Abstract
Background
Extemporaneous compounding is practiced globally by pharmacists to allow for dispensing of personalised doses of medicinal products not commercially available. Extemporaneous compounding must result in a product which is safe and effective. However, data on formulation and expiry of extemporaneous products may not be readily available. Pharmacists access various resources including compounding databases to obtain information on composition, preparation, and expiry of extemporaneous preparations.
Objectives
The aim of this study was to evaluate the type and frequency of extemporaneous compounding in hospital and community pharmacies in the Republic of Ireland (ROI) and to obtain contemporary information on compounding practices and resources used.
Methods
All community and hospital pharmacists registered with the Pharmaceutical Society of Ireland, were invited to participate in an on-line survey. The study was approved by the Royal College of Surgeons in Ireland (RCSI) research ethics committee.
Results
A total of 202 pharmacists responded to the survey, of which 145 were community-based, 52 hospital-based, and 5 practicing in both. On average, hospital and community pharmacists (n = 138) dispensed <2–10 prescriptions for extemporaneous products per month. Pharmacists reported compounding 13 different types of extemporaneous preparations. Of these, dermatological preparations and oral liquid formulations (OLFs) were most commonly compounded. Extemp.ie, an Irish compounding database, was the most frequently used resource for compounding guidance and product expiry.
Conclusions
The results of this study show that extemporaneous compounding is still practiced in hospital and community pharmacies in the ROI. The limited response of 4.6% obtained may reflect that extemporaneous compounding is concentrated in a relatively small number of pharmacies. There remains a clinical need for extemporaneous products in the ROI and extemporaneous compounding continues to be an invaluable skillset for pharmacists.
Keywords: Extemporaneous compounding, Hospital pharmacist, Community pharmacist, Compounding database, Expiry date, Survey
1. Introduction
Extemporaneous compounding is the science of preparing medicinal products for the treatment of a specific patient in the absence of an appropriate commercially available product.1,2 The practice of extemporaneous compounding typically involves the small scale manufacture of preparations, using raw materials or commercially available dosage forms to formulate a suitable dosage form at an appropriate strength for a specific patient.2
According to the World Health Organisation, the rational use of medicines has evolved into the quality use of medicines, which demands that appropriate medicines of quality, safety and efficacy be accessible to all patients at the right time, cost, dose and dosage form.3 Although commercially available medicines meet the therapeutic needs of most patients, lack of appropriate dosage forms, doses or strengths for certain patient groups is not uncommon.4, 5, 6, 7, 8 Notably, extemporaneously compounded medications are more frequently required in the pharmaceutical care of paediatric and geriatric patients. In the case of paediatric patients, licensed formulations may be unavailable. Unlicensed adult formulations may require manipulation to obtain an appropriate dose and/or an appropriate dosage form for oral administration.
Geriatric patients may also have difficulty in swallowing commercial solid dosage forms, due to poor or compromised swallowing reflexes.1 Extemporaneous compounding is also used in dermatological practice, where combinations of personalised topical dermatological preparations are compounded and dispensed by pharmacists to meet the clinical needs of individual patients.9 Additionally, extemporaneous compounding is warranted in many palliative care scenarios.10
Other reasons for the necessity to undertake extemporaneous compounding may include a patient's sensitivity or allergy to certain excipients and preservatives in available formulations, ongoing medicine shortages, and the discontinuation of medicines.11., 12., 13., 14., 15., 16 Extemporaneous compounding therefore has diverse applications and is critical for continuity of patient care and in optimizing treatment outcomes.
The quality of compounded preparations is ensured by adherence to the standards outlined in regulatory guidelines. In the Republic of Ireland, this is governed by the Pharmaceutical Society of Ireland (PSI), which specifies the standards required for ensuring the quality of extemporaneous products for the safety of patients.17 It is the responsibility of pharmacists to ensure the quality of extemporaneous preparations and that they possess the training to undertake extemporaneous compounding in accordance with acceptable compounding standards.18 Despite such measures, extemporaneously compounded products have inherent risks of quality and stability, as they have limited accompanying evidence-based studies to document stability, bioavailability, pharmacokinetics, pharmacodynamics, efficacy, and safety. Elevated risk is particularly seen in newborn infants, because they are more likely to be predisposed to adverse drug reactions due to their physiological immaturity.19,20 Liquid dosage forms prepared from commercially available medicines may contain preservatives; these excipients are considered to be largely inert in adults, but can lead to life threatening toxicity in paediatric patients if multiple doses of medications with these preservatives are employed.21,22 Reports have documented on the stability of individual extemporaneous preparations23,24; however, there remains a clear need for comprehensive stability studies and more detailed information on extemporaneous medicines as a collective.
