality (hazard ratio (HR) 0.86, 95% confidence interval (CI) 0.76 to 0.98 (random‐effects model)) and locoregional control (HR 0.79, 95% CI 0.70 to 0.89 (random‐effects model)). This statistically significant difference was not shown for disease free survival (the outcome with least available data), although the direction of effect was towards altered fractionation.
Comparing the current review's findings with the earlier individual patient data (IPD) meta‐analysis of hyperfractionated or accelerated radiotherapy in head and neck cancer (Bourhis 2006) shows similar results, despite slight discrepancies in trials included and methods used. Bourhis 2006 showed a statistically significant benefit in terms of total mortality (HR 0.92, 95% CI 0.86 to 0.97 (fixed‐effect model)) in favour of altered fractionation radiotherapy compared to conventional radiotherapy. Again, for locoregional control a statistically significant difference in favour of altered fractionation radiotherapy was shown (HR 0.82, 95% CI 0.77 to 0.88 (fixed‐effect model)).
The meta‐analysis by Bourhis 2006 reports a significantly higher survival benefit with hyperfractionated radiotherapy than with accelerated radiotherapy. Trials were classified differently in the current review and the meta‐analysis by Bourhis 2006, not allowing for a direct comparison. However, of trials included within the current review, those classed as purely 'hyperfractionated' were the only pooled group to show a statistically significant difference in favour of the altered fractionation for the outcome of total mortality (HR 0.78, 95% CI 0.68 to 0.90).
Comparisons between mixed beam versus conventional photon radiotherapy, and neutron versus photon radiotherapy showed no statistically significant difference between treatment groups for total mortality, locoregional control or disease free survival. This supports the findings reported by Duncan 1994 and Koh 1994 in evaluations of neutron therapy trials in a variety of cancers, including head and neck cancers. Neither show mixed beam or neutron therapy to be advantageous to photon radiotherapy and both raise concern over late morbidity associated with neutron therapy. Current evidence does not justify the use of mixed beam or neutron therapy for the treatment of head and neck cancers.
The addition of pre‐operative radiotherapy was evaluated in four trials; three looked at pre‐operative versus surgery alone (Ketcham 1969;Lawrence 1974;Terz 1981), one evaluated pre‐operative plus post‐operative versus post‐operative alone (Bergermann 1992). All trials were considered to be at unclear or high risk of bias and showed no statistically significant difference for any reported outcome. There is insufficient evidence to support or refute the addition of pre‐operative radiotherapy for the treatment of cancer of the oral cavity or oropharynx.
Similarly, there is insufficient evidence to support or refute the use of high dose rate interstitial radiotherapy over low dose rate interstitial radiotherapy (Inoue 2001).
Overall completeness and applicability of evidence
A limitation of the review is that it aims to evaluate the role of radiotherapy for the treatment of cancers of the oral cavity and oropharynx. The prevalence of trials of treatments of combined head and neck malignancies suggests that those undertaking the primary studies seldom confine trials to patients with a primary lesion in either oral cavity or oropharynx, probably for pragmatic reasons. Of the 30 included trials only two included recruited participants with oral cavity cancer only and a further four included only those with oropharyngeal cancer. The authors of two trials provided us with separate data (see Additional Table 2 for details) and one trial recruited only those with cancer of either the oral cavity or oropharynx. In the remaining included trials at least 50% of participants had either oral cavity or oropharyngeal cancer. As for previous reviews assessing the effectiveness of surgery and chemotherapy for the treatment of patients with cancers of the oral cavity/oropharynx, we have included these trials because we believe that they still contribute important information in the absence of separate data in the research literature. We acknowledge that data on oral cavity cancers or oropharynx cancers alone may provide better evidence upon which to inform clinical practice, and we encourage that in future researchers publish the data for the different primary tumour sites separately. A subgroup analysis was undertaken in the IPD meta‐analysis by Bourhis 2006. They report that the effect of altered fractionation on tumour control, when compared to conventional radiotherapy, did not differ significantly according to tumour site (oral cavity, oropharynx, larynx, hypopharynx).
This review does not present a comprehensive systematic review of adverse event data, but does report toxicity data presented in the included trials. The reporting of adverse events within the included trials varied greatly. It has previously been acknowledged that reliable collection and reporting on adverse events remains challenging for clinical trials in oncology, with no uniform method being used for summarising the key elements of such data (Trotti 2007). Adverse effects from radiotherapy are usually considered in two groups: acute effects, which occur within 90 days of the start of treatment and late effects which occur more than 90 days after the start of treatment. In general acute adverse effects of radiotherapy include mucositis and skin reactions. Late effects include fibrosis, necrosis, myelitis, xerostomia or dysphagia. However, this classification of acute and late adverse effects was developed to reflect observations from conventional fractionation. The development of altered fractionation and combined modality treatment has lead to the reporting of extended acute effects, lasting beyond 90 days (Trotti 2000).
The severity of acute adverse effects is increased with increased daily dose, both with schedules that use increased dose per fraction and those that include more than one radiotherapy fraction per day. Lengthening the interfraction interval to at least 6 hours appears to mitigate some of this increased acute toxicity. A treatment 'rest', as in split course regimens, does not appear to reduce acute toxicity.
Late adverse effects are also more severe in the accelerated regimens which use dose/fraction greater than 1.1 Gy and short (< 5 hours) interfraction interval. This is supported by Ang 2001 who reports on a large body of radiobiologic data "showing that fraction size rather than radiotherapy duration is the major determinant of radiotherapy‐induced injury to normal tissues manifesting as late complications."
It has been reported that the major limitation of altered fractionation radiotherapy (and combined radio‐chemotherapy) for head and neck cancer is increased acute reaction primarily acute mucositis (Fu 2000). The role of molecular targeted therapies and improved radiotherapy techniques including intensity modulated radiotherapy (IMRT) for maintaining acceptable toxicities need further evaluation. There are currently no trials of IMRT in this systematic review, however, the PARSPORT trial is underway and likely to complete follow‐up in 2013 (Nutting 2009a).
The management of head and neck cancer often requires a combination of chemotherapy, radiotherapy or surgery, although current standard treatment is predominantly chemoradiotherapy with standard fractionation. This review focuses purely on trials to which the treatment under evaluation is some form of radiotherapy. While altered fractionation radiotherapy has been shown to improve overall survival when compared to conventional radiotherapy, it has not been directly compared to chemoradiotherapy. Also, there are no completed trials of altered fractionation plus chemotherapy versus chemoradiotherapy with standard fractionation. It is perhaps due to the cost and resource considerations of altered fractionation (especially hyperfractionation) that chemoradiotherapy remains the standard. For patients who have relative contraindications to chemotherapy (or specifically platinums), there is evidence to recommend the use of altered fractionation radiotherapy alone (especially hyperfractionation). To get a more complete overview of the role of each treatment modality, this review needs to be considered alongside the findings of previous reviews of surgery and chemotherapy for the treatment of cancers of the oral cavity and oropharynx (Furness 2010; Oliver 2007).
Potential biases in the review process
A comparison between meta‐analyses of individual patient data and data obtained from published literature has previously been explored (Duchateau 2001). The study focused on meta‐analyses of randomised controlled trials of chemotherapy in head and neck cancer. The outcome of interest was survival. For the meta‐analysis of individual patient data, the estimate of effect was the hazard ratio and for the literature‐based meta‐analysis the odds ratio for death at particular time point was used. The two meta‐analyses differed substantially in terms of number of comparisons, patients and events examined. However, even though the data sets vary, the treatment effect estimates and their 95% confidence intervals show little variation. The authors report that the main source of difference between the results of the meta‐analyses is due to the fact that one is based on the hazard ratio and the other on the odds ratio.
In the current review, the hazard ratio was used as preferred estimate of effect. For 21 of the trials included in the review data for the calculation of a hazard ratio were available for the outcome of total mortality, 15 for locoregional control and 8 for disease free survival. It is acknowledged that where data are determined from Kaplan‐Meier graphs there is scope for bias. Few trials present hazard ratios themselves, or data that allow for the calculation of hazard ratios without having to determine the number of events from a graph. When available, individual patient data were used over data presented in the published trials.
Authors' conclusions
Implications for practice.
Altered fractionation radiotherapy is associated with an improvement in overall survival and locoregional control in patients with oral cavity and oropharyngeal cancers. The benefit may be greater with hyperfractionated regimens rather than accelerated regimens. More accurate methods of reporting adverse events are needed in order to truly assess the clinical performance of different radiotherapy regimens.
Implications for research.
The role of molecular targeted therapies and improved radiotherapy techniques including intensity modulated radiotherapy for maintaining acceptable toxicities needs further evaluation. In addition, further research on the relative efficacy and toxicity of altered fractionation radiotherapy (+/‐ chemotherapy or biologics) versus conventional chemoradiotherapy is needed, as conventional chemoradiotherapy is still considered the current standard.
Trialists are encouraged to follow the Consolidated Standards of Reporting Trials (CONSORT) guidelines when reporting on their trials. Ideally trials should report hazard ratios with 95% confidence intervals for survival data, or present data that allow for the calculation of this estimate of effect. In addition, reporting of outcomes by tumour site and stage would allow for greater understanding of patient selection for different treatment modalities.
Acknowledgements
Thanks to:
May Wong for her statistical input during data extraction
Emma Tavender for her contribution to the drafting of the original protocol for this review, and helping to secure funding for the review
Sylvia Bickley and Anne Littlewood ‐ Cochrane Oral Health Group, Trials Search Co‐ordinators
Phil Riley ‐ retrieval of papers
Chris O'Brien for his contribution to the CSROC Expert Panel
Dr KK Ang and Dr G Sanguineti for providing additional data.
Appendices
Appendix 1. Search strategy for MEDLINE via OVID
1. "Head and Neck Neoplasms"/ 2. "Mouth Neoplasms"/ 3. "Gingival Neoplasms"/ 4. "Palatal Neoplasms"/ 5. "Tongue Neoplasms"/ 6. ((cancer$ or tumour$ or tumor$ or neoplas$ or malignan$ or carcinoma$ or metatasta$) adj5 (oral$ or intra‐oral$ or intraoral$ or "intra oral$" or gingiva$ or oropharyn$ or mouth$ or tongue$ or cheek$ or gum$ or palatal$ or palate$ or "head and neck")).mp. 7. or/1‐6 8. exp Radiotherapy/ 9. (radiotherap$ or chemotherap$ or chemoradiotherap$ or chemo‐radiotherap$ or "radiation therap$" or bracytherap$ or irradiat$).ti,ab. 10. (adjuvant or neo‐adjuvant or "neo adjuvant").ti,ab. 11. (hyperfractionate$ or hyper‐fractionate$).mp. 12. exp Surgical Procedures, Operative/ 13. (dissect$ adj2 neck$).ti,ab. 14. (excision or excise or resect$).ti,ab. 15. Lymph Node Excision/ 16. (lymphadenectom$ or glossectom$).ti,ab. 17. exp Antineoplastic agents/ 18. (antineoplast$ or antitumor$ or anti‐tumor$ or anti‐neoplast$ or antitumour$ or anti‐tumour$).mp. 19. Antineoplastic combined chemotherapy protocols/ 20. exp Combined Modality Therapy/ 21. or/8‐20 22. 7 and 21
The above subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity maximising version (2009 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions, version 5.0.2 (updated September 2009):
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 2. Cochrane Oral Health Group's Trials Register search strategy
((mouth or oral or intraoral or intra‐oral or gingiva* or oropharyn* or cheek* or gum* or palat* or lip or tongue or “head and neck”) AND (tumour* or tumor* or cancer* or carcinoma* or neoplas* or malignan*))
Appendix 3. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 MeSH descriptor Head and Neck Neoplasms this term only #2 MeSH descriptor Mouth neoplasms this term only #3 MeSH descriptor Gingival Neoplasms this term only #4 MeSH descriptor Palatal neoplasms this term only #5 MeSH descriptor Tongue neoplasms this term only #6 ((cancer* near/5 oral*) or (cancer* near/5 intra‐oral*) or (cancer* near/5 intraoral*) or (cancer* near/5 “intra) and oral”*) or (cancer* near/5 gingiva*) or (cancer* near/5 oropharyn*) or (cancer* near/5 mouth*) or (cancer* near/5 tongue*) or (cancer* near/5 cheek*) or (cancer* near/5 gum*) or (cancer* near/5 palatal*) or (cancer* near/5 palate*) or (cancer* near/5 "head and neck")) #7 ((tumour* near/5 oral*) or (tumour* near/5 intra‐oral*) or (tumour* near/5 intraoral*) or (tumour* near/5 “intra) and oral”*) or (tumour* near/5 gingiva*) or (tumour* near/5 oropharyn*) or (tumour* near/5 mouth*) or (tumour* near/5 tongue*) or (tumour* near/5 cheek*) or (tumour* near/5 gum*) or (tumour* near/5 palatal*) or (tumour* near/5 palate*) or (tumour* near/5 "head and neck")) #8 ((tumor* near/5 oral*) or (tumor* near/5 intra‐oral*) or (tumor* near/5 intraoral*) or (tumor* near/5 “intra) and oral”*) or (tumor* near/5 gingiva*) or (tumor* near/5 oropharyn*) or (tumor* near/5 mouth*) or (tumor* near/5 tongue*) or (tumor* near/5 cheek*) or (tumor* near/5 gum*) or (tumor* near/5 palatal*) or (tumor* near/5 palate*) or (tumor* near/5 "head and neck")) #9 ((neoplas* near/5 oral*) or (neoplas* near/5 intra‐oral*) or (neoplas* near/5 intraoral*) or (neoplas* near/5 “intra) and oral”*) or (neoplas* near/5 gingiva*) or (neoplas* near/5 oropharyn*) or (neoplas* near/5 mouth*) or (neoplas* near/5 tongue*) or (neoplas* near/5 cheek*) or (neoplas* near/5 gum*) or (neoplas* near/5 palatal*) or (neoplas* near/5 palate*) or (neoplas* near/5 "head and neck")) #10 ((malignan* near/5 oral*) or (malignan* near/5 intra‐oral*) or (malignan* near/5 intraoral*) or (malignan* near/5 “intra) and oral”*) or (malignan* near/5 gingiva*) or (malignan* near/5 oropharyn*) or (malignan* near/5 mouth*) or (malignan* near/5 tongue*) or (malignan* near/5 cheek*) or (malignan* near/5 gum*) or (malignan* near/5 palatal*) or (malignan* near/5 palate*) or (malignan* near/5 "head and neck")) #11 ((carcinoma* near/5 oral*) or (carcinoma* near/5 intra‐oral*) or (carcinoma* near/5 intraoral*) or (carcinoma* near/5 “intra) and oral”*) or (carcinoma* near/5 gingiva*) or (carcinoma* near/5 oropharyn*) or (carcinoma* near/5 mouth*) or (carcinoma* near/5 tongue*) or (carcinoma* near/5 cheek*) or (carcinoma* near/5 gum*) or (carcinoma* near/5 palatal*) or (carcinoma* near/5 palate*) or (carcinoma* near/5 "head and neck")) #12 ((metatasta* near/5 oral*) or (metatasta* near/5 intra‐oral*) or (metatasta* near/5 intraoral*) or (metatasta* near/5 “intra) and oral”*) or (metatasta* near/5 gingiva*) or (metatasta* near/5 oropharyn*) or (metatasta* near/5 mouth*) or (metatasta* near/5 tongue*) or (metatasta* near/5 cheek*) or (metatasta* near/5 gum*) or (metatasta* near/5 palatal*) or (metatasta* near/5 palate*) or (metatasta* near/5 "head and neck")) #13 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12) #14 MeSH descriptor Radiotherapy explode all trees #15 (radiotherap* or chemotherap* or chemoradiotherap* or chemo‐radiotherap* or "radiation therap*" or bracytherap* or irradiat*) #16 (adjuvant or neo‐adjuvant or "neo adjuvant”) #17 (hyperfractionate* or hyper‐fractionate*) #18 MeSH descriptor Surgical Procedures, Operative explode all trees #19 (dissect* near/2 neck*) #20 (excision or excise* or resect*) #21 MeSH descriptor Lymph node excision this term only #22 (lymphadenectom* or glossectom*) #23 MeSH descriptor Antineoplastic agents explode all trees #24 (antineoplast* or antitumor* or anti‐tumor* or anti‐neoplast* or antitumour* or anti‐tumour*) #25 MeSH descriptor antineoplastic combined chemotherapy protocols this term only #26 MeSH descriptor combined modality therapy explode all trees #27 (#14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26) #28 (#13 and #27)
Appendix 4. EMBASE via OVID search strategy
1. "Head and Neck Neoplasms"/ 2. "Mouth Neoplasms"/ 3. "Gingival Neoplasms"/ 4. "Palatal Neoplasms"/ 5. "Tongue Neoplasms"/ 6. ((cancer$ or tumour$ or tumor$ or neoplas$ or malignan$ or carcinoma$ or metatasta$) adj5 (oral$ or intra‐oral$ or intraoral$ or "intra oral$" or gingiva$ or oropharyn$ or mouth$ or tongue$ or cheek$ or gum$ or palatal$ or palate$ or "head and neck")).mp. 7. or/1‐6 8. exp Radiotherapy/ 9. (radiotherap$ or chemotherap$ or chemoradiotherap$ or chemo‐radiotherap$ or "radiation therap$" or bracytherap$ or irradiat$).ti,ab. 10. (adjuvant or neo‐adjuvant or "neo adjuvant").ti,ab. 11. (hyperfractionate$ or hyper‐fractionate$).mp. 12. exp Surgical Procedures, Operative/ 13. (dissect$ adj2 neck$).ti,ab. 14. (excision or excise or resect$).ti,ab. 15. Lymph Node Excision/ 16. (lymphadenectom$ or glossectom$).ti,ab. 17. exp Antineoplastic agents/ 18. (antineoplast$ or antitumor$ or anti‐tumor$ or anti‐neoplast$).mp. 19. Antineoplastic combined chemotherapy protocols/ 20. exp Combined Modality Therapy/ 21. or/8‐20 22. 7 and 21
The above subject search was linked to the Cochrane Oral Health Group filter for EMBASE via OVID:
1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/ 16. HUMAN/ 17. 16 and 15 18. 15 not 17 19. 14 not 18
Data and analyses
Comparison 1. Altered fractionation versus conventional radiotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality (using IPD where available) | 13 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.82, 0.95] | |
| 1.1 Hyperfractionated versus conventional | 3 | Hazard Ratio (Fixed, 95% CI) | 0.78 [0.68, 0.90] | |
| 1.2 Hyperfractionated/accelerated versus conventional | 4 | Hazard Ratio (Fixed, 95% CI) | 0.87 [0.75, 1.00] | |
| 1.3 Hyperfractionated/accelerated/split versus conventional | 4 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.90, 1.17] | |
| 1.4 Accelerated versus conventional | 1 | Hazard Ratio (Fixed, 95% CI) | 0.27 [0.16, 0.47] | |
| 1.5 Accelerated/boost versus conventional | 3 | Hazard Ratio (Fixed, 95% CI) | 0.95 [0.80, 1.13] | |
| 2 Locoregional control (using IPD where available) | 11 | Hazard Ratio (Fixed, 95% CI) | 0.80 [0.73, 0.87] | |
| 2.1 Hyperfractionated versus conventional | 3 | Hazard Ratio (Fixed, 95% CI) | 0.74 [0.62, 0.89] | |
| 2.2 Hyperfractionated/accelerated versus conventional | 4 | Hazard Ratio (Fixed, 95% CI) | 0.84 [0.72, 0.99] | |
| 2.3 Hyperfractionated/accelerated/split versus conventional | 4 | Hazard Ratio (Fixed, 95% CI) | 0.86 [0.74, 1.01] | |
| 2.4 Accelerated versus conventional | 1 | Hazard Ratio (Fixed, 95% CI) | 0.26 [0.14, 0.50] | |
| 2.5 Accelerated/boost versus conventional | 1 | Hazard Ratio (Fixed, 95% CI) | 0.78 [0.62, 0.99] | |
| 3 Disease free survival | 8 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.80, 0.96] | |
| 3.1 Hyperfractionated versus conventional | 1 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.72, 1.07] | |
| 3.2 Hyperfractionated/accelerated versus conventional | 1 | Hazard Ratio (Fixed, 95% CI) | 0.87 [0.66, 1.14] | |
| 3.3 Hyperfractionated/accelerated/split versus conventional | 2 | Hazard Ratio (Fixed, 95% CI) | 1.04 [0.88, 1.24] | |
| 3.4 Accelerated versus conventional | 2 | Hazard Ratio (Fixed, 95% CI) | 0.68 [0.51, 0.91] | |
| 3.5 Accelerated/boost versus conventional | 4 | Hazard Ratio (Fixed, 95% CI) | 0.82 [0.69, 0.96] |
Comparison 2. Summary analyses for altered fractionation versus conventional radiotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 13 | Hazard Ratio (Random, 95% CI) | 0.86 [0.76, 0.98] | |
| 2 Locoregional control | 11 | Hazard Ratio (Random, 95% CI) | 0.79 [0.70, 0.89] | |
| 3 Disease free survival | 8 | Hazard Ratio (Random, 95% CI) | 0.85 [0.70, 1.03] |
2.1. Analysis.
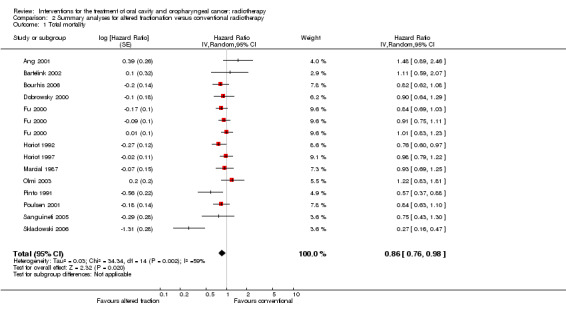
Comparison 2 Summary analyses for altered fractionation versus conventional radiotherapy, Outcome 1 Total mortality.