The literature shows that variability exists with regard to the extent of extemporaneous compounding over time, and by jurisdiction. Crommelin and Bouwman-Boer, writing in 2016, describe the waxing and waning of the practice of extemporaneous compounding over time but point to a revival of the practice.25 A broad survey of hospitals in 11 European countries in 201226 had revealed that in most countries, compounded medicines for oral use were those most frequently dispensed in pharmacies, although individual compounded medicines varied considerably throughout. The authors concluded that there was little consistency of compounding practices in Europe and that there was a need for common legislation, professional organisations, and information sources. More recently, the extent of compounding in Latvia was reported by Kiseļova through analysis of sales volumes in 2017, with 280 of 384 pharmacies submitting a report of sales, mostly in the capital.27 In other work, during a period between 2011 and 2014, three out of ten prescriptions issued by German dermatologists were extemporaneous formulations.9 Few studies explore the extent of extemporaneous compounding outside of Europe. AlKhatib showed that extemporaneous compounding is a common element of pharmaceutical care in pharmacies in Jordan.28 Of 423 pharmacies surveyed, 51.7% practiced extemporaneous compounding, primarily as a result of prescriptions from dermatologists. The authors stated that, based on their findings, there was a need for standardization of compounded product formularies, for product quality testing, and to improve the consistency in estimation of expiration dates of compounded products. Challenges faced by developing nations, with large paediatric populations, were highlighted in a recent narrative review.29 The example of Indonesia was highlighted, where as many as 71.5% of the drugs administered to children across a period of 2014 were compounded preparations. Compounding is also practiced in veterinary contexts. In New Zealand, such compounding is still significant, yet is not necessarily carried out by pharmacists.30 Emphasis on a more collaborative approach involving pharmacists in the practice was endorsed.
Cognisant of the evolving landscape of compounding both nationally and worldwide, the aim of this study was to evaluate the type and frequency of extemporaneous compounding by pharmacists in hospital and community pharmacies in the Republic of Ireland, and to obtain contemporary information on compounding practices and resources used.
2. Methods
Ethics approval for the study was received from the RCSI Research Ethics Committee (ID 212516287).
2.1. Participants
All pharmacists registered on the PSI register and working in hospital and/or community pharmacies were invited to participate in this study. At the time of the study, this included 3635 community pharmacists and 726 hospital pharmacists. Currently, in Ireland, there are 1980 registered pharmacy businesses, 75 of which are hospital pharmacies.
2.2. Data collection
For the purposes of the study, we sought to evaluate extemporaneous compounding as already defined (1], and as intended for a specific patient. Although bulk compounding may occur in some countries,31,32 and its use for stock preparations has been described,31 such practices were not considered within our remit. A questionnaire was specifically developed, based on the aims of this study. The survey design was literature-informed1,28,33,34 and constructed to obtain relevant quantitative and qualitative information. It contained eleven questions, mainly in multiple choice style format (see Supplementary Material). Ten of the questions aimed to elicit quantitative information on the nature and extent of extemporaneous compounding in Ireland, while one open question sought to elicit participants' opinions on the practice. The questionnaire was developed by two of the authors (HA and SC), under the supervision of the other authors (ZR and JB, both Senior Lecturers who are pharmacists with PhD qualifications and previous experience conducting qualitative research). Informed consent was an integral part of the survey questionnaire, and such consent was thus obtained from all subjects involved in the study. The questionnaire was wholly anonymous and self-administered. Piloting of the questionnaire was performed by two of the authors, engaged in the work as part of their Master's theses. Circulation among colleagues at the hospitals in which they worked informed iterative development of the questionnaire instrument. Administration of the questionnaire was via an on-line platform created using Microsoft Forms (Microsoft 365), a link to which was sent out via email by a nominated gatekeeper to all participants. The work was conducted between 01/01/2022 to 30/06/2022.
2.3. Data analysis
Quantitative data was analysed using Microsoft Excel (2007), while qualitative data was analysed using a descriptive approach. Answers to the opinion question were coded; themes were generated, reviewed, and defined, using the following steps. In a first draft, the 110 responses to the free form question were sorted into categories. In order to encompass all opinions as specifically as possible in the first draft, 47 initial categories emerged, many with only minor differences from one another. From the 47 initial categories, 10 overarching categories could be defined. For the second draft, the 110 responses were sorted based on their position in each category.