2.2. Analysis.
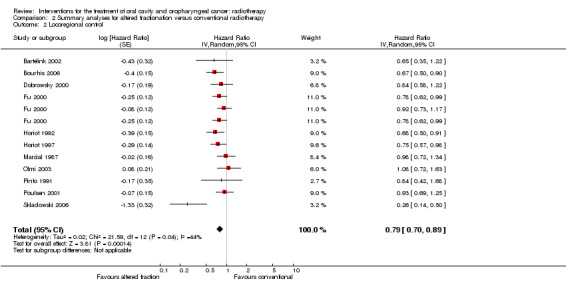
Comparison 2 Summary analyses for altered fractionation versus conventional radiotherapy, Outcome 2 Locoregional control.
2.3. Analysis.
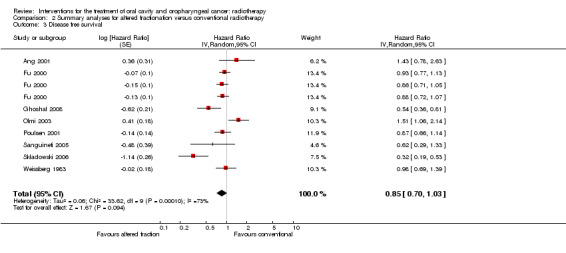
Comparison 2 Summary analyses for altered fractionation versus conventional radiotherapy, Outcome 3 Disease free survival.
Comparison 3. Neutron therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 4 | Hazard Ratio (Fixed, 95% CI) | 1.11 [0.96, 1.27] | |
| 1.1 Mixed beam (neutron/photon) versus photon | 1 | Hazard Ratio (Fixed, 95% CI) | 1.12 [0.92, 1.36] | |
| 1.2 Neutron versus photon | 3 | Hazard Ratio (Fixed, 95% CI) | 1.10 [0.90, 1.34] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ang 2001.
| Methods | Location of trial: US. Number of centres: 3. Funding: National Cancer Institute (grants CA‐06294 and CA‐16672), Gilbert H. Fletcher Chair, and Texas Tobacco Settlement Funds. Trial ID: not stated. |
|
| Participants | Inclusion criteria: histologically proven squamous cell carcinomas with advanced cancer (Stage II‐IV) of the oral cavity, oropharynx, larynx or hypopharynx, deemed likely to require treatment with a combination of surgery and post‐operative radiotherapy, and having a Zubrod performance status of 0‐2 were eligible for this trial. Median age 57 years. Exclusion criteria: not explicit. Recruitment period: August 1991 and August 1997. OC: 80/213 (38%). OP: 66/213 (31%). OC+OP: 146/213 (69%) (see notes). Number randomised: 151. Number analysed: 151. |
|
| Interventions |
Accelerated radiotherapy with boost versus conventional radiotherapy Accelerated/boost (n = 76): 1.8 Gy/fraction, 5 fractions per week for 3 weeks, followed by 10 fractions per week for 2 weeks (total 63 Gy). Conventional (n = 75): 1.8 Gy/fraction, 5 fractions per week for 7 weeks (total 63 Gy). Median interval between surgery and post‐operative radiotherapy was 31 days for those receiving accelerated radiotherapy and 29 days for those receiving conventional radiotherapy. |
|
| Outcomes | Primary: locoregional control. Secondary: overall survival, toxicity. Duration of follow‐up: unclear for randomised participants alone. |
|
| Notes | 258 participants underwent surgery and were classified as being low, intermediate or high risk according to pathologic risk features. Those classed as high risk were randomised to the 2 intervention groups (n = 151). HR calculated for OC/OP participants only, using data provided by authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Randomized trial." Not explicitly reported but undertaken by third party (data provided by author). |
| Allocation concealment? | Low risk | Third party allocation (data provided by author). |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Low risk | All randomised participants included in analysis. |
| Free of selective reporting? | Low risk | Important outcomes of locoregional control, overall survival and toxicity reported. |
| Free of other bias? | Low risk | Groups appear comparable. No other bias apparent. |
Bartelink 2002.
| Methods | Location of trial: Europe. Number of centres: 11. Funding: not stated. Trial ID: not stated (EORTC trial). |
|
| Participants | Inclusion criteria: locally advanced, inoperable head & neck cancer with primaries in oral cavity, oropharynx, larynx and hypopharynx. T2‐T4 included. Exclusion criteria: not explicit. Recruitment period: not stated. OC: 16/49 (33%). OP: 23/49 (47%). OC+OP: 39/49 (80%). Number randomised: 53. Number analysed: 49. |
|
| Interventions |
Hyperfractionated/accelerated/split course radiotherapy plus chemotherapy versus conventional radiotherapy plus chemotherapy Hyperfractionated/accelerated/split (n = 25): 1.6 Gy per fraction, 3 fractions per day on weeks 1, 4 & 7 (total dose 72 Gy) with 10 mg/m2 cisplatin IV administered daily between fractions 1 & 2. Interfraction interval varied between 3 & 4 hours. Conventional (n = 24): 2 Gy per fraction, 5 fractions per week for a total dose of 70 Gy over 7 weeks together with 6 mg/m2 cisplatin IV 30‐60 minutes prior to RT daily. |
|
| Outcomes | Primary: toxicity. Secondary: overall survival, locoregional control. Duration of follow‐up: minimum 24 months. |
|
| Notes | HR data taken from Kaplan‐Meier graphs (numbers at risk presented). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Patients were randomised between" ‐ method of sequence generation not described. |
| Allocation concealment? | Unclear risk | Insufficient information. |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Unclear risk | 3/28 (11%) patients in Gr A and 1/25 (4%) in Gr B did not receive allocated treatment. In this small trial this may have resulted in differences between groups with regard to prognostic factors and introduced bias. |
| Free of selective reporting? | Low risk | Primary outcomes are acute and late side effects, but also planned and reported treatment compliance, locoregional control and overall survival. |
| Free of other bias? | Unclear risk | There appear to be differences between treatment groups at baseline eg T stage, location of primary tumour and degree of tumour differentiation. No other bias apparent. |
Bergermann 1992.
| Methods | Location of trial: Germany. Number of centres: 2. Funding: not stated. Trial ID: not stated. |
|
| Participants | Inclusion criteria: histologically confirmed squamous cell carcinoma, primary tumour, T2, N0‐N2, M0, with no prior treatment. Tumour of the floor of the mouth, tongue edge and pars alveolaris (lower jaw) were included. Exclusion criteria: not explicitly stated in translation. Recruitment period: March 1982 to February 1987. OC: 100%. Number randomised: 100. Number analysed: 85. |
|
| Interventions |
Pre‐operative radiotherapy plus post‐operative radiotherapy versus post‐operative radiotherapy alone Pre‐operative (n = 44): 6 Gy on days 1‐3 followed by surgery on day 4. From day 21 saturation radiotherapy of 6 Gy/day (total 60 Gy). No pre‐operative (n = 41): surgery followed by saturation radiotherapy from day 21 of 6 Gy/day (total 60 Gy). |
|
| Outcomes | Primary outcome unclear. Overall survival, local recurrent disease, regional metastases, distant metastases, second tumour. Duration of follow‐up: 9 years. |
|
| Notes | Data from translation. Data taken from Kaplan‐Meier graphs (no numbers at risk). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Randomly allocated to treatment groups". No further information on method of sequence generation. |
| Allocation concealment? | Unclear risk | Insufficient information. |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Unclear risk | 15/100 randomised patients excluded from analysis (6 receiving radiotherapy; 9 not receiving radiotherapy). Reasons unclear. |
| Free of selective reporting? | Low risk | Trial reports outcomes of overall survival, local relapse, regional metastases, distant metastases, secondary tumours. No reporting of toxicity. |
| Free of other bias? | High risk | Substantial differences between groups in location of primary tumour. |
Bjarnason 2009.
| Methods | Location of trial: Canada. Number of centres: 12. Funding: National Cancer Institute of Canada. Trial ID: not stated. |
|
| Participants | Inclusion criteria: histologically proven squamous cell carcinomas of oral cavity, pharynx, larynx were eligible to receive radiotherapy without chemotherapy, 2 or more visible areas of oral mucosa in target area, ECOG performance status 0‐1, adequate haematological function. T1‐T4 included. Exclusion criteria: shift workers, patients with abnormal sleep habits, previous radiotherapy or chemotherapy within 6 months, planned use of radioprotective agents, connective tissue disease or AIDS. Recruitment period: August 1999 to November 2002. OC: 40/216 (19%). OP: 76/216 (35%). OC+OP: 116/216 (54%). Number randomised: 216. Number analysed: 216 (for overall survival); 205 (for toxicity). |
|
| Interventions |
Morning radiotherapy versus afternoon radiotherapy Morning (n = 108 ): radiotherapy between 8 &10 am. Afternoon (n = 108 ): radiotherapy between 4 & 6 pm. 4 different schedules were used, either 50 Gy in 25 fractions, or 60 Gy in 25‐30 fractions or 66 Gy in 33 fractions of 70 Gy in 25 fractions. Randomisation was stratified on planned total dose. |
|
| Outcomes | Primary: oral mucositis incidence of grade 3 or higher. Secondary: interval to development of grade 2 mucositis, duration of various grades of mucositis, treatment days lost due to toxicity, other acute/late toxicities, quality of life, weight loss during/after treatment, overall survival, locoregional control. Duration of follow‐up: maximum 5 years, 7 months. |
|
| Notes | Sample size calculation: hypothesised that incidence of grade 3 or greater mucositis with afternoon RT would be 35% and 17.5% for morning RT and it was estimated that 216 patients would be required to detect this difference with 80% power at a 2‐sided 0.05 level, after taking into account a potential 5% withdrawal rate. HR presented in text. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A minimisation procedure was used to randomise patients. Patients were stratified by treatment centre, pretreatment smoking status and planned total radiation dose. |
| Allocation concealment? | Unclear risk | Insufficient information. |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Low risk | Gr A 4/108 (4%) patients and Gr B 3/108 (3%) were found to be ineligible. In addition, Gr B 3/108 did not receive RT & 1/108 had no mucositis assessments recorded (4%). All randomised patients were included in survival outcome. |
| Free of selective reporting? | Low risk | Primary endpoint of study is toxicity, overall survival is a secondary outcome. |
| Free of other bias? | Low risk | Groups appear comparable at baseline. Possible co‐interventions clearly proscribed. |
Bourhis 2006.
| Methods | Location of trial: France. Number of centres: 11. Funding: not stated. Trial ID: not stated. |
|
| Participants | Inclusion criteria: patients with no previous history of cancer, or previous chemotherapy or radiotherapy, performance status 0‐2, squamous cell cancer of oral cavity, oropharynx, hypopharynx and larynx, T3‐T4, N0‐N3 not eligible for surgery. Exclusion criteria: not explicitly stated. Recruitment period: November 1994 to September 1998. OC: 36/266 (14%). OP: 205/266 (77%). OC+OP: 241/266 (91%). Number randomised: 268. Number analysed: 266. |
|
| Interventions |
Hyperfractionated/accelerated radiotherapy versus conventional radiotherapy Hyperfractionated/accelerated (n = 137): 62‐64 Gy in 31‐32 fractions over 22‐23 days, 2 Gy/fraction, 2 fraction/day, 20 Gy/week over 3 weeks. Interfraction interval 8 hours. Conventional (n = 129): 70 Gy in 35 fractions, 2 Gy per fraction, over 7 weeks. |
|
| Outcomes | Primary: locoregional control. Secondary: overall survival, disease free survival, toxicity. Duration of follow‐up: median > 6 years. |
|
| Notes | Sample size calculation "estimated that a minimum of 100 patients per group would be necessary to demonstrate an increase in the locoregional tumour control rate, from 30% in the conventional group to 55% in the very accelerated group, with an α = 5% and β = 5% (two tailed test)." Data from Kaplan‐Meier figures. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Centrally randomised at Institute Gustave Roussy Villejeuf, France from a computer generated list.... randomisation stratified by centre." |
| Allocation concealment? | Low risk | Treatment allocation made by telephone call to randomisation centre. |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Low risk | 1 patient had missing data, 1 refused treatment, and remainders of those randomised included in outcome evaluation. |
| Free of selective reporting? | Low risk | Important outcomes planned and reported ‐ locoregional control, toxicity and overall survival. |
| Free of other bias? | Low risk | Distribution of important patient and tumour characteristics well balanced between treatment arms. No other apparent bias. |
Cox 1990.
| Methods | Location of trial: US. Number of centres: multicentre (number unclear). Funding: National Cancer Institute, National Institute for Health (grants 21661, 32115, 12258, 13457, 20235, 21439, 29565, 12262). Trial ID: RTOG 83‐13. |
|
| Participants | Inclusion criteria: squamous cell carcinoma of the upper aerodigestive tract, Stages III & IV considered inoperable, with no prior resection or radiotherapy. Adequate bone marrow and renal function. T1‐T4 included. Exclusion criteria: history of previous malignant tumour. Prior chemotherapy within 6 weeks of randomisation. Recruitment period: April 1983 to February 1986 (Scheme A). OC: 47/237 (20%). OP: 104/237 (44%). OC+OP: 151/237 (64%). Number randomised: 260. Number analysed: 237. |
|
| Interventions |
Different doses of hyperfractionated radiotherapy Gr A (n = 63): 67.2 Gy. Gr B (n = 58): 72.0 Gy. Gr C (n = 116): 76.8 Gy. All fractions were 1.2 Gy given twice daily 5 days per week. Interval between fractions was permitted to be 4‐8 hours. Radiotherapy was administered with photons of 1.25 MV or greater with minimum source axis distance of 80 cm. |
|
| Outcomes | Primary: locoregional control. Secondary: overall survival, toxicity, late effects. Duration of follow‐up: minimum 2 years. |
|
| Notes | Trial also randomised patients to 81.6 Gy or 72.0 Gy between February 1986 to November 1987 (Scheme B). However, the trial focuses on data from Scheme A. HR data taken from Kaplan‐Meier graphs (no numbers at risk). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Randomized to 1 of 3 total doses". No details of method of sequence generation given. |
| Allocation concealment? | Unclear risk | Insufficient information. |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Unclear risk | 9% of patients randomised are excluded from analysis, but reasons and group allocation not described. |
| Free of selective reporting? | Unclear risk | Multiple publications addressing different outcomes and exposures. |
| Free of other bias? | Unclear risk | Possible contamination due to some patients having had prior chemotherapy. |
Dobrowsky 2000.
| Methods | Location of trial: Europe. Number of centres: 21. Funding: Medizinischwissenschaftlicher Fonds des Burgermeisters der Bundeshauptstadt Wien. Trial ID: not stated. |
|
| Participants | Inclusion criteria: squamous cell cancers originating in head and neck. Most were advanced tumours with lymph node involvement and were considered inoperable by the referring specialist. T1‐T4 included. Exclusion criteria: distant metastases. Recruitment period: October 1990 to December 1997. OC: 72/239 (30%). OP: 98/239 (41%). OC/OP: 170/239 (71%). Number randomised: 243. Number analysed: 239. |
|
| Interventions |
Hyperfractionated/accelerated radiotherapy versus conventional radiotherapy Hyperfractionated/accelerated (vCHART) (n = 78): 2.5 Gy on day 1 as single fraction, then 16 consecutive days of 1.65 Gy twice daily with interfraction interval of ≥6 hours to a total dose of 55.3 Gy. On weekdays therapy was performed with photons/electrons from a linear accelerator, and on weekends and holidays a Cobalt‐60 unit was used. Maximum dose to spinal cord was 38.8 Gy. Conventional (n = 81): 70 Gy delivered in 35 fractions over 7 weeks, 5 fractions of 2 Gy/week on weekdays using a linear accelerator to deliver photons & electrons. Maximum dose to spinal cord was 46 Gy. |
|
| Outcomes | Primary: overall survival. Secondary: locoregional response, recurrence, distant metastases, secondaries, toxicity. Duration of follow‐up: median follow‐up 48 months. |
|
| Notes | Sample size calculation reported. It was calculated that a sample size of 324 patients would be required to detect a "difference in survival of 15% (from 25% to 40%) after 3 years between 2 of the treatment groups with a probability of 85% at a significance level of 0.05." The trial had a third treatment arm not used in this review as intervention under assessment was chemotherapy not radiotherapy (V‐CHART+MMC). HR data taken from Kaplan‐Meier graphs. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Patients were randomised by the Documentation Office of the 1st Surgical University Clinic". Randomisation was stratified by site, age, performance status & gender. |
| Allocation concealment? | Low risk | Allocation was made by telephone call to the randomisation centre. |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Low risk | All randomised patients included in the analysis. |
| Free of selective reporting? | Low risk | Primary outcome of overall survival and secondary outcomes of toxicity, locoregional response and recurrence. |
| Free of other bias? | Low risk | Groups appear similar at baseline. No other apparent bias. |
Fu 1995.
| Methods | Location of trial: US. Number of centres: 5. Funding: National Cancer Institute, National Institute of Health. Trial ID: RTOG 88‐09. |
|
| Participants | Inclusion criteria: Stage III or IV of oral cavity, oropharynx, supraglottic larynx or nasopharynx, or Stage II, III, IV cancer of base of tongue or hypopharynx, age ≥ 18 years with Karnofsky performance status ≥ 60. No prior radiation therapy, chemotherapy or surgery. Exclusion criteria: prior or simultaneous malignancy, unless patient has been cancer free for > 5 years, metastases below clavicle. Recruitment period: February 1989 to January 1990. OC: 8/75 (11%). OP: 38/75 (51%). OC+OP: 46/75 (61%). Number randomised: 75. Number analysed: 70. |
|
| Interventions |
Hyperfractionated/accelerated split course radiotherapy versus accelerated radiotherapy with concomitant boost Hyperfractionated/accelerated/split course (AHFX‐S) (n = 38): radiotherapy to primary tumour and upper neck 1.6 Gy per fraction, twice daily with minimum 6‐hour interfraction interval, 5 times per week to a total dose of 38.4 Gy in 2.5 weeks. Rest from radiotherapy for 14 days then 1.6 Gy per fraction, twice daily to a reduced boost volume including primary tumour and positive nodes to further 28.8 Gy. Total dose 67.2 Gy. Accelerated/ boost accelerated (AFX‐C) Gr B (n = 32): 1.8 Gy per fraction, once daily, 5 times per week to total dose of 54 Gy in 30 fractions over 6 weeks. During the last 11 days of basic treatment a second daily dose of 1.5 Gy was given to a reduced boost volume. Total dose 70.5 Gy. |
|
| Outcomes | Primary: locoregional control. Secondary: overall survival, disease free survival, recurrence, toxicity. Duration of follow‐up: median follow‐up 2 years (0.03 to 4.87 years). |
|
| Notes | Dichotomous data only; unable to calculate HR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Stratified by Karnofsky performance status (60‐80 versus 90‐100) and randomised". No details of sequence generation given but assumed to be adequate as for other RTOG trials. |
| Allocation concealment? | Low risk | Patients entered into study by a telephone call to RTOG headquarters. |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Unclear risk | 5 patients excluded post‐randomisation (1 ineligible due to metastasis, 1 had prior CT, 3 refused treatment) but not ascribed to treatment groups. Unclear from paper how many patients are included in percentage figures given for locoregional control, disease free survival and overall survival. |
| Free of selective reporting? | Low risk | Trial planned to report locoregional control (success/failure) and tolerability and not powered for survival outcomes. |
| Free of other bias? | High risk | Some imbalance between the groups at baseline. The split course arm had higher percentage of OP primaries (63% versus 44%) and Stage IV disease (82% versus 50%), and a lower proportion of oral cavity lesions (3% versus 22%) and N0 disease 16% versus 31%. This imbalance has the potential to result in bias in the outcomes locoregional control, disease free survival and overall survival. |
Fu 2000.
| Methods | Location of trial: US, Canada. Number of centres: > 40. Funding: National Cancer Institute (grants CA21661, CA 37422, CA 32115, CA 06294). Trial ID: RTOG 9003. |
|
| Participants | Inclusion criteria: patients aged at least 18 years, with Karnofsky performance status ≥ 60%, no prior treatment, with Stage II‐IV disease M0 squamous cell carcinoma of oral cavity, oropharynx or supraglottic larynx or Stage II‐IV cancer of base of tongue or hypopharynx. T1‐T4 included. Exclusion criteria: prior or synchronous malignancy. Recruitment period: September 1991 to August 1997. OC: 110/1073 (10%). OP: 649/1073 (60%). OC+OP: 759/1073 (71%). Number randomised: 1113. Number analysed: 1073. |
|
| Interventions |
Hyperfractionated radiotherapy versus hyperfractionated/accelerated/split course radiotherapy versus accelerated/boost radiotherapy versus conventional radiotherapy Hyperfractionated (n = 263): 1.2 Gy per fraction, 2 fractions per day, interfraction interval 6 hours, 5 times per week to total dose of 81.6 Gy in 35 fractions over 7 weeks. Hyperfractionated/accelerated/split (n = 274): 1.6 Gy per fraction, 2 fractions per day, interfraction interval 6 hours, 5 times per week to 38.4 Gy then 2 weeks rest then resume as for Phase 1 with further 28.8 Gy for total of 67.2 Gy in 42 fractions over 6 weeks. Accelerated/boost (n = 268): 1.8 Gy per fraction, daily, interfraction interval 6 hours, 5 times per week together with a 1.5 Gy boost field for last 12 treatment days to a total of 72 Gy in 42 fractions over 6 weeks. Conventional (n = 268): 2 Gy per fraction, 5 fractions per week to a total of 70 Gy in 35 fractions over 7 weeks. |
|
| Outcomes | Primary: locoregional control at 2 years. Secondary: overall survival, disease free survival, acute and late toxicity. Duration of follow‐up: median follow‐up was 23 months for all analysable patients and 41.2 for surviving patients. |
|
| Notes | Sample size calculation. Study was designed to detect an increase in locoregional control from 40% to 55% with a type 1 error of 0.05 and power of 80%. Sample size was increased by 20% to allow for patients being found to be ineligible, lost to follow‐up or dying without locoregional failure within 2 years. Sample planned 1080 participants. Data from Kaplan‐Meier graphs (numbers at risk presented). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Stratified by Karnofsky performance status (90‐100 versus 60‐80), N stage (N+ versus N‐) and primary site. Randomisation was according to the scheme of Zelen, used to achieve balance in treatment assignment among the institutions. |
| Allocation concealment? | Low risk | Patients were enrolled by means of a telephone call to RTOG headquarters. |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Unclear risk | 1113 patients randomised, 28 were found to be ineligible, 5 refused protocol treatment or died before treatment started, 7 had inadequate data, total 40/1113 = 4% excluded. Exclusions not described by treatment group; appears likely that more excluded from hyperfractionated group (A) and fewer from split accelerated group (B). |
| Free of selective reporting? | Low risk | Important outcomes reported ‐ locoregional control, overall survival and disease free survival. |
| Free of other bias? | Unclear risk | Appears to be some difference between groups at baseline. |
Ghoshal 2008.
| Methods | Location of trial: India. Number of centres: 1. Funding: not stated. Trial ID: not stated. |
|
| Participants | Inclusion criteria: previously untreated patients with locally advanced squamous cell carcinoma of oropharynx, hypopharynx & larynx, Stage III & IV, M0, aged > 25 years, Karnofsky performance status ≥ 70, adequate haematological function, and no comorbidities. Exclusion criteria: large lymph nodes, extending beyond spinal cord where radiation therapy to spare cord area would be difficult. Recruitment period: June 1998 to June 2004. OC: 0/285 (0%). OP: 186/285 (65%). Number randomised: 290. Number analysed: 285. |
|
| Interventions |
Accelerated fractionation with concomitant boost versus conventional fractionation radiotherapy Accelerated/boost (n = 145): 1.8 Gy per fraction 5 times per week for 5 weeks to a total of 45 Gy with additional 1.5 Gy fraction given daily after 6‐hour interfraction interval for last 3 weeks for additional 22.5 Gy. Total dose 67.5 Gy. Conventional (n = 145): 2 Gy per fraction, 1 fraction per day, 5 times per week to a total dose of 66 Gy over 6.5 weeks. |
|
| Outcomes | Primary: disease free survival. Secondary: locoregional control, compliance with treatment protocol. Duration of follow‐up: median duration of follow‐up 2 years. |
|
| Notes | "Exploratory subgroup analyses were carried out on various prognostic variables." HR presented in text. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Permuted block randomisation using a computer generated in house system. Randomisation was not stratified. |
| Allocation concealment? | Unclear risk | Insufficient information. |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Low risk | 2 in each group did not receive treatment, 2 & 3 were lost to follow‐up and 2 & 3 discontinued treatment due to grade 3 mucositis. Remainder were included in outcome evaluation. |
| Free of selective reporting? | Low risk | Disease free survival and locoregional control were planned and reported. |
| Free of other bias? | Low risk | Groups appear comparable at baseline. No other apparent bias. |
Griffin 1984.
| Methods | Location of trial: USA Number of centres: 2 Funding: not stated. Trial ID: RTOG 7610a. |
|
| Participants | Inclusion criteria: previously untreated histologically proven inoperable squamous cell carcinoma, T2‐4, any N originating in oral cavity, oropharynx, supraglottic larynx or hypopharynx. Exclusion criteria: distant metastases, prior treatment for head & neck cancer. Recruitment period: February 1977 to April 1982. OC: 10/40 (25%). OP: 13/40 (33%). OC+OP: 23/40 (58%). Number randomised: 40. Number analysed: 35. |
|
| Interventions |
Neutron versus photon Neutron (n = 26): neutron dose equivalent to 66‐74 Gy megavoltage photon irradiation based on radiobiological effectiveness. Each fraction equivalent to 2.5 Gy photon irradiation 4 times per week. Uninvolved neck and supraclavicular region received equivalent of 46‐50 Gy photon irradiation in neutrons. Duration of treatment was 7‐8 weeks. Photon (n = 14): 66‐74 Gy megavoltage photon irradiation in fractions of 1.8 to 2 Gy per day, 5 fractions per week. Uninvolved neck and supraclavicular regions received 46‐50 Gy and duration of treatment was 7‐8 weeks. Patients from both groups who had either residual or recurrent disease after RT were directed to surgery if this was considered feasible. |
|
| Outcomes | Primary: unclear. Locoregional control, overall survival, disease free survival. Duration of follow‐up: minimum of 4 years. |
|
| Notes | HR data taken from Kaplan‐Meier graphs (no numbers at risk). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation stratified by region and stage of primary tumour and institution where treatment given. Randomised groups were "intentionally unbalanced" to give a greater proportion of patients in the mixed beam group. Method of sequence generation not described but assumed adequate as for other RTOG trials. |
| Allocation concealment? | Unclear risk | Insufficient information. |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Unclear risk | 5/40 excluded (3 from neutron group; 2 from photon group) due to ineligibility or cancelled. Unclear how many patients included in outcome evaluation. |
| Free of selective reporting? | Low risk | Important outcomes of overall survival, locoregional control and disease free survival planned and reported. |
| Free of other bias? | Unclear risk | Differences at baseline for age, gender and primary tumour. |
Griffin 1989.
| Methods | Location of trial: USA. Number of centres: 5. Funding: not stated. Trial ID: RTOG 7610b. |
|
| Participants | Inclusion criteria: previously untreated histologically proven inoperable squamous cell carcinoma, T2‐4, any N originating in oral cavity, oropharynx, supraglottic larynx or hypopharynx. Exclusion criteria: distant metastases, Karnofsky performance status < 60, prior treatment for head & neck cancer. Recruitment period: February 1977 to April 1982. OC: 80/297 (27%). OP: 154/297 (52%). OC+OP: 234/297 (79%). Number randomised: 327. Number analysed: 297. |
|
| Interventions |
Neutron/photon mixed beam versus photon Mixed beam (n = 163): combination of 40‐44 Gy megavoltage photons and 7.5 to 10 Gy neutrons, delivered as 3 fractions photons & 2 fractions neutrons per week. Each fraction 1.8 to 2 Gy or the equivalent based on the relative biological effectiveness of the neutron source. Uninvolved neck and supraclavicular region received 46‐50 Gy over 7‐8 weeks. Photon (n = 134): 66‐74 Gy megavoltage photon irradiation in fractions of 1.8 to 2 Gy per day, 5 fractions per week. Uninvolved neck and supraclavicular regions received 46‐50 Gy and duration of treatment was 7‐8 weeks. Patients from both groups who had either residual or recurrent disease after RT were directed to surgery if this was considered feasible. |
|
| Outcomes | Primary: tumour clearance rate. Secondary: locoregional control, overall survival. Duration of follow‐up: minimum of 6 years. |
|
| Notes | HR data taken from Kaplan‐Meier graphs (no numbers at risk). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation stratified by region and stage of primary tumour and institution where treatment given. Randomised groups were "intentionally unbalanced" to give a greater proportion of patients in the mixed beam group. Method of sequence generation not described but assumed adequate as for other RTOG trials. |
| Allocation concealment? | Low risk | Eligible patients were randomised by means of a phone call to the central office. |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Low risk | After randomisation 16 patients were found to be ineligible, 6 cancelled and 8 had inadequate data. Total of 15 patients per group excluded for these reasons; unlikely to have resulted in bias. |
| Free of selective reporting? | Unclear risk | Main outcomes are reported but it is not clear if the subgroup analyses were preplanned. |
| Free of other bias? | Low risk | Groups appear similar at baseline. No other apparent bias. |
Horiot 1992.
| Methods | Location of trial: Europe. Number of centres: 28. Funding: not stated. Trial ID: EORTC 22791. |
|
| Participants | Inclusion criteria: patients aged ≤ 75 years, with Karnofsky performance status ≥ 60 who have oropharyngeal cancer, T2 or T3, either N0 or N1 (providing there is a single node involved and it is less than 3 cm). Exclusion criteria: lesions in the base of the tongue, whether N0 or N1 (< 3 cm). Recruitment period: February 1980 to April 1987. OP: 356/356 (100%). Number randomised: 356. Number analysed: 325. |
|
| Interventions |
Hyperfractionated radiotherapy versus conventional fractionation radiotherapy Hyperfractionated (n = 166): 80.5 Gy in 70 fractions over 7 weeks. 2 fractions of 1.15 Gy daily with interfraction interval of 4‐6 hours. Conventional (n = 159): daily fraction of 1.75 to 2 Gy per fraction, to a total of 70 Gy in 35‐40. Fractions over 7‐8 weeks (the longer treatment time was used when large amounts of mucosa within target volume). |
|
| Outcomes | Primary: locoregional control. Secondary: overall survival, tolerance, late toxicity. Duration of follow‐up: mean follow‐up is 200 weeks, maximum follow up is 11 years. |
|
| Notes | HR data taken from Kaplan‐Meier graphs (numbers at risk presented). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Randomised". No further information regarding generation of random sequence presented. |
| Allocation concealment? | Unclear risk | Insufficient information. |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Unclear risk | 29 patients (8%) found to be ineligible. Reasons given in Table 3 but not per randomised group. Seems likely that more were excluded from conventional radiotherapy group and that this may have introduced a bias. |
| Free of selective reporting? | Low risk | Important outcomes of locoregional control, tolerance, survival and adverse effects reported. |
| Free of other bias? | Unclear risk | Little information presented on groups at baseline. |
Horiot 1997.
| Methods | Location of trial: 11 countries in Europe. Number of centres: 26. Funding: 4th Medical and Health Research Programme, concerted action 1989‐92, Quality Assurance in Cancer Clinical Research theme. Trial ID: EORTC 22851. |
|
| Participants | Inclusion criteria: squamous cell carcinoma of head & neck, T2‐T4. Patients aged ≤75 years, with WHO performance status 0‐2. Exclusion criteria: cancer of the hypopharynx. Recruitment period: December 1985 to April 1995. OC: 16%. OP: 64%. OC+OP: 80%. Number randomised: 512. Number analysed: 512. |
|
| Interventions |
Hyperfractionated/accelerated/split course radiotherapy versus conventional radiotherapy Hyperfractionated/accelerated/split (n = 257): Phase 1: 3 fractions of 1.6 Gy daily with minimum 4‐hour interfraction interval, for total of 28.8 Gy in 18 fractions over 8 days. 12‐14 day rest period. Phase 2: 43.2 Gy in 27 fractions of 1.6 Gy per fraction over 17 days starting on day 21. Total dose of 72 Gy in 45 fractions over 5 weeks. Conventional (n = 255): 2 Gy per fraction, 1 fraction per day, 5 days per week to total of 70 Gy in 35 fractions over 7 weeks. In both groups the target volume was reduced once or twice after 50 Gy and spinal cord dose remained less than 50 Gy. The boost techniques used in each institution varied according to institution policy. |
|
| Outcomes | Primary: locoregional control. Secondary: overall survival, disease specific survival, toxicity. Duration of follow‐up: median duration of follow‐up 4 years and 9 months. |
|
| Notes | Sample size calculation reported: "Assuming a 2 year locoregional control rate of 35% in the CF arm, it was estimated that 340 patients followed for 2 years .... would be enough to detect a 15% difference in the 2 year LRC rates with an accuracy of 80% and a type 1 error probability of 0.05." However, "an excess of 172 patients was entered justified by the need to increase the statistical power of a 10 year trial that would obviously be difficult to reproduce." HR presented in text. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Randomisation performed centrally at the EORTC data centre in Bristol" Randomisation used the minimisation techniques and was stratified by institution, site of primary tumour and stage (T2 versus T3‐4). |
| Allocation concealment? | Low risk | Allocation to treatment group obtained by a telephone call to randomisation centre. |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Low risk | 10/257 and 2/255 excluded from Gr A and Gr B respectively, due to ineligibility. These patients were included in the analysis. |
| Free of selective reporting? | Low risk | Important outcomes of locoregional control, survival, time to progression and toxicity were reported. |
| Free of other bias? | Low risk | Groups appear comparable at baseline. No other apparent bias. |
Hukku 1991.
| Methods | Location of trial: India. Number of centres: 1. Funding: not stated. Trial ID: not stated. |
|
| Participants | Inclusion criteria: histologically proven squamous cell carcinoma, T3‐4, N0‐3, with primary tumours in oral cavity, oropharynx, larynx and nasopharynx. Exclusion criteria: chronic medical problems and distant metastases. Recruitment period: January 1980 to August 1983. OC: 7/110 (6%). OP: 72/110 (65%). OC+OP: 79/110 (70%). Number randomised: 110. Number analysed: 110. |
|
| Interventions |
Split course radiotherapy versus accelerated radiotherapy Split course (n = 50): Phase 1: 15 fractions of 2.3 Gy over 3 weeks to primary tumour and bilateral neck. 2‐week break. Phase 2: 2.5 Gy per fraction for 10 fractions over 2 weeks to primary tumour and residual lymphatic disease if present or upper neck if lymph nodes not palpable. Conventional (n = 60): 4 Gy per fraction in 2 opposing fields, 5 fractions per week, total of 11 fractions and 44 Gy. Treatment delivered to primary tumour along with bilateral neck with reduction of neck field after 7 fractions. |
|
| Outcomes | Primary: locoregional control. Secondary: overall survival, disease free survival, toxicity. Duration of follow‐up: 2 years. |
|
| Notes | Dichotomous data only; unable to calculate HR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Randomisation of patients...". No details of method of sequence generation provided. |
| Allocation concealment? | Unclear risk | Insufficient information. |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Low risk | All randomised patients included in the outcome analyses. |
| Free of selective reporting? | Low risk | Important outcomes of overall survival, disease free survival, locoregional control and adverse events reported. |
| Free of other bias? | Low risk | Groups appear similar at baseline. No other apparent bias. |
Inoue 2001.
| Methods | Location of trial: Japan. Number of centres: 1. Funding: not stated. Trial ID: not stated. |
|
| Participants | Inclusion criteria: patients with early mobile tongue cancer, T1‐2, N0 which could be treated with a single plane implantation, localisation of tumour at lateral border of the tongue, and tumour thickness less that 10 mm, performance status 0‐3. Exclusion criteria: any severe concurrent disease. Recruitment period: April 1992 to October 1996. OC: 59/59 (100%). Number randomised: 59. Number analysed: 51. |
|
| Interventions |
Low dose rate interstitial radiotherapy versus high dose rate interstitial radiotherapy Low dose (n = 26): 0.30 to 0.93 Gy/h to total dose of 65‐75 Gy (median 70 Gy) over 75 to 217 hours (median 117 hours). High dose (n = 30): 0.99 to 4.1 Gy/min to total dose of 60 Gy in 10 fractions over 6‐9 days (median 7 days) with 2 fractions per day and interfraction interval of > 6 hours. |
|
| Outcomes | Primary: locoregional control. Secondary: cause specific survival. Duration of follow‐up: minimum of 46 months (median duration of follow‐up is 85 and 78 months in the low dose and high dose groups respectively). |
|
| Notes | HR data taken from Kaplan‐Meier graphs (no numbers at risk). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Randomly allocated into LDR & HDR groups according to Peto's balanced randomisation list." |
| Allocation concealment? | Unclear risk | Not mentioned. |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Unclear risk | 8 patients were excluded; 3 (10%) from low dose rate and 5 (20%) from high dose rate but reasons are not given for each treatment group. This is a possible cause of bias. |
| Free of selective reporting? | Unclear risk | Some important outcomes, locoregional control, survival and cause specific survival are reported but there are no data on toxicity. |
| Free of other bias? | Unclear risk | At baseline there is some difference between groups with regard to tumour thickness. Low dose rate group has more medium thickness tumours and high dose rate more very thick tumours. Tumour thickness is likely to be an important prognostic factor linked to outcome. |
Ketcham 1969.
| Methods | Location of trial: US. Number of centres: 1. Funding: not stated. Trial ID: not stated. |
|
| Participants | Inclusion criteria: squamous cell carcinoma of upper aerodigestive tract. Exclusion criteria: previous RT, recurrent disease, more than 1 primary lesion, cancer of the lip. If the tumour was "inadvertently cut across during surgery" or if surgical margins were positive, patient was excluded. Recruitment period: not stated. OC: 44/79 (56%). OP: unclear. OC+OP: > 56%. Number randomised: unclear. Number analysed: 79. |
|
| Interventions |
Pre‐operative radiotherapy versus pre‐operative sham radiotherapy Pre‐operative radiotherapy (n = 60): using a 2 MeV van der Graaf generator with output of 1 Gy per minute at 1 metre, a single dose of 10 Gy was administered over 15 minutes, 24 hours prior to surgery. Pre‐operative sham radiotherapy (n = 19). |
|
| Outcomes | Primary: surgical complications. Secondary: locoregional control, metastases. Duration of follow‐up: 36‐86 months. |
|
| Notes | Dichotomous data only (unclear timing of outcome evaluation reported in paper); unable to calculate HR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Sealed envelope selected randomly from 3 groups based on type of surgery patient required." Not clear how the envelopes were prepared. |
| Allocation concealment? | Low risk | Sealed envelope selected and taken by the patient to the radiotherapist, who opened it and delivered either RT or sham depending on envelope contents. |
| Blinding ‐ Outcome Assessors | Low risk | Double blinded ‐ both the patient and the surgeon were blinded to the treatment. Surgeons were asked to record whether they thought each patient has had RT. |
| Incomplete outcome data addressed? | Unclear risk | Unclear from the paper how many patients in total were randomised, how many were excluded post‐randomisation and how many were included in the outcome analyses. |
| Free of selective reporting? | Unclear risk | Outcomes reported are surgical complications, recurrent disease and metastases but not survival, or numbers of patients who had positive margins or tumours inadvertently cut. |
| Free of other bias? | High risk | Randomised treatment was pre‐operative, yet 2 of the exclusion criteria related to the surgery. It is possible that the large imbalance in number in the treatment and placebo groups is related to surgical exclusions. |
Lawrence 1974.