3. Results
3.1. Demographics
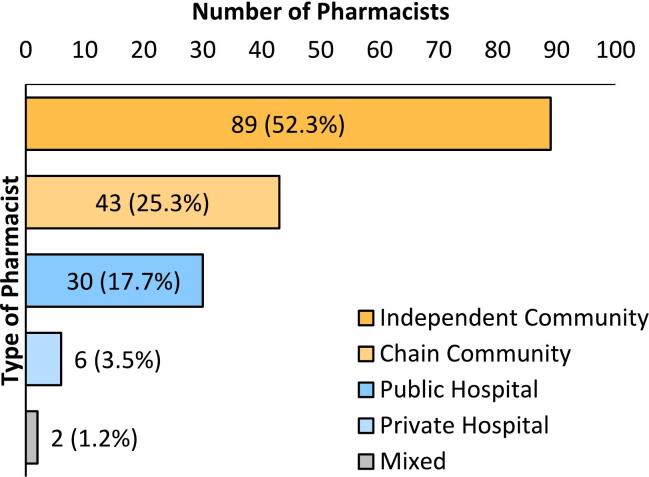
A total of 209 pharmacists completed the online survey. Of these respondents, 7 were excluded due to incomplete consent forms. The remaining 202 responses represented 4.6% of the 4361 pharmacists invited to participate. This included 145 (4%) of 3635 community pharmacists, 52 (7.2%) of 726 hospital pharmacists and 5 (0.1%) PSI registered pharmacists who practiced in two locations - hospitals and/or community pharmacies (Table 1). Of the 145 community pharmacists, 99 worked in independent community pharmacies and 46 worked in chain community pharmacies. Hospital pharmacist responders (n = 52) included 43 who worked in public hospitals and 9 who worked in private hospitals. The 5 respondents who worked across multiple areas of pharmacy (mixed) included 3 who worked in chain and independent community pharmacies, 1 who worked in public and private hospital pharmacies and 1 who worked in community and public hospital pharmacies. The community: hospital numbers of respondents was 145:52 (2.8:1 community: hospital pharmacists), significantly lower than the overall ratio of community: hospital pharmacists of 5:1 registered with the PSI.
Table 1.
Demographics of compounding and non-compounding pharmacist responders (% represents % of total (n = 202) responders).
| Pharmacist Type | Compounder | Non-compounder |
|---|---|---|
| Community Pharmacists | ||
| Independent (n = 99) | 89 (44%) | 10 (4.9%) |
| Chain (n = 46) | 43 (21.3%) | 3 (1.5%) |
| Hospital Pharmacists | ||
| Public (n = 43) | 30 (14.9%) | 13 (6.4%) |
| Private (n = 9) | 6 (3.0%) | 3 (1.5%) |
| Mixed-Practice Pharmacists | ||
| Mixed (n = 5) | 2 (1%) | 3 (1.5%) |
| Total | 170 (84.2%) | 32 (15.8%) |
Of the 202 respondents, 170 (84.2%) prepared extemporaneous products on foot of a prescription (compounders, Fig. 1). Of these, 132 (89 independent:43 chain) worked in community pharmacy, 36 in hospital pharmacy, mostly (n = 30) in public hospitals, while 2 respondents practiced across multiple types of pharmacies (1 chain-independent community, 1 chain community-public hospital).
Fig. 1.
Demographics of pharmacists who prepare extemporaneous products (compounders). Percentage displayed is that of total compounders (n = 170).
Of the 32 respondents who did not prepare extemporaneous products (non-compounders), 13 worked in community pharmacies (10:3 (3.3:1) independent: chain), 16 worked in hospital pharmacies (13:3 (4.3:1) public: private), and 3 worked in multiple types of pharmacies. Of the non-compounders in public hospital pharmacies, 3 cited the dispensing of unlicensed preparations procured from an unlicensed manufacturer (Supplemental Fig. S1).
3.2. Reasons for not compounding extemporaneous preparations
The dominant reason (n = 23, 71.9%) cited for not preparing extemporaneous preparations was the absence of receipt of any extemporaneous prescription (Rx), among both community and hospital pharmacists (Table 2). Others did not compound as a nearby pharmacy specialised in extemporaneous compounding, or due to unavailability of formulae or difficulty in compounding. Additional reasons cited for not compounding extemporaneous preparations were lack of material and/or equipment, lack of trained personnel, no reimbursement, and dispensing of unlicensed products / specials) (Table 2).
Table 2.
Reason(s) for NOT compounding extemporaneous preparations. Rx = prescription.
| Pharmacy Type | No Rx | Nearby Specializing pharmacy | Difficult procedure/ Unavailability of formula | Lack of materials/equipment | Lack of trained personnel | No reimburs-ement | Unlicensed products dispensed |
|---|---|---|---|---|---|---|---|
| Independent Community (n = 10) | 10 | 1 | 4 | 3 | 2 | ||
| Chain Community (n = 3) | 2 | 1 | 2 | 1 | 1 | ||
| Public Hospital (n = 13) | 6 | 1 | 2 | 1 | 2 | 3 | |
| Private Hospital (n = 3) | 3 | 1 | |||||
| Mixed (n = 3) | 2 | 2 | 1 | 2 | 2 | 1 | |
| Total | 23 | 6 | 9 | 7 | 5 | 3 | 3 |
3.3. Type of extemporaneous preparations compounded
The monthly average number of prescriptions for extemporaneous preparations received by compounding pharmacists over the past six months prior to the survey is shown in Tables 3 and S2. On average, hospital and community pharmacists (n = 138) received <2–10 prescriptions for extemporaneous products per month.
Table 3.
Average number of prescriptions received for extemporaneous compounding per month by hospital and community pharmacists in the Republic of Ireland. (n = 168).
| Pharmacist Type | Average Number of Extemporaneous Prescriptions per Month |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | <1 | <2 | <3 | 3–5 | 6–10 | 11–20 | 21–50 | 51–100 | |
| Community (n = 132) | 4 | 10 | 30 | 35 | 31 | 16 | 4 | 1 | 1 |
| Hospital (n = 34) | 3 | 1 | 7 | 5 | 4 | 9 | 3 | 1 | 1 |
| Mixed (n = 2) | 1 | 1 | |||||||
| Total | 7 | 12 | 37 | 41 | 35 | 25 | 7 | 2 | 2 |
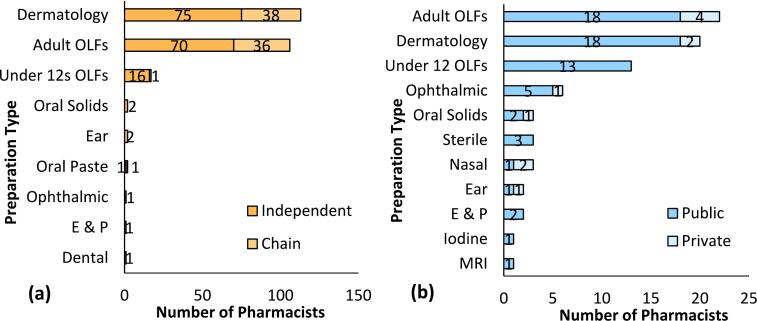
Regarding types of extemporaneous products, 13 different formulations were reported (Fig. 2a and b). Of these, 7 were prepared by both hospital and community pharmacists. These included dermatology preparations, adult and under-12 s' oral liquid formulations (OLFs), ophthalmic preparations, oral solid dosage forms, ear preparations, enemas and pessaries. OLFs and dermatology preparations were the two most commonly prepared types of extemporaneous product. Dermatology preparations were the most commonly prepared extemporaneous products by community pharmacists (n = 113), while adult OLFs were most commonly compounded by hospital pharmacists. Iodine, MRI drinks, nasal preparations and sterile parenteral preparations were reported only by hospital pharmacists, while oral pastes and dental preparations were reported only by community pharmacists. Little or no difference in types of compounding products, especially in dermatology and OLFs, were reported by independent and chain pharmacists or by public and private hospital pharmacists.
Fig. 2.
Types of extemporaneous preparations compounded by (a) community and (b) hospital pharmacists in the ROI. OLFs (Oral Liquid formulations), Oral solids (Oral solid formulations), Ear (Ear Preparations), E&P (Enemas and Pessaries), Dental (Dental Preparations). Ophthalmic (Ophthalmic Preparations), Sterile (Sterile Parenteral Preparations other than reconstituted products according to the SPC), Nasal (Nasal Preparations).
3.4. Frequency of dispensing different types of extemporaneous preparations
Pharmacists (n = 73) dispensed between 1- < 5 extemporaneous dermatology preparations per month, while n = 77 pharmacists reported dispensing 1- < 5 adult OLFs per month (Table 4). Only a small proportion of pharmacists reported the dispensing of higher numbers than these.
Table 4.