| Methods | Location of trial: US. Number of centres: 1. Funding: not stated. Trial ID: not stated. |
|
| Participants | Inclusion criteria: previously untreated Stage II‐IV squamous cell carcinoma of oral cavity, oropharynx, or pharynx with technically resectable disease. Exclusion criteria: Stage I cancer (usually treated by radiation therapy alone), patients with other cancers, including lip, paranasal sinus, nasopharynx, or glottic carcinoma of larynx. Recruitment period: January 1969 to December 1972. OC: 64/143 (45%). OP: 37/143 (26%). OC+OP: 101/143 (71%). Number randomised: 143. Number analysed: 143. |
|
| Interventions |
Pre‐operative radiotherapy plus surgery versus surgery alone Pre‐operative radiotherapy (n = 69): 2 fractions each of 1.4 Gy given 48 and 24 hours prior to surgery. RT delivered by Co‐60 unit using 80 cm source skin distance followed by radical resection of primary carcinoma and simultaneous radical neck dissection. Surgery alone (n = 74): radical resection of primary carcinoma & simultaneous unilateral or bilateral radical neck dissection. |
|
| Outcomes | Primary: surgical complications. Secondary: overall survival, local recurrence. Duration of follow‐up: 4 years. |
|
| Notes | Unable to use data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Patients...randomly assigned". Randomisation stratified by site of primary tumour and stage of disease, but method of sequence generation not described. |
| Allocation concealment? | Unclear risk | Insufficient information. |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Low risk | All randomised patients are included in the analyses. |
| Free of selective reporting? | Low risk | Important outcomes of surgical complications, mortality and local recurrence reported. |
| Free of other bias? | Low risk | Groups appear comparable at baseline. No other apparent bias. |
MacDougall 1990.
| Methods | Location of trial: Edinburgh, Scotland. Number of centres: 1. Funding: Medical Research Council, Cancer Research Campaign, Scottish Home and Health Department, Lothian Health Board. Trial ID: not stated. |
|
| Participants | Inclusion criteria: previously untreated patients with histologically confirmed squamous cell carcinoma of oral cavity, oropharynx, larynx or hypopharynx, less than 80 years old, deemed fit for radiotherapy. Exclusion criteria: primary tumours with high probability of local control with photon treatment. Recruitment period: 1977 to 1984. OC: 66/165 (40%). OP: 35/165 (21%). OC+OP: 101/165 (61%). Number randomised: 165. Number analysed: 165. |
|
| Interventions |
Fast neutron radiotherapy versus photon radiotherapy Fast neutron (n = 85): 20 daily fractions over 4 weeks. Total absorbed dose of 15.6 to 16.7 Gy. Photon (n = 80): 20 daily fractions over 4 weeks. Total absorbed dose 54‐56 Gy. |
|
| Outcomes | Primary: locoregional control. Secondary: 5 and 10 year survival, disease free survival at 5 years, cause specific survival, late radiation necrosis. Duration of follow‐up: minimum of 5 years, up to 11 years. |
|
| Notes | Sample size calculation: based on predicted increase of locoregional control from 40% to 70% it was estimated that 164 patients would be required to show this difference with power of 90% and α = 0.05 on a tow tailed test of significance. Part of multicentre trial but full data from 2 centres not available. Dichotomous data only for outcomes of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation stratified on site of primary tumour and presence/absence of malignant lymph nodes. Envelopes containing treatment allocation prepared by trial statistician in Edinburgh, and held by Neutron Clinic secretary. |
| Allocation concealment? | Low risk | Sequentially numbered sealed envelopes were drawn. |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Low risk | All randomised patients included in analysis. |
| Free of selective reporting? | Unclear risk | No planned outcomes listed in methods section. Important outcomes reported. |
| Free of other bias? | Unclear risk | Gender imbalance between the groups, unclear if this would introduce a bias. |
Maor 1986.
| Methods | Location of trial: US. Number of centres: 4. Funding: National Cancer Institute (core grant CA23113). Trial ID: RTOG 7808. |
|
| Participants | Inclusion criteria: untreated squamous cell carcinoma, T2‐T4, with any N but M0. Patients referred to trial following unsatisfactory response to initial radiotherapy. Exclusion criteria: more than 1 primary tumour or Karnofsky status < 50. Recruitment period: October 1978 to August 1982. OC: 30/115 (26%). OP: 59/115 (51%). OC+OP: 89/115 (77%). Number randomised: 118. Number analysed: 115. |
|
| Interventions |
Neutron boost versus photon boost Neutron boost (n = 57): to include only areas involved by gross tumour, primary, or nodes plus a margin of 2 cm. Boost given in 4‐6 fractions in 2‐3 weeks. Neutron dose depended on radiobiological effectiveness, equivalent to 25‐30 Gy photons. Photon boost (n = 58): to include areas with gross tumour plus a 1 cm margin (2 cm with cobalt). 25‐30 Gy over 2‐3 weeks in 5 daily fractions per week. All patients received 45‐50 Gy photons in daily fractions of 1.8‐2 Gy. |
|
| Outcomes | Primary outcome unclear. Tumour clearance, locoregional control, overall survival. |
|
| Notes | Data taken from Kaplan‐Meier graphs (no numbers at risk). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation stratified by institution, T stage and region and performed by central office. No details given but assumed adequate as for other RTOG trials. |
| Allocation concealment? | Unclear risk | Insufficient information. |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Low risk | 3/118 excluded from the analysis. Unlikely to affect results. |
| Free of selective reporting? | Low risk | Important outcomes of overall survival, local clearance, locoregional control planned and reported. |
| Free of other bias? | High risk | 1 patient in neutron boost group and 5 in photon boost group received interstitial implants to deliver boost. |
Maor 1995.
| Methods | Location of trial: US and UK. Number of centres: 5 (4 US and 1 UK). Funding: National Cancer Institute, Department of Health and Human Services (grants CA06294, CA16672, CM57775) and Medical Research Council in UK. Trial ID: not stated. |
|
| Participants | Inclusion criteria: patients with Stage III‐IV tumours in the oral cavity, oropharynx, hypopharynx and larynx, T3‐4 with any N or T2 with N > 1 or T1N3, Karnofsky performance status > 60. Exclusion criteria: no distant metastases, no history of another cancer. Recruitment period: April 1986 to March 1991. OC: 39/169 (23%). OP: 87/169 (51%). OC+OP: 126/169 (75%). Number randomised: 178. Number analysed: 169. |
|
| Interventions |
Neutrons versus photons Neutrons (n = 83): 20.4 Gy neutrons delivered in 12 fractions of 1.7 Gy over 4 weeks (3 fractions per week). Photons (n = 86): 70 Gy of photons delivered in 35 fractions, 2 Gy per fraction over 7 weeks (except in UK centre, 66 Gy in 30 fractions over 6 weeks). |
|
| Outcomes | Primary: tumour clearance. Secondary: locoregional control/relapse, overall survival, late toxicity. Duration of follow‐up: median duration of follow‐up 3.5 years (range 3 months to 6.7 years). |
|
| Notes | HR data taken from Kaplan‐Meier graphs (numbers at risk presented). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Patients...randomized to receive". Details of sequence generation not described. Stratification by site and stage of primary tumour and treating institution. |
| Allocation concealment? | Unclear risk | Insufficient information. |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Low risk | 6/178 patients died before treatment started, 2 had other cancers and 1 was lost to follow‐up (total 9 excluded 5% ‐ reasons not described by treatment group). All remaining patients included in analyses. |
| Free of selective reporting? | Low risk | Important outcomes of locoregional control, survival and toxicity reported. |
| Free of other bias? | Unclear risk | Characteristics of the treatment groups at baseline differed with regard to number of patients with hypopharynx (more in photon group) and supraglottic larynx (more in neutron group). Paper states that this is likely to mean more patients in the neutron group have worse prognosis. |
Marcial 1987.
| Methods | Location of trial: US. Number of centres: unclear. Funding: National Cancer Institute/National Institute for Health (grants CA12258, CA32115, CA20235, CA21661). Trial ID: RTOG 79‐13. |
|
| Participants | Inclusion criteria: advanced squamous cell carcinoma of oral cavity, pharynx, larynx and paranasal sinus whose only planned therapy was radiation (with possible surgical salvage). Patients had Stage III or IV tumours, or Stage II tumours of base of tongue, nasopharynx or maxillary sinus. T1‐T4 included. Exclusion criteria: metastatic disease, 2 primary tumours, previous chemotherapy or radiotherapy or surgery, Karnofsky performance status < 60%. Recruitment period: August 1979 to June 1983. OC: 28/187 (15%). OP: 86/187 (46%). OC+OP: 114/187 (61%). Number randomised: 210. Number analysed: 187. |
|
| Interventions |
Hyperfractionated/accelerated radiotherapy versus conventional radiotherapy Hyperfractionated/accelerated (n = 94): 1.2 Gy per fraction, 10 fractions per week with interfraction interval of 3‐6 hours to total dose of 60 Gy over 5 weeks. Conventional (n = 93): 1.8 to 2 Gy per fraction, 5 fractions per week, to total dose of 66‐73.8 Gy over 7‐8 weeks. Co‐60 or higher energy used, dose specified to mid plane from parallel opposed fields covering primary tumour and extensions with 1.5 cm margin. After 50 Gy lateral port was reduced to cover primary tumour only. |
|
| Outcomes | Primary: tumour clearance. Secondary: locoregional control, overall survival, early and late toxicity. Duration of follow‐up: estimated to be 30 months. |
|
| Notes | HR data taken from Kaplan‐Meier graphs (no numbers at risk). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation was undertaken at RTOG headquarters in Philadelphia, and was stratified by site of primary tumour and stage of disease. No details on method of sequence generation are provided but it is assumed to be adequate. |
| Allocation concealment? | Low risk | Treatment allocation made by phone call to RTOG headquarters. |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Unclear risk | 23 patients (11%) excluded. 10 were ineligible, 2 cancelled before treatment started and 11 had insufficient data. It is not stated which treatment groups these patients are from, and this may result in bias. |
| Free of selective reporting? | Low risk | Important outcomes of locoregional control, survival and toxicity are reported. |
| Free of other bias? | High risk | Groups are not balanced at baseline in the distribution of Karnofsky performance scores, which are likely to be linked with prognosis. Randomisation was not stratified by treating institution. |
Marcial 1993.
| Methods | Location of trial: US. Number of centres: approximately 7. Funding: National Cancer Institute (grants CA12258 and CA32115). Trial ID: RTOG trial (number unclear). |
|
| Participants | Inclusion criteria: patients with all stages of untreated cancer of the tonsillar fossa. Exclusion criteria: patients aged > 80 years, with adenocarcinoma, other cancers (previous or present except for skin cancers), presence of distant metastases, medical conditions which made treatment completion unlikely, or patient deemed unlikely to complete follow‐up. Recruitment period: 1971 to 1976. OP: 147/147 (100%). Number randomised: 147. Number analysed: 137. |
|
| Interventions |
Accelerated split course radiotherapy versus continuous radiotherapy Split course (n = 63): Phase 1: 3 Gy per fraction, 10 fractions over 2 weeks total of 30 Gy. 3 weeks rest. Phase 2: 3 Gy per fraction and further 10 fractions over 2 weeks. Continuous (n = 74): 2.0‐2.2 Gy per fraction, with 30‐33 fractions over 6 weeks to a total dose of 66 Gy. Original protocol was modified to allow 2 Gy fractions to a total of 60‐66 Gy. Spinal cord protection was required after 50 Gy in Phase 2. Source was teletherapy energy at 1 MeV or higher with minimal source skin distance of 75 cm. Surgical salvage was permitted at least 2 months after completion of radiotherapy. |
|
| Outcomes | Primary: tumour clearance. Secondary: locoregional control, overall survival, acute and late toxicity. Duration of follow‐up: minimum of 7 years (4% of patients lost to follow‐up prior to 7 years). |
|
| Notes | Dichotomous data only; unable to calculate HR. Locoregional control data presented as percentage but unclear of denominator. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation done at RTOG headquarters in Philadelphia and was stratified by institution, T stage, N stage and gender. No details on method of sequence generation provided but it is assumed to be adequate. |
| Allocation concealment? | Low risk | Treatment allocation made by telephone call to RTOG headquarters. |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Unclear risk | 10 patients (7%) excluded. 17 reasons for exclusions (3 cancellation, 3 ineligible, 11 no data) for 10 people ‐ unclear how many and which treatment group they were from. |
| Free of selective reporting? | Low risk | Important outcomes of tumour response, locoregional control, overall survival and toxicity reported. |
| Free of other bias? | Low risk | No significant differences between the groups at baseline. No other apparent bias. |
Olmi 2003.
| Methods | Location of trial: Italy. Number of centres: 18. Funding: Consiglio Nazionale delle Richerche. Trial ID: ORO‐93 01. |
|
| Participants | Inclusion criteria: histologically proven squamous cell carcinoma of oropharynx, Stage III or IV, M0 no prior surgery, radiotherapy or chemotherapy, age < 70 years, Karnofsky performance status ≥70 or ECOG 0‐2, adequate bone marrow reserve, renal, hepatic cardiac and pulmonary function, available for follow‐up, informed consent. Exclusion criteria: T1N1 & T2N1, previous tumours, active infectious disease, psychosis. Recruitment period: January 1993 to June 1998. OP: 192/192 (100%). Number randomised: 192. Number analysed: 182. |
|
| Interventions |
Conventional radiotherapy plus concomitant chemotherapy versus hyperfractionated/accelerated/split course radiotherapy versus conventional radiotherapy Conventional radiotherapy plus concomitant chemotherapy (n = 64): 66‐70 Gy in 33‐35 fractions (2 Gy/fraction) 5 times per week over 6.5 to 7 weeks. 50 Gy to uninvolved neck nodes, tolerance dose for spinal cord 44 Gy. Carboplatin 75 mg/m2 IV over 30 minutes on days 1‐4 of RT, and 5FU 1000 mg/m2/day IV continuous over 96 hours on days 1‐4, repeated on weeks 5 and 9 of RT. Hyperfractionated/accelerated/split (n = 65): 64‐67.2 Gy ‐ 2 fractions each of 1.6 Gy daily with 4‐6 hour interfraction interval, 5 times per week. After 38.4 Gy over 2 weeks 2‐week split planned, followed by a repeat of phase 1. Conventional (n = 63): 66‐70 Gy in 33‐35 fractions (2 Gy/fraction) 5 times per week over 6.5 to 7 weeks. 50 Gy to uninvolved neck nodes, tolerance dose for spinal cord 44 Gy. |
|
| Outcomes | Primary: survival at 5 years. Secondary: overall survival, relapse free survival, locoregional control, acute and late toxicity. Duration of follow‐up: median follow‐up 8.35 years (4.8 to 10.2 years). |
|
| Notes | Trial closed prior to planned accrual of 260 due to slowed accrual rate. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomisation performed by the Instituto Mario Negri, Milan. Patients were stratified by centre and disease stage (Stage III & IV N0‐N1 versus Stage IV N2‐3). No details on sequence generation given. |
| Allocation concealment? | Unclear risk | Insufficient information. |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Low risk | 10 patients are excluded from analysis (across groups), 8 of these are due to death during treatment, reasons for other unclear. Unlikely to have introduced bias to results. |
| Free of selective reporting? | Low risk | Important outcomes of locoregional control, mortality, acute and late toxicity reported. |
| Free of other bias? | Low risk | Groups appear similar at baseline. No other apparent bias. |
Pinto 1991.
| Methods | Location of trial: Brazil. Number of centres: 1. Funding: not stated. Trial ID: not stated. |
|
| Participants | Inclusion criteria: patients with previously untreated, histopathologically confirmed squamous cell carcinoma, Stage III & IV oropharyngeal cancer, aged < 70, no previous malignancy (except basal cell carcinoma of skin), no trismus, no metastases and Karnofsky performance status ≥ 50%. Exclusion criteria: not explicitly stated. Recruitment period: April 1986 to May 1989. OP: 112/112 (100%). Number randomised: 112. Number analysed: 98. |
|
| Interventions |
Hyperfractionated radiotherapy versus conventional radiotherapy Hyperfractionated (n = 56): 64 fractions of 1.1 Gy given twice daily to a total dose of 70.4 Gy over 6.5 weeks with minimum interfraction interval of 6 hours. Conventional (n = 54): 33 fractions of 2 Gy per fractions given 5 times per week over 6.5 weeks to total dose of 66 Gy. The spinal cord was protected after 46.2 Gy and 46 Gy in the hyperfractionated and conventional groups respectively. Radiation delivered from Co‐60 machine at distance of 80 cm. |
|
| Outcomes | Primary: overall survival. Secondary: locoregional control, early and late toxicity. Duration of follow‐up: median follow‐up 22.5 months (7‐41 months). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "...randomly allocated .... after stratification by site of primary tumour, T stage (T1‐2 vs T3‐4) N stage (N0 vs N1 vs N2‐3) and lymph node size (> or < 6 cm)". No details on method of sequence generation provided. |
| Allocation concealment? | Unclear risk | Not mentioned. |
| Blinding ‐ Outcome Assessors | Unclear risk | Assessment of late radiation induced fibrosis conducted by clinician blinded to treatment allocation. |
| Incomplete outcome data addressed? | Low risk | 14 patients (12%) excluded from the analyses. In hyperfractionated group 2 died during treatment and 4 stopped treatment early and in conventional RT group 3 died and 5 stopped treatment early. This is unlikely to have resulted in bias. |
| Free of selective reporting? | Low risk | Important outcomes of overall survival, locoregional control and toxicity reported. |
| Free of other bias? | Low risk | Groups well balanced at baseline for main prognostic factors. |
Poulsen 2001.
| Methods | Location of trial: Australia and New Zealand. Number of centres: 14. Funding: Queensland Cancer Fund. Trial ID: not stated. |
|
| Participants | Inclusion criteria: invasive squamous cell carcinoma in oral cavity, oropharynx, hypopharynx or larynx, disease at Stage III or IV, ECOG performance status 0‐2, age ≤ 80 years, weight > 40 kg, loss of body weight < 15%. Exclusion criteria: prior radiotherapy, chemotherapy, or therapeutic surgery, other active malignancy, intercurrent illness likely to reduce life expectancy or exacerbate toxicity. Recruitment period: 1991 to 1998. OC: 37/343 (11%). OP: 229/343 (67%). OC+OP: 266/343 (78%). Number randomised: 350. Number analysed: 343. |
|
| Interventions |
Hyperfractionated/accelerated radiotherapy versus conventional radiotherapy Hyperfractionated/accelerated (n = 172): 1.8 Gy per fraction twice daily to a dose of 39.6 Gy in 22 fractions over 16 days with macroscopic disease receiving a dose of 59.4 Gy in 33 fractions over 24 days. Spinal cord dose was initially limited to 42 Gy but this was decreased to 40 Gy after a case of myelitis. Conventional (n = 171): large volume comprising primary site and all draining lymph nodes at risk were treated with 2 Gy per fraction 5 fractions per week to total of 50 Gy in 25 fractions over 35 days. Macroscopic disease with 1 cm margin was boosted to 70 Gy in 35 fractions over 49 days. Spinal cord dose was limited to 45 Gy. |
|
| Outcomes | Primary: disease free survival at 5 years. Secondary: disease specific survival, locoregional control, toxicity. Duration of follow‐up: median 53 months (14‐101 months). |
|
| Notes | Sample size: estimated that 342 patients were required to enable a difference of 15% in disease free survival from 40% to 55% to be detected with 80% power at 5% level of significance using a 2‐sided test. HR presented in text. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation done by Data Management Office of Queensland Radium Institute, stratified by primary tumour site and stage (4 groups). No details of the method of sequence generation provided but assumed to be adequate. |
| Allocation concealment? | Low risk | Allocation made by telephone call to randomisation centre. |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Low risk | 7 (2%) patients excluded from analysis: 3 refused treatment, 3 found to be ineligible, 1 died before treatment started. Not stated which groups these were from but numbers small and unlikely to have introduced a bias. |
| Free of selective reporting? | Low risk | Important outcomes of disease free survival, survival, locoregional control and toxicity were reported. |
| Free of other bias? | Low risk | Authors state that groups were comparable at baseline for the variables examined. |
Sanguineti 2005.