Frequency of dispensing of each type of extemporaneous product per month over the last 6 months. (n = 169).
| Type of Extemporaneous Product | Number of Pharmacists Reporting Monthly Dispensing Frequency |
|||
|---|---|---|---|---|
| <1 | <5 | 10–25 | 25+ | |
| Dermatology Preparations | 45 | 73 | 10 | 4 |
| Adults' Oral liquid formulations | 33 | 77 | 13 | 3 |
| Under-12 s' Oral liquid formulations | 10 | 15 | 3 | 5 |
| Ophthalmic preparations | 5 | 3 | 1 | 0 |
| Oral solid dosage forms | 3 | 3 | 1 | 0 |
| Ear preparations | 4 | 2 | 0 | 0 |
| Sterile parenteral products | 4 | 1 | 0 | 0 |
| Nasal preparations | 2 | 2 | 1 | 0 |
| Enemas and pessaries | 2 | 1 | 0 | 0 |
| Other | 4 | 2 | 1 | 1 |
3.5. Compounding information resources used by pharmacists
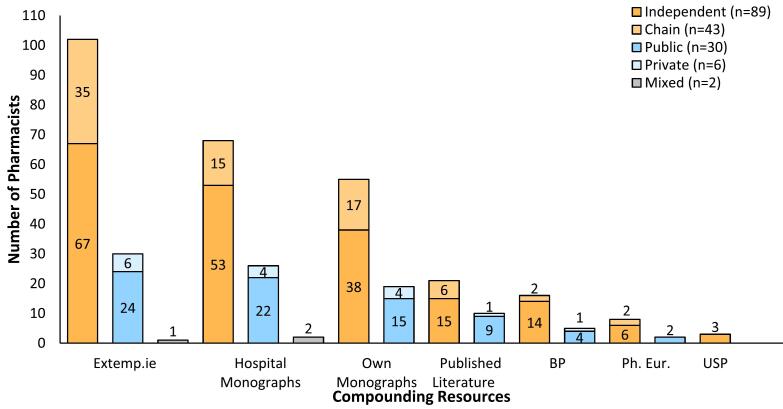
The most common resource for compounding information used by community and hospital pharmacists was the www.extemp.ie database, an Irish database established in 2006 (n = 133, Fig. 3).35 Hospital monographs and in-house monographs were the second-most frequently used resources. Pharmacists used published literature, including the Pharmaceutical Press Handbook of Extemporaneous Preparations. Pharmacists also accessed PubMed, and the United States, British and European Pharmacopoeias. Most pharmacists (n = 114, 67.1%) used multiple sources of technical information for compounding.
Fig. 3.
Technical information resources used by pharmacists to compound safely and accurately.
For assigning an expiry date to extemporaneous preparations, hospital and community pharmacists used the same resources as for compounding guidance. (Supplemental Fig. S2). A majority (n = 129) used the www.extemp.ie database, followed by hospital monographs or their own monographs, for assigning expiry dates.
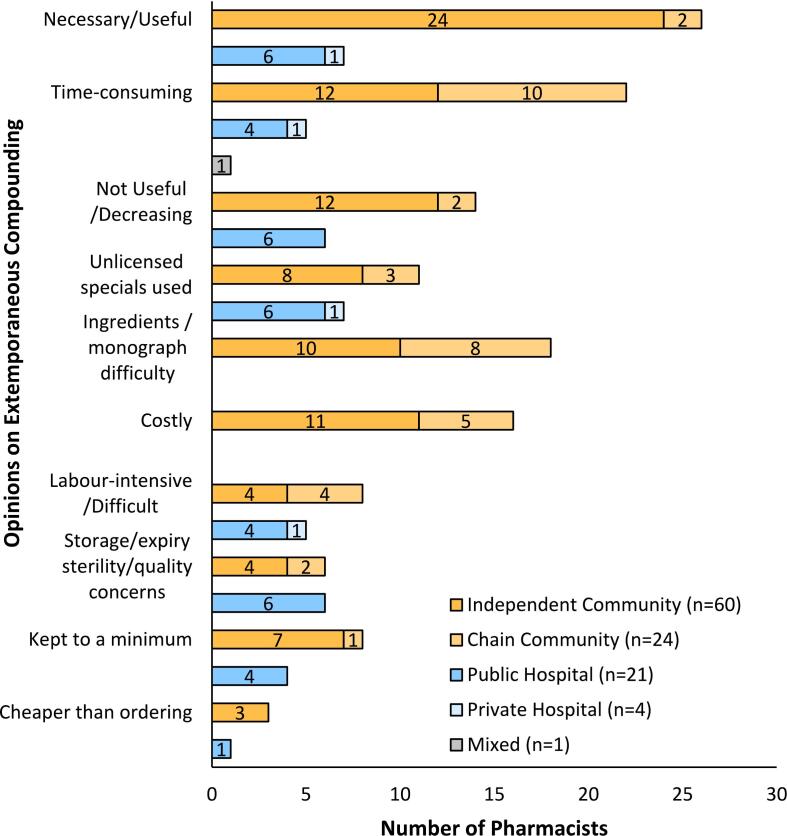
3.6. Pharmacists' opinions on extemporaneous compounding
The survey instrument contained a section seeking pharmacists' opinions on extemporaneous compounding (See Fig. 4). This was a ‘free text’ box on the questionnaire. A total of 110 compounding pharmacists expressed their opinion(s). Upon analysis, ten themes, hereafter referred to as opinions, emerged. Of these, 84 were from community pharmacists, 25 were from hospital pharmacists, and 1 from a pharmacist who worked in multiple areas of pharmacy.