| Methods | Location of trial: Italy. Number of centres: 4. Funding: not stated. Trial ID: not stated. |
|
| Participants | Inclusion criteria: pathologically proven squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx or larynx, and major surgical resection of primary disease and clinically involved neck lymph nodes without macroscopic residual; ECOG performance status ≤ 2 before radiotherapy, no distant metastases. Exclusion criteria: patients with other concurrent or previous (within 5 years) cancers other than basal cell carcinoma of the skin and in situ squamous cell carcinoma of the cervix. Recruitment period: March 1994 to August 2000. OC: 44/226 (19%). OP: 40/226 (18%). OC+OP: 84/226 (37%) (see notes). Number randomised: 226. Number analysed: 226. |
|
| Interventions |
Accelerated radiotherapy with boost versus conventional radiotherapy Accelerated/boost (n = 113 (46 OC/OP patients)): 2 Gy/fraction, 5 fractions a week for 5 weeks. A concomitant boost of 1.4 Gy/fraction during first week and 1.6 Gy/fraction during fifth week of radiotherapy was given (total dose 64 Gy). Conventional (n = 113 (38 OC/OP patients)): 2 Gy/fraction, 5 fractions a week for 5 weeks (total 50 Gy) to areas at low risk of macroscopic disease and 6 weeks (total 60 Gy) to areas at high risk. All radiotherapy had to commence within 8 weeks following surgery. Surgery consisted of major surgical resection of both primary disease and clinically involved neck lymph nodes without macroscopic residual. |
|
| Outcomes | Primary: locoregional control. Secondary: overall survival, toxicity. Duration of follow‐up: 10 years. |
|
| Notes | Sample size: estimated that 224 patients would provide power of 80% (with an α error of 5%, 2‐sided) to detect improvement of 81% in the probability of locoregional control at 2 years in the accelerated radiotherapy group compared with 70% in the conventional group. HR calculated for OC/OP patients only using data supplied by authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Computer generated list and stratified according to center and balanced by variable blocks." |
| Allocation concealment? | Low risk | "A centralized telephone call procedure to the unit of clinical epidemiology and trials in Genoa." |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Low risk | All randomised participants included in analysis for locoregional control and overall survival. For acute toxicity 221/226 participants analysed and for late toxicity 214/226 participants analysed. |
| Free of selective reporting? | Low risk | Important outcomes of overall survival, locoregional control and toxicity were reported. |
| Free of other bias? | Unclear risk | Groups appear similar at baseline. No other apparent bias. |
Skladowski 2006.
| Methods | Location of trial: Poland. Number of centres: 1. Funding: Polish Scientific Research Committee (grant #4PO5 B15208). Trial ID: not stated. |
|
| Participants | Inclusion criteria: histologically proven squamous cell carcinoma with primary tumour in oropharynx, hypopharynx oral cavity or supraglottic larynx, T2‐4, N0‐1, aged ≤ 70 years, WHO performance status ≤ 2 and no other neoplastic disorders. Exclusion criteria: weight loss more than 10% in past 3 months, radiologically confirmed infiltration of mandible or thyroid cartridge or refusal. Recruitment period: December 1993 to June 1996. OC: 22/100 (22%). OP: 28/100 (28%). OC+OP: 50/100 (50%). Number randomised: 100. Number analysed: 100. |
|
| Interventions |
Accelerated radiotherapy versus conventional radiotherapy Accelerated (n = 51): 2 Gy per fraction, 7 daily fractions per week to total of 66 Gy ± 2 Gy for T2, & 70 Gy ± 2 Gy for T3‐4 with overall treatment time of 33‐36 days. Large fields covering the whole clinical target volume were used Monday to Friday and at weekends a smaller field, limited to primary tumour and involved nodes only, was irradiated. Patients were hospitalised for the duration of the treatment. Conventional (n = 49): 2 Gy per day, 5 times per week, to a total of 66 Gy ± 2 Gy for T2 and 70 Gy ± 2 Gy for T3‐4 with overall treatment time of 47‐50 days. Small fields were used as a shrinking fields technique during last week of treatment. From 1995 the fraction size was changed from 2 Gy to 1.8 Gy in both arms "due to the high rate of mucosal necrosis." |
|
| Outcomes | Primary: local tumour control. Secondary: overall survival, morbidity free survival, disease free survival, acute and late toxicity. Duration of follow‐up: median follow‐up 96 months (59‐123 months). |
|
| Notes | Sample size calculation: to detect an expected increase in local tumour control in the CAIR arm of 24% it was estimated that about 200 patients were required with α = 0.05 and 1‐β = 0.90 (2‐sided test). Investigators planned an interim analysis and possible change to protocol, to detect unacceptable treatment toxicity, or unexpectedly high benefit. HR data taken from graphs (no numbers at risk). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Simple randomisation stratified by site of primary tumour, TNM Stage with 1:1 arm allocation, was made at Bureau of Trials at the Instiute using random numbers." |
| Allocation concealment? | Low risk | Sealed envelope method used. |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Low risk | No drop outs post‐randomisation. 2 in accelerated group and 1 in conventional group did not complete treatment. |
| Free of selective reporting? | Low risk | Important outcomes of response, survival and toxicity reported. |
| Free of other bias? | Unclear risk | Fraction size reduced in both groups from 2 Gy to 1.8 Gy in 1995 in response to planned interim analysis. Accelerated group were hospitalised throughout treatment and received a higher rate of systemic corticosteroids and/or antibiotics for severe mucositis ‐ 90% compared to 48% in control group. |
Terz 1981.
| Methods | Location of trial: USA. Number of centres: 2. Funding: not stated. Trial ID: not stated. |
|
| Participants | Inclusion criteria: previously untreated squamous cell carcinoma Stages II‐IV of oral cavity, oropharynx or hypopharynx if disease appeared to be technically resectable. Exclusion criteria: Stage 1 disease, patients with primary tumours of lip, nasopharynx, paranasal sinuses or larynx. Recruitment period: January 1969 to September 1975. OC: 94/248 (38%). OP: 72/248 (29%). OC+OP: 166/248 (67%). Number randomised: 248. Number analysed: unclear. |
|
| Interventions |
Pre‐operative radiotherapy versus surgery alone Pre‐operative radiotherapy (n = 126): 1.4 Gy in 2 equal fractions 48 and 24 hours prior to surgery. Radiotherapy administered through a pair of opposed fields to cover both primary lesion and entire cervical lymph drainage area. Co‐60 source with source to skin = 80 cm. Followed by resection of primary tumour with uni or bilateral neck dissection. Surgery alone (n = 122): resection of primary tumour with uni or bilateral neck dissection. |
|
| Outcomes | Primary: complications, operative mortality. Secondary: recurrence, 5‐year disease free survival. Duration of follow‐up: 4‐9 years. |
|
| Notes | HR taken from Kaplan‐Meier graph (no numbers at risk). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No details on method of sequence generation used. Randomisation was stratified on site of primary tumour and stage of disease. |
| Allocation concealment? | Unclear risk | Not mentioned. |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Unclear risk | Unclear how many patients are included in the analysis as only percentages are reported. |
| Free of selective reporting? | Unclear risk | Many outcomes reported ‐ complications, operative mortality, locoregional recurrence, disease free survival. Subgroup analyses were also undertaken and it is not clear if these were preplanned. |
| Free of other bias? | Low risk | Groups appear similar at baseline. No other apparent bias. |
Weissberg 1983.
| Methods | Location of trial: US. Number of centres: 1. Funding: US Public Health Service (grant CA 06519). Trial ID: not stated. |
|
| Participants | Inclusion criteria: inoperable advanced biopsy proven squamous cell carcinoma of head and neck. Exclusion criteria: metastatic disease beyond neck nodes, Karnofsky performance status < 60%, other malignant neoplasms in past 5 years. Recruitment period: 1973 to 1979. OC: 12/64 (19%). OP: 29/64 (45%). OC+OP: 41/64 (64%). Number randomised: 64. Number analysed: 56. |
|
| Interventions |
Accelerated radiotherapy versus conventional radiotherapy Accelerated (n = 33): high fractional dose of 4 Gy per day to total dose of 40‐48 Gy in 2‐3 weeks. Bilateral neck regions irradiated to 28 Gy and spinal cord dose limited to 28 Gy. Conventional (n = 31): 2 Gy per day to total of 60‐70 Gy over 6‐7 weeks. Bilateral neck regions irradiated to 44 Gy and spinal cord dose limited to 44 Gy. Radiotherapy from 2 MeV van der Graff generator or 4 or 6 MeV linear accelerator. |
|
| Outcomes | Primary: disease free survival at 5 years. Secondary: disease free survival, regression, toxicity. Duration of follow‐up: 5‐7 years. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Randomized" but no details on method of sequence generation used. |
| Allocation concealment? | Unclear risk | Not mentioned. |
| Blinding ‐ Outcome Assessors | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Low risk | Gr A 1 patient withdrew and 2 died during treatment; Gr B 3 died during treatment, 1 stroke and 1 withdrew due to metastatic disease. Total 13% post‐randomisation exclusion but numbers and reasons similar in each group. |
| Free of selective reporting? | Low risk | Tumour regression, survival and toxicity reported. |
| Free of other bias? | Unclear risk | Imbalance between groups with regard to oral cancer primaries (10 versus 2). Unclear whether this indicates bias. |
CT = chemotherapy; ECOG = Eastern Cooperative Oncology Group; EORTC = European Organisation for Research and Treatment of Cancer; HR = hazard ratio; N = node; OC = oral cavity; OP = oropharynx; RT = radiotherapy; RTOG = Radiation Therapy Oncology Group; T = tumour; WHO = World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arimoto 2003 | Less than 50% of participants in trial have oral cavity or oropharyngeal cancer. |
| Awwad 1992 | Less than 50% of participants in trial have oral cavity or oropharyngeal cancer. |
| Awwad 2002 | Less than 50% of participants in trial have oral cavity or oropharyngeal cancer. |
| Baumann 2001 | 2 commentaries on Fu 2000; no additional data. |
| Catterall 1977 | Less than 50% of participants in trial have oral cavity or oropharyngeal cancer. |
| CHART 1997 | Less than 50% of participants in trial have oral cavity or oropharyngeal cancer. |
| Cummings 2007 | Less than 50% of participants in trial have oral cavity or oropharyngeal cancer. |
| DAHANCA 2003 | Less than 50% of participants in trial have oral cavity or oropharyngeal cancer. |
| Datta 1989 | Abstract only. No subsequent publication identified. Unclear percentage of oral cavity and oropharyngeal cancer patients included. |
| Dieckmann 1990 | Uncertain if truly randomised ‐ abstract only, no subsequent publication identified. |
| Dvivedi 1978 | 59% of patients have squamous cell carcinoma of the head and neck but not stated how many of these have oral cavity or oropharyngeal cancer. No longer possible to contact authors. |
| Flores 1996 | Unclear how many of these have oral cavity or oropharyngeal cancer. |
| Garden 2004 | 3 treatment groups, each with different chemotherapy regimen, therefore results cannot be attributed to radiotherapy. |
| Giglio 1997 | Comparison of radiotherapy regimen, but results confounded by use of chemotherapy in 1 arm. |
| Hansen 1997 | Reanalysis of data from 2 DAHANCA studies. |
| Hering 1981 | Randomisation not mentioned. |
| Hintz 1979 | Less than 50% of participants in trial have oral cavity or oropharyngeal cancer. |
| Holsti 1988 | Inadequate randomisation ‐ odd versus even birth dates. |
| Jackson 1997 | Some trial participants are randomly allocated to treatment, and some are not. Minority of participants have oral cavity or oropharyngeal cancer. Attempts to contact author failed. |
| Janot 2008 | Patients had recurrent disease. |
| Johnson 1995 | Conference abstract. No information on proportion of included participants with oral cavity or oropharyngeal cancer. No subsequent publication identified. |
| Katori 2007 | Less than 50% of participants in trial have oral cavity or oropharyngeal cancer. |
| Klima 1988 | Patients with metastatic disease included. |
| Kokal 1988 | Less than 50% of participants in trial have oral cavity or oropharyngeal cancer. |
| Kramer 1987 | Less than 50% of participants in trial have oral cavity or oropharyngeal cancer. |
| Maor 1983 | Includes recurrent cancer patients. |
| Mishra 1996 | Only some of the patients are randomised but all are analysed together. "However some surgeons preferred to put more clinically node‐positive cases into the post‐operative radiotherapy group." |
| Nissenbaum 1984 | Less than 6 months follow‐up. |
| Noel 1997 | Unclear how many patients have oral cavity or oropharyngeal cancer. |
| Noel 1997a | Unclear how many patients have oral cavity or oropharyngeal cancer. |
| Noel 1997b | Unclear how many patients have oral cavity or oropharyngeal cancer. |
| Noel 2001 | Commentary on 4 trials ‐ no data. |
| Rink 1989 | 3 groups but not randomised. |
| Robertson 1998 | Comparison of 2 different radiotherapy regimens, 1 of which includes surgery. Results confounded. |
| Sanchiz 1990 | Less than 50% of participants in trial have oral cavity or oropharyngeal cancer. |
| Singh 1984 | Unclear if true RCT. Data presented as percentages; unclear denominator. |
| Snow 1981 | Less than 50% of participants in trial have oral cavity or oropharyngeal cancer. Authors have not responded to request for separate data. |
| Srivastava 2001 | Less than 50% of participants in trial have oral cavity or oropharyngeal cancer. |
| Strong 1978 | Less than 50% of participants in trial have oral cavity or oropharyngeal cancer. |
| Suwinski 2008 | Less than 50% of participants in trial have oral cavity or oropharyngeal cancer. |
| Tupchong 1991 | Less than 50% of participants in trial have oral cavity or oropharyngeal cancer. |
RCT = randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Ang 2007.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Abstract only ‐ full text due maybe in 2010. |
Ghosh 2006.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Abstract only ‐ awaiting full paper. |
Nutting 2009a.
| Methods | PARSPORT study. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | American Society of Clinical Oncology (ASCO) abstract 2009 ‐ outcome = xerostomia, full report due in 2010 or 2011. |
Rodrigo 2004.
| Methods | Randomised controlled trial. |
| Participants | Head and neck cancer patients. |
| Interventions | Post‐operative radiotherapy versus surgery. |
| Outcomes | Total mortality, disease specific survival, locoregional control. |
| Notes | Awaiting translation. |
Rosenthal 2006.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Abstract only ‐ unclear of oral cavity/oropharyngeal percentages in full group. |
Skladowski 2001.
| Methods | Randomised controlled trial. |
| Participants | Head and neck cancer patients (oral cavity, oro‐hypopharynx and larynx in Stage T2‐4, N0‐1, M0). |
| Interventions | 7 fractions in 7 days versus 7 fractions in 5 days. |
| Outcomes | Toxicity (additional outcomes unclear). |
| Notes | Information taken from published abstracts. |
Characteristics of ongoing studies [ordered by study ID]
Moergel 2009.
| Trial name or title | |
| Methods | Non‐blinded, multicentre randomised controlled trial. |
| Participants | Histologically verified diagnosis of a primary squamous cell carcinoma of the oral cavity or the oropharynx are eligible. "‐ Maximum tumor diameter less than 4 cm in the pathohistological specimen irrespective of histological grading (pT1 or pT2) ‐ Concomitant histological verification of a singular ipsilateral lymph node metastasis less than 3 cm in diameter (pN1) without penetration of the lymph node's capsule and without presence of lymphangiosis carcinomatosa ‐ Radical resection of the tumor within adequate resection margins (R0) ‐ Written informed consent from the patient ‐ Adequate performance status ECOG Index greater or equal to 2 Patients younger than 18 and pregnant women are to be excluded." |
| Interventions | Surgery plus radiotherapy versus surgery alone. |
| Outcomes | Primary outcome: overall survival. Secondary outcomes: incidence and time to tumour relapse, quality of life and time from surgery to orofacial rehabilitation. |
| Starting date | September 2009. |
| Contact information | Maximilian Moergel (moergel@mkg.klinik.uni‐mainz.de). |
| Notes |
Differences between protocol and review
Types of studies: As the primary outcome for this review is total mortality we have added a requirement that included studies have a minimum of 6 months of follow‐up of participants after the end of treatment.
Types of participants: We have only included studies where at least 50% of the participants have either oral cavity or oropharyngeal cancer, or where data for the oral cavity and oropharyngeal patients only are available.
Types of interventions: The intervention under evaluation must be radiotherapy. Trials where all participants receive the same radiotherapy regimen and are randomised to other treatments were excluded.
Types of outcomes: The protocol for this review stated that quality of life would be a primary outcome for this review. Quality of life is an important outcome, for both patients with oral cavity and oropharyngeal cancers and their doctors. However, quality of life is infrequently and inconsistently reported in trials which address a primary outcome of overall survival. Therefore we have opted to transfer this outcome to the list of secondary outcomes to be considered in future updates of this review as appropriate.
Search methods: The search strategy has been updated.
Quality assessment has been replaced by the new risk of bias tool.
Data synthesis has been updated. The primary outcome is total mortality expressed as a hazard ratio. A meta‐analysis of individual patient data (IPD) for altered fractionation versus conventional fractionation has previously been published (Bourhis 2006). For trials included in the Bourhis meta‐analysis, the IPD were used instead of data presented in the published reports. For dichotomous outcomes, the estimates of effect of an intervention were expressed as risk ratios together with 95% confidence intervals. Dichotomous data were only used for primary outcomes where hazard ratios were unavailable or could not be calculated.
Contributions of authors
Conceiving the review and writing of protocol: Helen Worthington (HW), Jan Clarkson (JC), Anne‐Marie Glenny (AMG), Richard Oliver (RO)
Designing the review: HW, JC, AMG, RO, Sue Pavitt (SP), Michaelina Macluskey (MM), David Conway (DC) and CSROC Expert Panel
Co‐ordinating the review: AMG, Susan Furness (SF)
Data collection for the review: AMG, HW, JC, SF, MM, DC
Designing search strategies: Anne Littlewood (AL) (Trials Seach Co‐ordinator, Cochrane Oral Health Group) in collaboration with SF and AMG
Undertaking searches: AL
Screening search results: SF, HW, AMG
Organizing retrieval of papers: AMG, SF, SP
Screening retrieved papers against eligibility criteria: AMG, SF, HW, JC, RO, SP, MM, DC
Appraising risk of bias: AMG, SF, Paul Brocklehurst (PB)
Extracting data from papers: AMG, HW, SF, PB
Writing to authors of papers for additional information: SP, SF
Data management for the review: AMG, SF, HW, SP
Entering data into RevMan: AMG, SF
Analysis of data: AMG, SF, HW
Interpretation of data: AMG, SF, HW, Kelvin KW Chan (KC)
Writing the review: AMG, SF, HW, KC.
The CSROC Expert Panel comprises: Bertrand Baujat, Gerry Humphris, Iain Hutchison, Jean‐Pierre Pignon, Gerry Robertson, Simon Rogers, Jatin Shah, Nick Slevin, Phil Sloan, David Soutar, Erich Sturgis, Jan Vermorken, Steve Wardell, Saman Warnakulasuriya, Keith Webster.
Sources of support
Internal sources
School of Dentistry, The University of Manchester, UK.
Cochrane Oral Health Group, UK.
The University of Dundee, UK.
The University of Glasgow, UK.
Manchester Academic Health Sciences Centre (MAHSC) and NIHR Manchester Biomedical Research Centre, UK.
External sources
National Institute of Health, National Institute of Dental & Craniofacial Research, USA.
Central Manchester & Manchester Children's University Hospitals NHS Trust, UK.
Declarations of interest
None known.