Fig. 4.
Opinions on extemporaneous compounding from compounding community and hospital pharmacists in the Republic of Ireland.
Views of extemporaneous compounding as a necessary or useful aspect of full pharmaceutical care were the most commonly expressed opinions among both community (n = 26) and hospital (n = 7) pharmacists. These included the statements ‘Necessary’, ‘Useful’ ‘Important skill for a pharmacist’ and ‘Happy to compound’. The prevalence of opinions (n = 33, 30%) in the ‘Necessary/Useful’ category indicated that, among both hospital and community pharmacists in the ROI, the main opinion held about compounding was related to the needs of patients and was part of pharmaceutical care (Supplemental Fig. S3). ‘Time-consuming’ was the second-most common opinion held about extemporaneous preparation among respondents (n = 28, 25.5%). This was sometimes mentioned in conjunction with extemporaneous compounding being labour intensive and/or difficult to provide training in or to perform.
Twenty respondents (n = 20, 18%) reported that extemporaneous compounding was decreasing and/or was a practice that had become less useful than formerly. The use of unlicensed manufacturers to fulfil some, or all, of the extemporaneous prescriptions received was reported by 18 pharmacists; 8 reported that their use of such premade products had increased. The difficulty of sourcing ingredients and/or monographs was mentioned by 18 respondents. The cost of extemporaneous compounding, cost of materials required, low frequency of extemporaneous compounding, and delayed and/or insufficient reimbursement were concerns expressed by community pharmacists (n = 16) (Supplemental Fig. S3). One pharmacist mentioned that the skills required to prepare extemporaneous products were ‘not being maintained’, due to the rarity of extemporaneous prescriptions. Hospital pharmacists were less likely to express opinions about the cost of extemporaneous preparation.
Extemporaneous compounding was viewed as difficult or labour intensive by 13 respondents; 3 reported that staff disliked or lacked confidence in their ability to prepare extemporaneous products. Concerns about storage, expiry, sterility and/or quality of extemporaneous products were expressed by 12 pharmacists. Pharmacists (n = 12) reported that they kept extemporaneous compounding to a minimum. The preparation of extemporaneous compounding rather than ordering from unlicensed manufacturers was noted as being cheaper by 4 pharmacists.
3.7. Extemporaneous preparations for which pharmacists would like to have an improved formulation and/or stability data
Fifty pharmacists (n = 35 community, n = 14 hospital, n = 1 mixed practice) responded to an optional question which allowed them to identify up to 3 extemporaneous preparations for which they would like an improved formulation and/or stability data. A total of 38 different extemporaneous products were identified (Supplemental Fig. S4). Dermatology preparations (n = 45) and BMX mouthwash were the most common (n = 22) extemporaneous products identified as requiring revised formulation and/or stability data. Among the dermatology products, creams and ointments containing coal tar (n = 17) or betametasone (Betnovate®) (n = 15), and nipple creams and ointments (n = 9) had high demand for improved formulations and/or stability data. Fifty-eight hospital and community pharmacists also indicated their willingness to share extemporaneous monographs on the www.extemp.ie database.
4. Discussion
An on-line survey on extemporaneous compounding practices in the ROI was sent to all community and hospital pharmacists (4361) registered with the PSI. A total of 202 responses were obtained and used in this study, representing a response rate of 4.6%. The community: hospital number of respondents was 145:52 (2.8:1), significantly lower than the overall ratio of 5:1 community: hospital pharmacists registered with the PSI. No difference in providing extemporaneous compounding as part of patient pharmaceutical care was observed among pharmacists practicing in the different types of community or hospital pharmacies. Previously, we reported a high response rate of 79% when we surveyed hospital pharmacies in the Republic of Ireland regarding extemporaneous captopril liquid formulations.21 A high level of response (97%) was also reported by AlKhatib et al. in 2019, who surveyed a selected sample of 444 community and hospital pharmacists in Jordan regarding the practice of extemporaneous compounding.26 The higher responses in these two studies may be due to more targeted sampling, although we wished to obtain a national picture, hence our broader sampling approach.