New
References
References to studies included in this review
Ang 2001 {published data only}
- Ang KK, Trotti A, Brown BW, Garden AS, Foote RL, Morrison WH, et al. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head‐and‐neck cancer. International Journal of Radiation Oncology, Biology, Physics 2001;51(3):571‐8. [DOI] [PubMed] [Google Scholar]
Bartelink 2002 {published data only}
- Bartelink H, Bogaert W, Horiot JC, Jager J, Glabbeke M. Concomitant cisplatin and radiotherapy in a conventional and modified fractionation schedule in locally advanced head and neck cancer: a randomised phase II EORTC trial. European Journal of Cancer 2002;38(5):667‐73. [DOI] [PubMed] [Google Scholar]
Bergermann 1992 {published data only}
- Bergermann M, Dieckmann J, Machtens E. Preoperative short‐term preliminary irradiation with 3 x 6 Gy, immediate radical tumor resection and postoperative booster irradiation to 60 Gy as an effective therapy concept for the treatment of T2 squamous cell carcinomas of the mouth. Results of a prospective randomized bi‐center study. Fortschritte der Kiefer und Gesichts Chirurgie 1992;37:17‐20. [PubMed] [Google Scholar]
Bjarnason 2009 {published data only}
- Bjarnason GA, Mackenzie RG, Nabid A, Hodson ID, El‐Sayed S, Grimard L, et al. Comparison of toxicity associated with early morning versus late afternoon radiotherapy in patients with head‐and‐neck cancer: a prospective randomized trial of the National Cancer Institute of Canada Clinical Trials Group (HN3). International Journal of Radiation Oncology, Biology, Physics 2009;73(1):166‐72. [DOI] [PubMed] [Google Scholar]
Bourhis 2006 {published data only}
- Bourhis J, Lapeyre M, Tortochaux J, Rives M, Aghili M, Bourdin S, et al. Phase III randomized trial of very accelerated radiation therapy compared with conventional radiation therapy in squamous cell head and neck cancer: a GORTEC trial. Journal of Clinical Oncology 2006;24(18):2873‐8. [DOI] [PubMed] [Google Scholar]
Cox 1990 {published data only}
- Cox JD, Pajak TF, Marcial VA, Coia L, Mohiuddin M, Fu KK, et al. ASTRO plenary: interfraction interval is a major determinant of late effects, with hyperfractionated radiation therapy of carcinomas of upper respiratory and digestive tracts: results from Radiation Therapy Oncology Group protocol 8313. International Journal of Radiation Oncology, Biology, Physics 1991;20(6):1191‐5. [DOI] [PubMed] [Google Scholar]
- Cox JD, Pajak TF, Marcial VA, Coia L, Mohiuddin M, Fu KK, et al. Interruptions adversely affect local control and survival with hyperfractionated radiation therapy of carcinomas of the upper respiratory and digestive tracts. New evidence for accelerated proliferation from Radiation Therapy Oncology Group Protocol 8313. Cancer 1992;69(11):2744‐8. [DOI] [PubMed] [Google Scholar]
- Cox JD, Pajak TF, Marcial VA, Hanks GE, Mohiuddin M, Fu KK, et al. Dose‐response for local control with hyperfractionated radiation therapy in advanced carcinomas of the upper aerodigestive tracts: preliminary report of radiation therapy oncology group protocol 83‐13. International Journal of Radiation Oncology, Biology, Physics 1990;18(3):515‐21. [DOI] [PubMed] [Google Scholar]
- Fu KK, Cox JD, Pajak TF, Marcial VA, Coia LR, Mohiuddin M, et al. Carcinomas of the oropharynx treated with hyperfractionated radiation therapy on Radiation Therapy Oncology Group Protocol 8313. Recent Results in Cancer Research 1994;134:145‐54. [DOI] [PubMed] [Google Scholar]
- Fu KK, Pajak TF, Marcial VA, Ortiz HG, Rotman M, Asbell SO, et al. Late effects of hyperfractionated radiotherapy for advanced head and neck cancer: long‐term follow‐up results of RTOG 83‐13. International Journal of Radiation Oncology, Biology, Physics 1995;32(3):577‐88. [DOI] [PubMed] [Google Scholar]
- Herrmann T. Late effects of hyperfractionated radiotherapy for advanced head and neck cancer: long‐term follow‐up results of RTOG 83‐13. Radiation Therapy Oncology Group. Strahlentherapie und Onkologie 1995;171(4):244‐5. [PubMed] [Google Scholar]
Dobrowsky 2000 {published data only}
- Dobrowsky W, Dobrowsky E, Naude J, Millesi W, Pavelka R, Kautzky M, et al. Conventional vs accelerated fractionation in head and neck cancer. British Journal of Cancer ‐ Supplement 1996;27:S279‐81. [PMC free article] [PubMed] [Google Scholar]
- Dobrowsky W, Naude J. Continuous hyperfractionated accelerated radiotherapy with/without mitomycin C in head and neck cancers. Radiotherapy & Oncology 2000;57(2):119‐24. [DOI] [PubMed] [Google Scholar]
Fu 1995 {published data only}
- Fu KK, Clery M, Ang KK, Byhardt RW, Maor MH, Beitler JJ. Randomized phase I/II trial of two variants of accelerated fractionated radiotherapy regimens for advanced head and neck cancer: results of RTOG 88‐09. International Journal of Radiation Oncology, Biology, Physics 1995;32(3):589‐97. [DOI] [PubMed] [Google Scholar]
Fu 2000 {published data only}
- Dubben HH, Beck‐Bornholdt HP. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard‐fractionation radiotherapy for head‐and‐neck squamous cell carcinomas: first report of RTOG 9003: in regard to Fu et al. IJROBP 2000;48:7‐16. Actuarial estimates of late normal‐tissue effects...now!. International Journal of Radiation Oncology, Biology, Physics 2001;51(2):563. [DOI] [PubMed] [Google Scholar]
- Fu KK, Pajak TF, Trotti A, Jones CU, Spencer SA, Phillips TL, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. International Journal of Radiation Oncology, Biology, Physics 2000;48(1):7‐16. [DOI] [PubMed] [Google Scholar]
- Konski AA, Winter K, Cole BF, Ang KK, Fu KK. Quality‐adjusted survival analysis of Radiation Therapy Oncology Group (RTOG) 90‐03: phase III randomized study comparing altered fractionation to standard fractionation radiotherapy for locally advanced head and neck squamous cell carcinoma. Head and Neck 2009;31:207‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logie MB. Radiation therapy oncology: in regard to Fu et al., IJROBP 2000;48:7‐16. International Journal of Radiation Oncology, Biology, Physics 2002;52(4):1148. [DOI] [PubMed] [Google Scholar]
Ghoshal 2008 {published data only}
- Ghoshal S, Goda JS, Mallick I, Kehwar TS, Sharma SC. Concomitant boost radiotherapy compared with conventional radiotherapy in squamous cell carcinoma of the head and neck‐‐a phase III trial from a single institution in India. Clinical Oncology (Royal College of Radiologists) 2008;20(3):212‐20. [DOI] [PubMed] [Google Scholar]
Griffin 1984 {published data only}
- Griffin TW, Davis R, Hendrickson FR, Maor MH, Laramore GE. Fast neutron radiation therapy for unresectable squamous cell carcinomas of the head and neck: the results of a randomized RTOG study. International Journal of Radiation Oncology, Biology, Physics 1984;10(12):2217‐21. [DOI] [PubMed] [Google Scholar]
Griffin 1989 {published data only}
- Griffin TW, Davis R, Laramore GE, Maor MH, Hendrickson FR, Rodriguez‐Antunez A, et al. Mixed beam radiation therapy for unresectable squamous cell carcinomas of the head and neck: the results of a randomized RTOG study. International Journal of Radiation Oncology, Biology, Physics 1984;10(12):2211‐5. [DOI] [PubMed] [Google Scholar]
- Griffin TW, Pajak TF, Maor MH, Laramore GE, Hendrickson FR, Parker RG, et al. Mixed neutron/photon irradiation of unresectable squamous cell carcinomas of the head and neck: the final report of a randomized cooperative trial. International Journal of Radiation Oncology, Biology, Physics 1989;17(5):959‐65. [DOI] [PubMed] [Google Scholar]
Horiot 1992 {published data only}
- Bernier J, Thames HD, Smith CD, Horiot JC. Tumor response, mucosal reactions and late effects after conventional and hyperfractionated radiotherapy. Radiotherapy & Oncology 1998;47(2):137‐43. [DOI] [PubMed] [Google Scholar]
- Horiot JC. Controlled clinical trials of hyperfractionated and accelerated radiotherapy in otorhinolaryngologic cancers. Bulletin de l'Academie Nationale de Medecine 1998;182(6):1247‐60. [PubMed] [Google Scholar]
- Horiot JC, Begg AC, Fur R, Schraub S, Bogaert W, Glabbeke M, et al. Present status of EORTC trials of hyperfractionated and accelerated radiotherapy on head and neck carcinoma. Recent Results in Cancer Research 1994;134:111‐9. [DOI] [PubMed] [Google Scholar]
- Horiot JC, Bontemps P, Begg AC, Fur R, Bogaert W, Bolla M, et al. Hyperfractionated and accelerated radiotherapy in head and neck cancers: results of the EORTC trials and impact on clinical practice. Bulletin du Cancer. Radiotherapie 1996;83(4):314‐20. [PubMed] [Google Scholar]
- Horiot JC, Fur R, N'Guyen T, Chenal C, Schraub S, Alfonsi S, et al. Hyperfractionated compared with conventional radiotherapy in oropharyngeal carcinoma: an EORTC randomized trial. European Journal of Cancer 1990;26(7):779‐80. [DOI] [PubMed] [Google Scholar]
- Horiot JC, Fur R, N'Guyen T, Chenal C, Schraub S, Alfonsi S, et al. Hyperfractionation versus conventional fractionation in oropharyngeal carcinoma: final analysis of a randomized trial of the EORTC cooperative group of radiotherapy. Radiotherapy & Oncology 1992;25(4):231‐41. [DOI] [PubMed] [Google Scholar]
- Horiot JC, Fur R, NGuyen T, Schraub S, Chenal C, Pauw M, et al. Two fractions per day versus a single fraction per day in the radiotherapy of oropharynx carcinoma: results of an EORTC randomized trial. International Journal of Radiation Oncology, Biology, Physics 1988;15:179. [Google Scholar]
Horiot 1997 {published data only}
- Horiot JC. Controlled clinical trials of hyperfractionated and accelerated radiotherapy in otorhinolaryngologic cancers. Bulletin de l'Academie Nationale de Medecine 1998;182(6):1247‐60. [PubMed] [Google Scholar]
- Horiot JC, Bontemps P, Begg AC, Fur R, Bogaert W, Bolla M, et al. Hyperfractionated and accelerated radiotherapy in head and neck cancers: results of the EORTC trials and impact on clinical practice. Bulletin du Cancer. Radiotherapie 1996;83(4):314‐20. [PubMed] [Google Scholar]
- Horiot JC, Bontemps P, Bogaert W, Fur R, Weijngaert D, Bolla M, et al. Accelerated fractionation (AF) compared to conventional fractionation (CF) improves loco‐regional control in the radiotherapy of advanced head and neck cancers: results of the EORTC 22851 randomized trial. Radiotherapy & Oncology 1997;44(2):111‐21. [DOI] [PubMed] [Google Scholar]
Hukku 1991 {published data only}
- Hukku S. Comparison of high fractional dose continuous versus conventional split course radiation therapy in advanced head and neck cancer. Indian Journal of Radiology and Imaging 1991;I(Suppl 1 (Pt 1)):434‐9. [Google Scholar]
Inoue 2001 {published data only}
- Inoue T, Inoue T, Teshima T, Murayama S, Shimizutani K, Fuchihata H, et al. Phase III trial of high and low dose rate interstitial radiotherapy for early oral tongue cancer. International Journal of Radiation Oncology, Biology, Physics 1996;36(5):1201‐4. [DOI] [PubMed] [Google Scholar]
- Inoue T, Inoue T, Yoshida K, Yoshioka Y, Shimamoto S, Tanaka E, et al. Phase III trial of high‐ vs. low‐dose‐rate interstitial radiotherapy for early mobile tongue cancer. International Journal of Radiation Oncology, Biology, Physics 2001;51(1):171‐5. [DOI] [PubMed] [Google Scholar]
Ketcham 1969 {published data only}
- Ketcham AS, Hoye RC, Chretien PB, Brace KC. Irradiation twenty‐four hours preoperatively. American Journal of Surgery 1969;118(5):691‐7. [DOI] [PubMed] [Google Scholar]
Lawrence 1974 {published data only}
- Lawrence W Jr, Terz JJ, Rogers C, King RE, Wolf JS, King ER. Proceedings: Preoperative irradiation for head and neck cancer: a prospective study. Cancer 1974;33:318‐23. [DOI] [PubMed] [Google Scholar]
MacDougall 1990 {published data only}
- Arnott SJ, Duncan W. An interim assessment of the experience of fast neutron therapy in patients with head and neck cancer in Edinburgh. Journal Europeen de Radiotherapie 1984;5:138‐41. [Google Scholar]
- Duncan W, Arnott SJ, Battermann JJ, Orr JA, Schmitt G, Kerr GR. Fast neutrons in the treatment of head and neck cancers: the results of a multi‐centre randomly controlled trial. Radiotherapy & Oncology 1984;2(4):293‐300. [DOI] [PubMed] [Google Scholar]
- Duncan W, Arnott SJ, Orr JA, Kerr GR. The Edinburgh experience of fast neutron therapy. International Journal of Radiation Oncology, Biology, Physics 1982;8(12):2155‐7. [DOI] [PubMed] [Google Scholar]
- Duncan W, Orr JA, Arnott SJ, Jack WJ, Kerr GR, Williams JR. Fast neutron therapy for squamous cell carcinoma in the head and neck region: results of a randomized trial. International Journal of Radiation Oncology, Biology, Physics 1987;13(2):171‐8. [DOI] [PubMed] [Google Scholar]
- MacDougall RH, Orr JA, Kerr GR, Duncan W. Fast neutron treatment for squamous cell carcinoma of the head and neck: final report of Edinburgh randomised trial. BMJ 1990;301(6763):1241‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maor 1986 {published data only}
- Maor MH, Schoenfeld DA, Hendrickson FR, Davis LW, Laramore GE, Thomas FJ, et al. Evaluation of a neutron boost in head and neck cancer. Results of the randomized RTOG trial 78‐08. American Journal of Clinical Oncology 1986;9(1):61‐6. [DOI] [PubMed] [Google Scholar]
Maor 1995 {published data only}
- Cranberg L. Regarding Maor et al. IJROBP 1995;32(3):599‐604. International Journal of Radiation Oncology, Biology, Physics 1996;34(4):972. [DOI] [PubMed] [Google Scholar]
- Laramore GE, Griffin TW. Fast neutron radiotherapy: where have we been and where are we going? The jury is still out‐‐regarding Maor et al., IJROBP 32:599‐604; 1995. International Journal of Radiation Oncology, Biology, Physics 1995;32(3):879‐82. [DOI] [PubMed] [Google Scholar]
- Maor MH, Errington RD, Caplan RJ, Griffin TW, Laramore GE, Parker RG, et al. Fast‐neutron therapy in advanced head and neck cancer: a collaborative international randomized trial. International Journal of Radiation Oncology, Biology, Physics 1995;32(3):599‐604. [DOI] [PubMed] [Google Scholar]
Marcial 1987 {published data only}
- Marcial VA, Pajak TF, Chang C. Hyperfractionated photon radiation therapy in the treatment of advanced squamous cell carcinoma of the oral cavity, pharynx, larynx and sinuses, using radiotherapy as the only planned modality: a Radiation Therapy Oncology Group report. International Journal of Radiation Oncology, Biology, Physics 1985;11:142. [DOI] [PubMed] [Google Scholar]
- Marcial VA, Pajak TF, Chang C, Tupchong L, Stetz J. Hyperfractionated photon radiation therapy in the treatment of advanced squamous cell carcinoma of the oral cavity, pharynx, larynx, and sinuses, using radiation therapy as the only planned modality: (preliminary report) by the Radiation Therapy Oncology Group (RTOG). International Journal of Radiation Oncology, Biology, Physics 1987;13(1):41‐7. [DOI] [PubMed] [Google Scholar]
Marcial 1993 {published data only}
- Marcial VA, Hanley JA, Hendrickson F, Ortiz H. Split‐course radiation therapy of carcinoma of the base of the tongue: results of a prospective national collaborative clinical trial conducted by the Radiation Therapy Oncology Group. International Journal of Radiation Oncology, Biology, Physics 1983;9(4):437‐43. [DOI] [PubMed] [Google Scholar]
- Marcial VA, Pajak TF, Rotman M, Brady LW, Amato D. 'Compensated' split‐course versus continuous radiation therapy of carcinoma of the tonsillar fossa. Final results of a prospective randomized clinical trial of the Radiation Therapy Oncology Group. American Journal of Clinical Oncology 1993;16(5):389‐96. [DOI] [PubMed] [Google Scholar]
- Ortiz H, Marcial VA, Hanley JA, Ramirez C, Pacheco JC. Protocol variations and their influence on the final outcome: critical review of the split‐course base of tongue study of the RTOG. International Journal of Radiation Oncology, Biology, Physics 1985;11:885‐92. [DOI] [PubMed] [Google Scholar]
Olmi 2003 {published data only}
- Crispino S, Olmi P, Fallai C, Rossi F, Marsoni S, Tinazzi A, et al. Conventional radiotherapy (RT) versus accelerated hyperfractionated RT versus conventional RT and concomitant chemotherapy in locally advanced oropharyngeal carcinoma (ORO‐93/01). Proceedings of the Annual Meeting of the American Society of Clinical Oncology. 1997:Abs No 1423.