Among those not providing extemporaneous compounding services, the main reason cited was the absence of receiving any prescriptions for extemporaneous preparations (n = 23, 71.9%), (Table 2). This was in line with previous literature.26,31,32 Under Irish and European legislation, compounding and supply of extemporaneous preparations occurs only when a pharmacist is presented with a prescription for such products, to fulfil the needs of an individual patient.15 Lack of equipment and supplies was also cited, aligning with previous work.28
Dermatological preparations and OLFs were the most commonly compounded preparations reported in our study, also similar to previous studies.28 Given potential variation in practices and formulations used, especially for those preparations most commonly encountered, the development of initiatives to optimise safety, accuracy, and communication has been advocated for.36
Obtaining accurate and accessible information on aspects of compounding is central to this aim. In this study, most compounding pharmacists (n = 133) reported use of the www.extemp.ie database, with 96 (56.5%) using hospital monographs and 74 (43.5%) using their own monographs. However, the number of pharmacists relying on either their own monographs or the published literature was similar to that reported by AlKhatib et al.28
Opinions of pharmacists on extemporaneous compounding were obtained as free-form text, which differed from previous literature that has used predefined answers derived or refined using a pilot group of pharmacists.26,31,32 A dominant theme emerging from the reported opinions was that extemporaneous compounding was deemed to be Necessary/Useful. AlKhatib et al. found that necessity, due to lack of an alternative commercially available medication, lack of a dosage form, or required dose, to be key reasons for extemporaneous compounding.28 A reduction in the practice was partially attributable to more widespread reliance on procurement from unlicensed manufacturers. This had been reported in a prior study of captopril liquid formulations by hospital pharmacies in Ireland.23
The free text format also allowed us to capture a more nuanced impression of extemporaneous compounding, with pharmacists reporting the time-consuming aspect and quality and safety risks of compounding. The impact of COVID-19 was also noted, as some responders noted a recent increase (‘within the past few years/since COVID’) in ordering from unlicensed manufacturers in lieu of compounding in the pharmacy setting. Analogous concerns emerged in a recent study of knowledge, perception, and practice of healthcare practitioners toward compounded medications, notably antimicrobial products, almost half of respondents believed that compounded medications might lack quality and have poor patient compliance.37 Additionally, a majority expressed concerns regarding potentially inappropriate preparation processes and under-dose administration of compounded medications, with a fear this might contribute to antimicrobial resistance. Whatever the trends in compounding, our study, and others, show that the practice seems destined to stay, in one guise or another. Reflecting on the evolving concept of personalised medicine, Beer et al. explored the opportunities and challenges presented by 3D printing technologies as a future face of compounding, in various scenarios.38 Although economic, ethical and organisational challenges were identified, the need to have pharmacist involvement at the centre of such initiatives was highlighted. We echo this sentiment and advocate for the continued emphasis on compounding as an expert role of the pharmacist, in both undergraduate education and dynamic practice,
4.1. Limitations
The low response rate of this study is a limiting factor, although anecdotally we know that compounding is mostly carried out by a limited number of specialised pharmacies, and therefore those responding may reflect this particular cohort.
5. Conclusions
Extemporaneous compounding remains a necessary part of pharmaceutical care in the ROI. The limited response of 4.6% obtained may reflect that extemporaneous compounding is concentrated in a relatively small number of pharmacies Dermatology preparations and OLFs were the most commonly compounded products reported. Pharmacists in the ROI reported the use of the www.extemp.ie database and hospital monographs as the predominant resource for guidance on extemporaneous compounding and expiry dates. Opinions of pharmacists on extemporaneous compounding reflected its continued importance in fulfilling prescriptions for patients in the absence of suitably licensed formulations, but also highlighted ongoing concerns regarding he time, cost, and quality considerations relevant to extemporaneous compounding. The identification of particular formulations for which stability and other data was felt to be deficient may inform future work, to support the needs of pharmacists, and ultimately, patients.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Zebunnissa Ramtoola: Conceptualization, Methodology, Validation, Formal analysis, Resources, Data curation, Writing – original draft, Writing – review & editing, Supervision, Project administration. Ayumi Catibusic: Validation, Formal analysis, Writing – original draft, Writing – review & editing. Hitam Ameen: Methodology, Formal analysis, Investigation, Writing – original draft. James W. Barlow: Conceptualization, Methodology, Validation, Resources, Writing – review & editing, Supervision, Project administration.
Declaration of Competing Interest
None.
Acknowledgments
The authors wish to acknowledge all the hospital and community pharmacists in the Republic of Ireland who participated in this study. The authors wish to acknowledge the contribution of Ms. Sarah Cullen in carrying out the survey as part of her final year thesis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rcsop.2023.100380.
Appendix A. Supplementary data
Supplementary material
Data availability statement
Research data is available upon request.