- Fallai C, Bolner A, Signor M, Gava A, Franchin G, Ponticelli P, et al. Long‐term results of conventional radiotherapy versus accelerated hyperfractionated radiotherapy versus concomitant radiotherapy and chemotherapy in locoregionally advanced carcinoma of the oropharynx. Tumori 2006;92(1):41‐54. [DOI] [PubMed] [Google Scholar]
- Olmi P, Crispino S, Fallai C, Torri V, Rossi F, Bolner A, et al. Locoregionally advanced carcinoma of the oropharynx: conventional radiotherapy vs. accelerated hyperfractionated radiotherapy vs. concomitant radiotherapy and chemotherapy‐‐a multicenter randomized trial. International Journal of Radiation Oncology, Biology, Physics 2003;55(1):78‐92. [DOI] [PubMed] [Google Scholar]
Pinto 1991 {published data only}
- Pinto LH, Canary PC, Araujo CM, Bacelar SC, Souhami L. Prospective randomized trial comparing hyperfractionated versus conventional radiotherapy in stages III and IV oropharyngeal carcinoma. International Journal of Radiation Oncology, Biology, Physics 1991;21(3):557‐62. [DOI] [PubMed] [Google Scholar]
- Pinto LH, Canary PC, Araujo CM, Bacelar SC, Souhami L. Prospective randomized trial comparing hyperfractionated versus conventional radiotherapy in stages III and IV oropharynx carcinoma. International Journal of Radiation Oncology, Biology, Physics 1990;19:140‐1. [DOI] [PubMed] [Google Scholar]
Poulsen 2001 {published data only}
- Daly T, Poulsen MG, Denham JW, Peters LJ, Lamb DS, Krawitz H, et al. The effect of anaemia on efficacy and normal tissue toxicity following radiotherapy for locally advanced squamous cell carcinoma of the head and neck. Radiotherapy & Oncology 2003;68(2):113‐22. [DOI] [PubMed] [Google Scholar]
- Poulsen M, Denham J, Spry N, Lamb D, Peters L, Krawitz H, et al. Acute toxicity and cost analysis of a phase III randomized trial of accelerated and conventional radiotherapy for squamous carcinoma of the head and neck: a Trans‐Tasman Radiation Oncology Group study. Australasian Radiology 1999;43(4):487‐94. [DOI] [PubMed] [Google Scholar]
- Poulsen MG, Denham JW, Peters LJ, Lamb DS, Spry NA, Hindley A, et al. A randomised trial of accelerated and conventional radiotherapy for stage III and IV squamous carcinoma of the head and neck: a Trans‐Tasman Radiation Oncology Group Study. Radiotherapy & Oncology 2001;60(2):113‐22. [DOI] [PubMed] [Google Scholar]
Sanguineti 2005 {published data only}
- Antogoni P, Bignardi M, Richetti A, Corvo R, Sormani M, Gabriele P, et al. Accelerated versus conventional fractionated postoperative radiotherapy for advanced head and neck cancer: results of a multicenter Phase III study. International Journal of Radiation Oncology, Biology, Physics 2003;57(2):S154. [DOI] [PubMed] [Google Scholar]
- Sanguineti G, Richetti A, Bignardi M, Corvo R, Gabriele P, Sormani MP, et al. Accelerated versus conventional fractionated postoperative radiotherapy for advanced head and neck cancer: results of a multicenter Phase III study. International Journal of Radiation Oncology, Biology, Physics 2005;61(3):762‐71. [DOI] [PubMed] [Google Scholar]
Skladowski 2006 {published data only}
- Maciejewski B, Skladowski K, Pilecki B, Taylor JM, Withers RH, Miszczyk L, et al. Randomized clinical trial on accelerated 7 days per week fractionation in radiotherapy for head and neck cancer. Preliminary report on acute toxicity. Radiotherapy & Oncology 1996;40(2):137‐45. [DOI] [PubMed] [Google Scholar]
- Skladowski K, Maciejewski B, Golen M, Pilecki B, Przeorek W, Tarnawski R. Randomized clinical trial on 7‐day‐continuous accelerated irradiation (CAIR) of head and neck cancer ‐ report on 3‐year tumour control and normal tissue toxicity. Radiotherapy & Oncology 2000;55(2):101‐10. [DOI] [PubMed] [Google Scholar]
- Skladowski K, Maciejewski B, Golen M, Tarnawski R, Slosarek K, Suwinski R, et al. Continuous accelerated 7‐days‐a‐week radiotherapy for head‐and‐neck cancer: long‐term results of phase III clinical trial. International Journal of Radiation Oncology, Biology, Physics 2006;66(3):706‐13. [DOI] [PubMed] [Google Scholar]
Terz 1981 {published data only}
- Terz JJ, King ER, Lawrence W Jr. Preoperative irradiation for head and neck cancer: results of a prospective study. Surgery 1981;89(4):449‐53. [PubMed] [Google Scholar]
Weissberg 1983 {published data only}
- Percarpio B, Fischer JJ. Irradiation of advanced head and neck cancer with large daily fractions. Preliminary results of a prospectively randomized clinical trial. Radiology 1976;121(2):489‐90. [DOI] [PubMed] [Google Scholar]
- Weissberg JB, Pillsbury H, Sasaki CT, Son YH, Fischer JJ. High fractional dose irradiation of advanced head and neck cancer. Implications for combined radiotherapy and surgery. Archives of Otolaryngology 1983;109(2):98‐102. [DOI] [PubMed] [Google Scholar]
- Weissberg JB, Son YH, Percarpio B, Fischer JJ. Randomized trial of conventional versus high fractional dose radiation therapy in the treatment of advanced head and neck cancer. International Journal of Radiation Oncology, Biology, Physics 1982;8(2):179‐85. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arimoto 2003 {published data only}
- Arimoto T, Yamazaki A, Yonesaka A, Matsuzawa T, Kanai N. Clinical application of AcMAR (accelerated multiple‐arc radiotherapy) for head and neck tumors ‐ Results of a randomized, two‐dose study in Kitami Red‐Cross General Hospital. Journal of JASTRO 2003;15(3):203‐11. [Google Scholar]
Awwad 1992 {published data only}
- Awwad HK, Khafagy Y, Barsoum M, Ezzat S, el‐Attar I, Farag H, et al. Accelerated versus conventional fractionation in the postoperative irradiation of locally advanced head and neck cancer: influence of tumour proliferation. Radiotherapy & Oncology 1992;25(4):261‐6. [DOI] [PubMed] [Google Scholar]
Awwad 2002 {published data only}
- Awwad HK, Lotayef M, Shouman T, Begg AC, Wilson G, Bentzen SM, et al. Accelerated hyperfractionation (AHF) compared to conventional fractionation (CF) in the postoperative radiotherapy of locally advanced head and neck cancer: influence of proliferation. British Journal of Cancer 2002;86(4):517‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baumann 2001 {published data only}
- Baumann M, Klages HT. Comparison of various fractionation schedule in curative radiotherapy alone of locally advanced head and neck tumors. Strahlentherapie und Onkologie 2001;177(3):162‐5. [PubMed] [Google Scholar]
Catterall 1977 {published data only}
- Catterall M. First randomized clinical trial of fast neutrons compared with photons in advanced carcinoma of the head and neck. Clinical Otolaryngology & Allied Sciences 1977;2(4):359‐72. [DOI] [PubMed] [Google Scholar]
- Catterall M, Bewley DK, Sutherland I. Second report on results of a randomised clinical trial of fast neutrons compared with chi or gamma rays in treatment of advanced tumours of head and neck. British Medical Journal 1977;1(6077):1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall M, Sutherland I, Bewley DK. First results of a randomized clinical trial of fast neutrons compared with X or gamma rays in treatment of advanced tumours of the head and neck. Report to the Medical Research Council. British Medical Journal 1975;2(5972):653‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
CHART 1997 {published data only}
- Baumann M, Herrmann T. Outcome of CHART radiotherapy in squamous cell carcinoma of the head and neck and in non‐small cell bronchial carcinoma. Strahlentherapie und Onkologie 1997;173(6):341‐3. [DOI] [PubMed] [Google Scholar]
- Dische S, Saunders M, Barrett A, Harvey A, Gibson D, Parmar M. A randomised multicentre trial of CHART versus conventional radiotherapy in head and neck cancer. Radiotherapy & Oncology 1997;44(2):123‐36. [DOI] [PubMed] [Google Scholar]
- Griffiths GO, Parmar MK, Bailey AJ. Physical and psychological symptoms of quality of life in the CHART randomized trial in head and neck cancer: short‐term and long‐term patient reported symptoms. CHART Steering Committee. Continuous hyperfractionated accelerated radiotherapy. British Journal of Cancer 1999;81(7):1196‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders MI, Dische S, Barrett A, Parmar MK, Harvey A, Gibson D. Randomised multicentre trials of CHART vs conventional radiotherapy in head and neck and non‐small‐cell lung cancer: an interim report. CHART Steering Committee. British Journal of Cancer 1996;73(12):1455‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders MI, Rojas AM, Parmar MKB, Dische S, CHART Trial Collaborators. Mature results of a randomized trial of accelerated hyperfractionated versus conventional radiotherapy in head‐and‐neck cancer. International Journal of Radiation Oncology, Biology, Physics 2010;77:3‐8. [DOI] [PubMed] [Google Scholar]
Cummings 2007 {published data only}
- Cummings B, Keane T, Pintilie M, Warde P, Waldron J, Payne D, et al. Five year results of a randomized trial comparing hyperfractionated to conventional radiotherapy over four weeks in locally advanced head and neck cancer. Radiotherapy & Oncology 2007;85(1):7‐16. [DOI] [PubMed] [Google Scholar]
DAHANCA 2003 {published data only}
- Overgaard J, Hansen H, Specht L, Grau C, Overgaard M, Andersen E, et al. The Danish Head and Neck Cancer Study Group DAHANCA 6&7 randomized trial of 5 versus 6 fractions per week of conventional radiotherapy of squamous cell carcinoma of the head and neck. Proceedings of the American Society of Clinical Oncology. 2003:Abs No 1994.
- Overgaard J, Hansen HS, Specht L. Erratum: Five compared with six fractions per week of conventional radiotherapy of squamous‐cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial (Lancet 2003;362:933‐40). Lancet 2003;362(9395):1588. [DOI] [PubMed] [Google Scholar]
- Overgaard J, Hansen HS, Specht L, Overgaard M, Grau C, Andersen E, et al. Five compared with six fractions per week of conventional radiotherapy of squamous‐cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet 2003;362(9388):933‐40. [DOI] [PubMed] [Google Scholar]
- Overgaard J, Sand HH, Overgaard M, Grau C, Specht L, Andersen E, et al. A randomized trial with 1485 patients evaluating the importance of accelerated versus conventional fractionated radiotherapy in squamous cell carcinoma of the head and neck. Final results of the DAHANCA 6&7 study. European Journal of Cancer 2003; Vol. 1, issue 5:S318.
- Overgaard J, Sand Hansen H, Sapru W, Overgaard M, Grau C, Jorgensen K, et al. Conventional radiotherapy as the primary treatment of squamous cell carcinoma (SCC) of the head and neck. A randomised multicentre study of 5 versus 6 fractions per week. Preliminary report from the Dahanca 6 and 7 trial. Radiotherapy & Oncology 1996;40 Suppl 1:S31. [Google Scholar]
- Toubiana T, Racadot S, Mazeron JJ. Five compared with six fractions per week of conventional radiotherapy of squamous‐cell carcinoma of head and neck: DAHANCA 6&7 randomised controlled trial. Bulletin du Cancer/Radiotherapie 2004;8(3):209‐10. [Google Scholar]
Datta 1989 {published data only}
- Datta NR, Dutta Choudhry A, Gupta S, Bose AK. Twice a day versus once a day radiation therapy in head and neck cancer. International Journal of Radiation Oncology, Biology, Physics 1989;17(Suppl 1):132‐3. [Google Scholar]
Dieckmann 1990 {published data only}
- Dieckmann J. Combined pre‐and postoperative radiotherapy (RT) in the treatment of carcinomas of the floor of the mouth. Journal of Cancer Research & Clinical Oncology 1990;116:797. [Google Scholar]
Dvivedi 1978 {published data only}
- Dvivedi M, Pradhan DG. Immediate results of weekly fractionation in external radiotherapy. International Journal of Radiation Oncology, Biology, Physics 1978;4(7‐8):573‐8. [DOI] [PubMed] [Google Scholar]
Flores 1996 {published data only}
- Flores AD, Kandil A, Jamshed A, Khafaga Y, Schultz H, Rostom A, et al. The Saudi experience with neutron therapy in locally advanced head and neck cancers. Bulletin du Cancer/Radiotherapie 1996;83 Suppl:106s‐9s. [DOI] [PubMed] [Google Scholar]
Garden 2004 {published data only}
- Garden AS, Harris J, Vokes EE, Forastiere AA, Ridge JA, Jones C, et al. Preliminary results of Radiation Therapy Oncology Group 97‐03: a randomized phase ii trial of concurrent radiation and chemotherapy for advanced squamous cell carcinomas of the head and neck. Journal of Clinical Oncology 2004;22(14):2856‐64. [DOI] [PubMed] [Google Scholar]
Giglio 1997 {published data only}
- Giglio R, Mickiewicz E, Pradier R, Roth B, Gatica G, Califano L, et al. Chemotherapy + alternating hyperfractionated radiotherapy vs hyperfractionated radiotherapy alone in patients with inoperable stage III and IV epidermoid carcinoma. Prensa Medica Argentina 1997; Vol. 84, issue 5.
Hansen 1997 {published data only}
- Hansen O, Overgaard J, Hansen HS, Overgaard M, Hoyer M, Jorgensen KE, et al. Importance of overall treatment time for the outcome of radiotherapy of advanced head and neck carcinoma: dependency on tumor differentiation. Radiotherapy & Oncology 1997;43(1):47‐51. [DOI] [PubMed] [Google Scholar]
Hering 1981 {published data only}
- Dieckmann J, Morgenroth K, Hering KG, Will CH. Histo‐morphologic findings and first clinical results after preoperative short term preliminary irradiation with telecobalt in patients with epidermoid cancers of the mouth floor. Strahlentherapie. Sonderbande 1981;76:206‐11. [PubMed] [Google Scholar]
Hintz 1979 {published data only}
- Hintz B, Charyulu K, Chandler JR, Sudarsanam A, Garciga C. Randomized study of local control and survival following radical surgery or radiation therapy in oral and laryngeal carcinomas. Journal of Surgical Oncology 1979;12:61‐74. [DOI] [PubMed] [Google Scholar]
Holsti 1988 {published data only}
- Holsti LR, Mantyla M. Split‐course versus continuous radiotherapy. Analysis of a randomized trial from 1964 to 1967. Acta Oncologica 1988;27(2):153‐61. [DOI] [PubMed] [Google Scholar]
Jackson 1997 {published data only}
- Jackson SM, Weir LM, Hay JH, Tsang VH, Durham JS. A randomised trial of accelerated versus conventional radiotherapy in head and neck cancer. Radiotherapy & Oncology 1997;43(1):39‐46. [DOI] [PubMed] [Google Scholar]
Janot 2008 {published data only}
- Janot F, Raucourt D, Benhamou E, Ferron C, Dolivet G, Bensadoun RJ, et al. Randomized trial of postoperative reirradiation combined with chemotherapy after salvage surgery compared with salvage surgery alone in head and neck carcinoma. Journal of Clinical Oncology 2008;26(34):5518‐23. [DOI] [PubMed] [Google Scholar]
Johnson 1995 {published data only}
- Johnson CR, Schmidt‐Ullrich RK, Arthur DW, Huang DT, Duffy EW. Standard once‐daily versus thrice‐daily concomitant boost accelerated superfractionated irradiation for advanced squamous cell carcinoma of the head and neck: preliminary results of a prospective randomized trial. International Journal of Radiation Oncology, Biology, Physics 1995;32(971):162. [Google Scholar]
Katori 2007 {published data only}
- Katori H, Tsukuda M, Watai K. Comparison of hyperfractionation and conventional fractionation radiotherapy with concurrent docetaxel, cisplatin and 5‐fluorouracil (TPF) chemotherapy in patients with locally advanced squamous cell carcinoma of the head and neck (SCCHN). Cancer Chemotherapy & Pharmacology 2007;60(3):399‐406. [DOI] [PubMed] [Google Scholar]
Klima 1988 {published data only}
- Klima A, Bergmann I, Szepesi S. Multimodality treatment in stage IV squamous cell carcinoma of the head and neck. A long‐term follow‐up. Journal of Laryngology & Otology 1992;106(3):234‐7. [DOI] [PubMed] [Google Scholar]
- Klima A, Klippstein TH, Bergmann L, Szepesi S, Ilberg CV. Adjuvant chemotherapy in stage III and IV squamous cell carcinoma of the head and neck. Journal of Laryngology & Otology 1988;102:337‐40. [DOI] [PubMed] [Google Scholar]
- Klima A, Ilberg C, Bergmann L, Klippstein T, Zoubek A, Mitrou PS, et al. The effect of radio‐ and chemotherapy on survival in advanced head and neck tumors. Strahlentherapie und Onkologie 1987;163(5):297‐300. [PubMed] [Google Scholar]
- Klima A, Ilberg C, Bergmann L, Zoubek A, Klippstein T, Mitrou PS, et al. Chemotherapy or chemotherapy and radiotherapy in advanced head and neck tumors. A prospective, randomized study comparing the 2 therapeutic modalities. Head & Neck Oncology (HNO) 1987;35(2):78‐83. [PubMed] [Google Scholar]
- Klima A, Ilberg C, Klippstein T, Szepesi S. Chemotherapy of advanced head and neck tumors. A prospective randomized study comparing the effectiveness of chemotherapy alone to a chemo‐radiotherapy procedure. Laryngologie, Rhinologie, Otologie 1987;66(4):205‐10. [PubMed] [Google Scholar]
Kokal 1988 {published data only}
- Kokal WA, Neifeld JP, Eisert D, Lipsett JA, Lawrence W, Beatty JD, et al. Postoperative radiation as adjuvant treatment for carcinoma of the oral cavity, larynx, and pharynx: preliminary report of a prospective randomized trial. Journal of Surgical Oncology 1988;38:71‐6. [DOI] [PubMed] [Google Scholar]
Kramer 1987 {published data only}
- Kramer S, Gelber RD, Snow JB, Marcial VA, Lowry LD, Davis LW, et al. Combined radiation therapy and surgery in the management of advanced head and neck cancer: final report of study 73‐03 of the Radiation Therapy Oncology Group. Head & Neck Surgery 1987;10(1):19‐30. [DOI] [PubMed] [Google Scholar]
Maor 1983 {published data only}
- Maor MH, Hussey DH. Experience with fast neutron radiotherapy at the M.D. Anderson Hospital with emphasis on the randomized head and neck trial. Strahlentherapie. Sonderbande 1981;77:11‐6. [PubMed] [Google Scholar]
- Maor MH, Hussey DH, Barkley HT Jr, Peters LJ. Neutron therapy for head and neck cancer: II. Further follow‐up on the M.D. Anderson TAMVEC randomized clinical trial. International Journal of Radiation Oncology, Biology, Physics 1983;9:1261‐5. [DOI] [PubMed] [Google Scholar]
- Maor MH, Hussey DH, Barkley HT, Jesse RH, Fletcher GH. Further follow‐up on the M. D. Anderson trial on fast neutron therapy for head and neck cancer. International Journal of Radiation Oncology, Biology, Physics 1981;7(9):1212‐3. [Google Scholar]
- Maor MH, Hussey DH, Fletcher GH, Jesse RH. Fast neutron therapy for locally advanced head and neck tumors. International Journal of Radiation Oncology, Biology, Physics 1981;7(2):155‐63. [DOI] [PubMed] [Google Scholar]
Mishra 1996 {published data only}
- Mishra RC, Singh DN, Mishra TK. Post‐operative radiotherapy in carcinoma of buccal mucosa, a prospective randomized trial. European Journal of Surgical Oncology 1996;22(5):502‐4. [DOI] [PubMed] [Google Scholar]
Nissenbaum 1984 {published data only}
- Nissenbaum M, Browde S, Bezwoda WR, Moor NG, Derman DP. Treatment of advanced head and neck cancer: multiple daily dose fractionated radiation therapy and sequential multimodal treatment approach. Medical & Pediatric Oncology 1984;12(3):204‐8. [DOI] [PubMed] [Google Scholar]
Noel 1997 {published data only}
- Noel G, Feuvret L, Coeffic D, Germain I, Mazeron JJ. Combined postoperative radiotherapy and weekly cisplatin infusion for locally advanced head and neck carcinoma: final report of a randomized trial. Cancer/Radiotherapie 1997;1(3):265‐6. [Google Scholar]
Noel 1997a {published data only}
- Noel G, Feuvret L, Coeffic D, Germain I, Mazeron JJ. Five‐year update of a randomized trial alternating radiotherapy and chemotherapy compared with radiotherapy in treatment of unresectable squamous cell carcinoma of the head and neck. Cancer/Radiotherapie 1997;1(3):262‐3. [Google Scholar]
Noel 1997b {published data only}
- Noel G, Feuvret L, Coeffic D, Germain I, Mazeron JJ. Randomised multicentre trials of CHART vs conventional radiotherapy in head and neck and non‐small cell lung cancer: An interim report. Cancer/Radiotherapie 1997;1(3):263‐4. [Google Scholar]
Noel 2001 {published data only}
- Noel G, Mazeron JJ. Randomized clinical trial on 7‐day continuous accelerated irradiation (CAIR) of head and neck cancer. Report on 3‐year tumour control and normal tissue toxicity. Cancer/Radiotherapie 2001;5(2):207‐8. [DOI] [PubMed] [Google Scholar]
Rink 1989 {published data only}
- Rink B, Neumann HJ, Kuchler I. Clinical experience with different modes of preoperative radiotherapy in advanced carcinomas of the tongue and the floor of the mouth. Deutsche Zeitschrift fur Mund‐, Kiefer‐, und Gesichts‐Chirurgie 1989;13(4):286‐90. [PubMed] [Google Scholar]
Robertson 1998 {published data only}
- Robertson AG, Soutar DS, Paul J, Webster M, Leonard AG, Moore KP, et al. Early closure of a randomized trial: surgery and postoperative radiotherapy versus radiotherapy in the management of intra‐oral tumours. Clinical Oncology 1998;10:155‐60. [DOI] [PubMed] [Google Scholar]
Sanchiz 1990 {published data only}
- Sanchiz F, Milla A, Torner J, Bonet F, Artola N, Carreno L, et al. Single fraction per day versus two fractions per day versus radiochemotherapy in the treatment of head and neck cancer. International Journal of Radiation Oncology, Biology, Physics 1990;19(6):1347‐50. [DOI] [PubMed] [Google Scholar]
Singh 1984 {published data only}
- Singh R, Rai G, Singh SK, Tandon VK, Bhadury S, Shrivastava M, et al. Fractionation study on 3f/wk or 5f/wk treatment of oral carcinoma. Indian Journal of Radiology and Imaging 1984;38(2):153‐9. [Google Scholar]
Snow 1981 {published data only}
- Marcial VA, Gelber R, Kramer S, Snow JB, Davis LW, Vallecillo LA. Does preoperative irradiation increase the rate of surgical complications in carcinoma of the head and neck? A Radiation Therapy Oncology Group Report. Cancer 1982;49:1297‐1301. [DOI] [PubMed] [Google Scholar]
- Snow JB, Gelber RD, Kramer S, Davis LW, Marcial VA, Lowry LD. Comparison of preoperative and postoperative radiation therapy for patients with carcinoma of the head and neck. Interim report. Acta Otolaryngologica 1981;91(5‐6):611‐26. [DOI] [PubMed] [Google Scholar]
Srivastava 2001 {published data only}
- Srivastava K, Srivastava M. Concomitant boost radiotherapy vs conventional radiotherapy in advanced oral cavity and oropharynx cancers. Indian Journal of Radiology and Imaging 2001;11(3):127‐30. [Google Scholar]
Strong 1978 {published data only}
- Strong MS, Vaughan CW, Kayne HL, Aral IM, Ucmakli A, Feldman M, et al. A randomized trial of preoperative radiotherapy in cancer of the oropharynx and hypopharynx. American Journal of Surgery 1978;136(4):494‐500. [DOI] [PubMed] [Google Scholar]
Suwinski 2008 {published data only}
- Suwinski R, Bankowska‐Wozniak M, Majewski W, Idasiak A, Maciejewski A, Ziolkowska E, et al. Randomized clinical trial on 7‐days‐a‐week postoperative radiotherapy for high‐risk squamous cell head and neck cancer. Radiotherapy & Oncology 2008;87(2):155‐63. [DOI] [PubMed] [Google Scholar]
- Suwinski R, Bankowska‐Wozniak M, Majewski W, Sowa A, Galwas K, Ziolkowska E, et al. A randomized clinical trial on continuous 7‐day‐a‐week postoperative radiotherapy in high‐risk squamous cell head and neck cancer: a preliminary report on acute normal tissue reactions. International Journal of Radiation Oncology, Biology, Physics 2003;57(2 Suppl):S403. [Google Scholar]
- Suwinski R, Bankowska‐Wozniak M, Majewski W, Sowa A, Idasiak A, Ziolkowska E, et al. Randomized clinical trial on continuous 7‐days‐a‐week postoperative radiotherapy for high‐risk squamous cell head‐and‐neck cancer: a report on acute normal tissue reactions. Radiotherapy & Oncology 2005;77(1):58‐64. [DOI] [PubMed] [Google Scholar]
- Suwinski R, Jaworska M, Nikiel B, Bankowska‐Wozniak M. E22 ‐ Randomized clinical trial on 7‐days‐a‐week postoperative radiotherapy for high risk head and neck cancer: factors influencing loco‐regional control. Strahlentherapie und Onkologie 2007;183(Suppl 2):81‐3. [Google Scholar]
Tupchong 1991 {published data only}
- Tupchong L, Scott CB, Blitzer PH, Marcial VA, Lowry LD, Jacobs JR, et al. Randomized study of preoperative versus postoperative radiation therapy in advanced head and neck carcinoma: long‐term follow‐up of RTOG study 73‐03. International Journal of Radiation Oncology, Biology, Physics 1991;20(1):21‐8. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Ang 2007 {published data only}
- Ang K, Pajak T, Rosenthal DI, Nguyen F, Lu C, Kim H, et al. A phase III trial to compare standard versus accelerated fractionation in combination with concurrent cisplatin for head and neck carcinomas (RTOG 0129): report of compliance and toxicity. International Journal of Radiation Oncology, Biology, Physics 2007;69(3 Suppl 1):S12‐3, Abs No 21. [Google Scholar]
Ghosh 2006 {published data only}
- Ghosh S, Agarwal JP, Bhutani R, Vora A, Prabhas K, D'Cruz A, et al. Randomized trial of conventional fractionated RT (CFRT) vs. concomitant chemo radiotherapy (CTRT) and accelerated radiotherapy (ACRT) in patients with advanced, non nasopharyngeal, squamous cell cancers of the head and neck region. International Journal of Radiation Oncology, Biology, Physics 2006;66(3):S191. [Google Scholar]
Nutting 2009a {published data only}
- Nutting C, A'Hern R, Rogers MS, Sydenham MA, Adab F, Harrington K, et al. First results of a phase III multicenter randomized controlled trial of intensity modulated (IMRT) versus conventional radiotherapy (RT) in head and neck cancer (PARSPORT: ISRCTN48243537; CRUK/03/005). Proceedings from the 45th American Society of Clinical Oncology (ASCO) Annual Meeting, Orlando, FL, USA, May 29‐June 2 2009. Journal of Clinical Oncology 2009;27(15S):Abs No LBA6006. [Google Scholar]
Rodrigo 2004 {published data only}
- Rodrigo JP, Maseda E, Maldonado M, Aldama P, Puente M, Llorente JL, et al. Efficacy of postoperative radiation therapy for squamous cell carcinoma of the head and neck: results of a prospective randomised clinical trial. Acta Otorrinolaringologica Espanola 2004;55(9):415‐9. [DOI] [PubMed] [Google Scholar]
Rosenthal 2006 {published data only}
- Peters LJ, Goepfert H, Ang KK, Byers RM, Maor MH, Guillamondegui O, et al. Evaluation of the dose for postoperative radiation therapy of head and neck cancer: first report of a prospective randomized trial. International Journal of Radiation Oncology, Biology, Physics 1993;26(1):3‐11. [DOI] [PubMed] [Google Scholar]
- Rosenthal DI, Peters LJ, Morrison WH, Garden AS, Oswald MJ, El‐Naggar A, et al. The final report of a prospective randomized trial to evaluate the dose‐response relationship for postoperative radiation therapy (PORT) and pathologic risk groups in patients with head and neck cancer (HNC). International Journal of Radiation Oncology, Biology, Physics 2006;66(3):S411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skladowski 2001 {published data only}
- Skladowski K, Golen M, Wygoda A, Przeorek W, Sygula M, Pilecki B, et al. What is new with ,,CAIR" fractionation? ‐ A next Gliwice trial on 7 fractions in 7 days versus 7 fractions in 5 days is undergoing. In: Kogelnik HD, Lukas P, Sedlmayer F editor(s). Progress in Radio‐Oncology VII, Proceedings of the 7th International Meeting on Progress in Radio Oncology ‐ ICRO/OGRO 7. Italy: Monduzzi Editore, 2002:459‐64. [Google Scholar]
- Skladowski K, Hutnik M, Wygoda A, Golen M, Pilecki B, Rutkowski T, et al. Randomised clinical trial on two accelerated radiation treatments for patients with head and neck cancer. First report on early results of Cair‐2 study. Strahlentherapie und Onkologie 2007;183:A07. [Google Scholar]
- Skladowski K, Hutnik M, Wygoda A, Sasiadek W, Rutkowski T, Golen M, et al. Two accelerated radiation treatments have produced identical acute mucosal toxicity profile. An interim report on CAIR‐2 phase III trial for head and neck cancer patients. Radiotherapy & Oncology 2007;82:235. [Google Scholar]
- Skladowski K, Hutnik M, Wygoda A, Sygula M, Golen M, Pilecki B, et al. 7 fractions in 7 days versus 7 fractions in 5 days ‐ an interim report on early treatment tolerance in CAIR‐2 randomized clinical trial for head and neck cancer patients. Radiotherapy & Oncology 2004;73:S16‐7. [Google Scholar]
- Skladowski K, Wygoda A, Maciejewski A, Rutkowski T, Poltorak S, Maciejewski B. Definitive accelerated radiotherapy for head and neck cancer patients ‐ The results of organ preservation and prognostic‐factor analysis of CAIR‐2 phase III trial. Oral Oncology 2009:198.
- Skladowski KA, Hutnik M. Sublethal‐damage‐repair and clonogen repopulation during accelerated radiation therapy of head and neck cancer ‐ The biological interpretation of CAIR‐2 trial. International Journal of Radiation Oncology, Biology, Physics 2009;75(3):S180. [Google Scholar]
- Skladowski KA, Hutnik M, Rutkowski T, Golen M, Wygoda A, Heyda A, et al. Weekend breaks have no impact on the outcome of head and neck cancer when definitive radiotherapy is accelerated‐results of phase III clinical trial (CAIR‐2). International Journal of Radiation Oncology, Biology, Physics 2008;72(1):S97. [Google Scholar]
- Skladowski KA, Hutnik M, Wygoda A, Sasiadek W, Golen M, Rutkowski T, et al. Identity of acute effects in two accelerated radiation treatments: 7 fractions in 7 days versus 7 fractions in 5 days. An interim report on early tolerance of CAIR‐2 randomized clinical trial for head and neck cancer patients. International Journal of Radiation Oncology, Biology, Physics 2006;66(3 Suppl 1):S187. [Google Scholar]
References to ongoing studies
Moergel 2009 {published data only}
- Moergel M, Jahn‐Eimermacher A, Krummernauer F, Reichert TE, Wagner W, Wendt TG, et al. Effectiveness of adjuvant radiotherapy in patients with oropharyngeal and floor of mouth squamous cell carcinoma and concomitant histological verification of singular ipsilateral cervical lymph node metastasis (pN1‐state)‐‐a prospective multicenter randomized controlled clinical trial using a comprehensive cohort design. Trials 2009;10:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Attner 2010
- Attner P, Du J, Nasman A, Hammarstedt L, Ramqvist T, Lindholm J, et al. The role of human papillomavirus in the increased incidence of base of tongue cancer. International Journal of Cancer 2010;126:2879‐84. [DOI] [PubMed] [Google Scholar]
Bernier 2005
- Bernier J. Alteration of radiotherapy fractionation and concurrent chemotherapy: a new frontier in head and neck oncology?. Nature Clinical Practice. Oncology 2005;2(6):305‐14. [DOI] [PubMed] [Google Scholar]
Bernier 2006
- Bernier J, Bentzen SM. Radiotherapy for head and neck cancer: latest developments and future perspectives. Current Opinion in Oncology 2006;18(3):240‐6. [DOI] [PubMed] [Google Scholar]
Bjordal 1992
- Bjordal K, Kaasa S. Psychometric validation of the EORTC Core Quality of Life Questionnaire, 30‐item version and a diagnosis‐specific module for head and neck cancer patients. Acta Oncologica 1992;31(3):311‐21. [DOI] [PubMed] [Google Scholar]
Bourhis 2006
- Bourhis J, Overgaard J, Audry H, Ang K, Saunders M, Bernier J, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta‐analysis. Lancet 2006;368:843‐54. [DOI] [PubMed] [Google Scholar]
Conway 2008
- Conway DI, Petticrew M, Marlborough H, Berthiller J, Hashibe M, Macpherson LM. Socioeconomic inequalities and oral cancer risk: a systematic review and meta‐analysis of case‐control studies. International Journal of Cancer 2008;122(12):2811‐9. [DOI] [PubMed] [Google Scholar]
CRUK 2009
- Cancer Research UK. About chemotherapy for mouth cancer. http://www.cancerhelp.org.uk/help/default.asp?page=13205.
D'Souza 2007
- D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case‐control study of human papillomavirus and oropharyngeal cancer. New England Journal of Medicine 2007;356(19):1944‐56. [DOI] [PubMed] [Google Scholar]
Day 1992
- Day GL, Blot WJ. Second primary tumors in patients with oral cancer. Cancer 1992;70(1):14‐9. [DOI] [PubMed] [Google Scholar]
Day 2003
- Day TA, Davis BK, Gillespie MB, Joe JK, Kibbey M, Martin‐Harris B, et al. Oral cancer treatment. Current Treatment Options in Oncology 2003;4(1):27‐41. [DOI] [PubMed] [Google Scholar]
Deleyiannis 1997
- Deleyiannis FW, Weymuller EA Jr, Coltrera MD. Quality of life of disease‐free survivors of advanced (stage III or IV) oropharyngeal cancer. Head & Neck 1997;19(6):466‐73. [DOI] [PubMed] [Google Scholar]
Duchateau 2001
- Duchateau L, Pignon JP, Bijnens L, Bertin S, Bourhis J, Sylvester R. Individual patient‐versus literature‐based meta‐analysis of survival data: time to event and event rate at a particular time can make a difference, an example based on head and neck cancer. Controlled Clinical Trials 2001;22(5):538‐47. [DOI] [PubMed] [Google Scholar]
Duncan 1994
- Duncan W. An evaluation of the results of neutron therapy trials. Acta Oncologica 1994;33(3):299‐306. [DOI] [PubMed] [Google Scholar]
Faggiano 1997
- Faggiano F, Partanen T, Kogevinas M, Boffetta P. Socioeconomic differences in cancer incidence and mortality. In: Kogevinas M, Pearce N, Susser M, Boffetta P editor(s). Social Inequalities and Cancer. Lyon: IARC Scientific Publications No 138. International Agency for Research in Cancer, 1997. [PubMed] [Google Scholar]
Fakhry 2006
- Fakhry C, Gillison ML. Clinical implications of human papillomavirus in head and neck cancers. Journal of Clinical Oncology 2006;24(17):2606‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fakhry 2008
- Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus‐positive head and neck squamous cell carcinoma in a prospective clinical trial. Journal of the National Cancer Institute 2008;100(4):261‐9. [DOI] [PubMed] [Google Scholar]
Fanucchi 2006
- Fanucchi M, Khuri FD, Shin D, Johnstone PAS, Chen A. Update in the management of head and neck cancer. Update on Cancer Therapeutics 2006;1(2):211‐9. [Google Scholar]
Freedman 2007
- Freedman ND, Abnet CC, Leitzmann MF, Hollenbeck AR, Schatzkin A. Prospective investigation of the cigarette smoking‐head and neck cancer association by sex. Cancer 2007;110(7):1593‐601. [DOI] [PubMed] [Google Scholar]
Furness 2010
- Furness S, Glenny AM, Worthington HV, Pavitt S, Oliver R, Clarkson JE, et al. Interventions for the treatment of oral cavity and oropharyngeal cancer: chemotherapy. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD006386.pub2] [DOI] [PubMed] [Google Scholar]
Garg 2004
- Garg M, Beitler JJ. Controversies in management of the neck in head and neck cancer. Current Treatment Options in Oncology 2004;5(1):35‐40. [DOI] [PubMed] [Google Scholar]
Hammarstedt 2006
- Hammarstedt L, Lindquist D, Dahlstrand H, Romanitan M, Dahlgren LO, Joneberg J, et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. International Journal of Cancer 2006;119(11):2620‐3. [DOI] [PubMed] [Google Scholar]
Hammerlid 1997
- Hammerlid E, Bjordal K, Ahlner‐Elmqvist M, Jannert M, Kaasa S, Sullivan M, et al. Prospective, longitudinal quality‐of‐life study of patients with head and neck cancer: a feasibility study including the EORTC QLQ‐C30. Otolaryngology Head and Neck Surgery 1997;116(6 Pt 1):666‐73. [DOI] [PubMed] [Google Scholar]
Harari 2005
- Harari PM. Promising new advances in head and neck radiotherapy. Annals of Oncology 2005;16 Suppl 6:vi13‐vi19. [DOI] [PubMed] [Google Scholar]
Hassan 1993
- Hassan SJ, Weymuller EA Jr. Assessment of quality of life in head and neck cancer patients. Head & Neck 1993;15(6):485‐96. [DOI] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.0.2 (updated September 2009). The Cochrane Collaboration 2009. Available from www.cochrane‐handbook.org.
Hindle 1996
- Hindle I, Downer MC, Speight PM. The epidemiology of oral cancer. British Journal of Oral and Maxillofacial Surgery 1996;34(5):471‐6. [DOI] [PubMed] [Google Scholar]
Koh 1994
- Koh WJ, Griffin TW, Laramore GE, Stelzer KJ, Russell KJ. Fast neutron radiation therapy. Results of phase III randomized trials in head and neck, lung, and prostate cancers. Acta Oncologica 1994;33(3):293‐8. [DOI] [PubMed] [Google Scholar]
La Vecchia 1997
- Vecchia C, Tavani A, Franceschi S, Levi F, Corrao G, Negri E. Epidemiology and prevention of oral cancer. Oral Oncology 1997;33(5):302‐12. [DOI] [PubMed] [Google Scholar]
Licitra 2006
- Licitra L, Perrone F, Bossi P, Suardi S, Mariani L, Artusi R, et al. High‐risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. Journal of Clinical Oncology 2006;24(36):5630‐6. [DOI] [PubMed] [Google Scholar]
Macfarlane 1995
- Macfarlane GJ, Zheng T, Marshall JR, Boffetta P, Niu S, Brasure J, et al. Alcohol, tobacco, diet and the risk of oral cancer: a pooled analysis of three case‐control studies. European Journal of Cancer. Part B, Oral Oncology 1995;31B(3):181‐7. [DOI] [PubMed] [Google Scholar]
McGurk 2005
- McGurk M, Chan C, Jones J, O'Regan E, Sherriff M. Delay in diagnosis and its effect on outcome in head and neck cancer. British Journal of Oral and Maxillofacial Surgery 2005;43(4):281‐4. [DOI] [PubMed] [Google Scholar]
Oliver 2007
- Oliver R, Clarkson JE, Conway D, Glenny AM, Macluskey M, Pavitt S, et al. Interventions for the treatment of oral and oropharyngeal cancers: surgical treatment. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006205.pub2] [DOI] [PubMed] [Google Scholar]
Parkin 1999
- Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. International Journal of Cancer 1999;80(6):827‐41. [DOI] [PubMed] [Google Scholar]
Parkin 2001
- Parkin DM. Global cancer statistics in the year 2000. Lancet Oncology 2001;2(9):533‐43. [DOI] [PubMed] [Google Scholar]
Parkin 2005
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: a Cancer Journal for Clinicians 2005;55(2):74‐108. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analysis of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Partridge 2000
- Partridge M, Li SR, Pateromichelakis S, Francis R, Phillips E, Huang XH, et al. Detection of minimal residual cancer to investigate why oral tumors recur despite seemingly adequate treatment. Clinical Cancer Research 2000;6(7):2718‐25. [PubMed] [Google Scholar]
Pavitt 2007
- Pavitt S, Clarkson JE, Conway D, Glenny AM, Macluskey M, Oliver R, et al. Interventions for the treatment of oral and oropharyngeal cancer: immunotherapy/biotherapy. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006845] [DOI] [PubMed] [Google Scholar]
Pignon 2000
- Pignon JP, Sylvester R, Bourhis J. Hyperfractionated and/or accelerated radiotherapy versus conventional radiotherapy for head and neck cancer. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002026] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pignon 2007
- Delbaldo C, Michiels S, Rolland E, Syz N, Soria JC, Chevalier T, Pignon J. Second or third additional chemotherapy drug for non‐small cell lung cancer in patients with advanced disease. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD004569.pub2] [DOI] [PubMed] [Google Scholar]
Robinson 2003
- Robinson KL, Macfarlane GJ. Oropharyngeal cancer incidence and mortality in Scotland: are rates still increasing?. Oral Oncology 2003;39(1):31‐6. [DOI] [PubMed] [Google Scholar]
Ryerson 2008
- Ryerson AB, Peters ES, Coughlin SS, Chen VW, Gillison ML, Reichman ME, et al. Burden of potentially human papillomavirus‐associated cancers of the oropharynx and oral cavity in the US, 1998‐2003. Cancer 2008;113(10 Suppl):2901‐9. [DOI] [PubMed] [Google Scholar]
Shah 1990
- Shah JP. Cervical lymph node metastases‐‐diagnostic, therapeutic, and prognostic implications. Oncology (Williston Park, NY) 1990;4(10):61‐9. [PubMed] [Google Scholar]
Sturgis 2007
- Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence. Cancer 2007;110(7):1429‐35. [DOI] [PubMed] [Google Scholar]
Trotti 2000
- Trotti A. Toxicity in head and neck cancer: a review of trends and issues. International Journal of Radiation Oncology, Biology, Physics 2000;47(1):1‐12. [DOI] [PubMed] [Google Scholar]
Trotti 2007
- Trotti A, Pajak TF, Gwede CK, Paulus R, Cooper J, Forastiere A, et al. TAME: development of a new method for summarising adverse events of cancer treatment by the Radiation Therapy Oncology Group. Lancet 2007;8:613‐24. [DOI] [PubMed] [Google Scholar]
Warnakulasuriya 2009
- Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncology 2009;45(4‐5):309‐16. [DOI] [PubMed] [Google Scholar]
WHO 1992
- WHO. ICD‐O. International Statistical Classification of Diseases and Related Health Problems, 1989 Revision. Geneva: World Health Organization, 1992. [Google Scholar]
Woolgar 2003
- Woolgar JA, Rogers SN, Lowe D, Brown JS, Vaughan ED. Cervical lymph node metastasis in oral cancer: the importance of even microscopic extracapsular spread. Oral Oncology 2003;39(2):130‐7. [DOI] [PubMed] [Google Scholar]