References
- 1.Falconer J.R., Steadman K.J. Extemporaneously compounded medicines. Aust Prescr. 2017;40(1):5–8. doi: 10.18773/austprescr.2017.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell E.H., Elston D.M., Straughan C.L. A review of the clinical indications, general principles and techniques related to compounding. J Am Acad Dermatol. 2020 Jul;83(1):179–183. doi: 10.1016/j.jaad.2019.10.038. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organisation Promoting rational use of medicines. https://www.who.int/activities/promoting-rational-use-of-medicines (accessed on May 19, 2023)
- 4.Tiengkate P., Lallemant M., Charoenkwan P., Angkurawaranon C., Kanjanarat P., Suwannaprom Borriharn P. Gaps in accessibility of pediatric formulations: a cross-sectional observational study of a teaching Hospital in Northern Thailand. Children (Basel) 2022;9(3):301. doi: 10.3390/children9030301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dooms M., Carvalho M. Compounded medication for patients with rare diseases. Orphanet J Rare Dis. 2018;13:1. doi: 10.1186/s13023-017-0741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiménez López Y., Pérez Cano E., Merino Almazán M., Claramunt García R., Sierra Torres M.I., Caba Porras I. 3PC-025 Magistral formulation for a patient with multiple food allergy. Eur J Hosp Pharm. 2020;27(suppl 1):A33. doi: 10.1136/ejhpharm-2020-eahpconf.72. [DOI] [Google Scholar]
- 7.Vanhoorne V., Peeters E., Van Tongelen I., et al. Pharmaceutical compounding of orphan active ingredients in Belgium: how community and hospital pharmacists can address the needs of patients with rare diseases. Orphanet J Rare Dis. 2019 Aug 1;14(1):186. doi: 10.1186/s13023-019-1154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner C., Adams V., Overley C. Alternate dosage formulations of oral targeted anticancer agents. J Oncol Pharm Pract. 2021 Dec;27(8):1963–1981. doi: 10.1177/10781552211037976. Epub 2021 Sep 24. PMID: 34558356. [DOI] [PubMed] [Google Scholar]
- 9.Staubach P., Salzmann S., Peveling-Oberhag A., et al. Extemporaneous formulations in Germany - relevance for everyday clinical practice. J Dtsch Dermatol Ges. 2018;16(5):566–574. doi: 10.1111/ddg.13519. [DOI] [PubMed] [Google Scholar]
- 10.Tayeb B.O., Winegarden J.A., Alashari R.A., et al. Scoping review of off-label topical analgesia in palliative, hospice and cancer care: Towards flexibility in evidence-based medicine. J Pain Res. 2021;14:3003–3009. doi: 10.2147/JPR.S263845. Published 2021 Sep 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Health Products Regulatory Authority Medicines Shortages. 2023. http://www.hpra.ie/homepage/medicines/medicines-information/medicines-shortages Accessed on May 24.
- 12.National Health Products Catalogue Medicine Shortages. https://nhpc.ie/medicine-shortages/ February 2023. (Accessed on May 10, 2023)
- 13.European Association of Hospital Pharmacists 2019 Medicines Shortages Survey. 2019. https://www.eahp.eu/sites/default/files/eahp_2019_medicines_shortages_report.pdf (Accessed on May 29, 2023)
- 14.European Medicines Agency Public information on medicine shortages, EMA shortages catalogue. https://www.ema.europa.eu/en/medicines/ema_group_types/ema_document-supply_shortage/field_ema_shortage_status/0?sort=field_ema_computed_date_field&order=desc Accessed on May 24, 2023.
- 15.U.S. Food and Drug Administration. Public information on medicine shortages, FDA Drug Shortages. https://www.accessdata.fda.gov/scripts/drugshortages/default.cfm (Accessed on May 24, 2023).
- 16.Jovanović Lješković N., Jovanović Galović A., Stojkov S., Jojić N., Gigov S. Medicine shortages in Serbia: Pharmacists’ standpoint and potential solutions for a non-EU country. Pharmaceutics. 2021;13(4):448. doi: 10.3390/pharmaceutics13040448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pharmaceutical Society of Ireland Guidance for Pharmacists on Extemporaneous Dispensing. Published 2015. https://www.thepsi.ie/Libraries/Folder_Pharmacy_Practice_Guidance/02_Guidance_for_Pharmacists_on_Extemporaneous_Dispensing_V1_0.sflb.ashx (Accessed May 23, 2023)
- 18.Pharmaceutical Inspection Co-operation Scheme Guide to Good Practices for the Preparation of Medicinal Products in Healthcare Establishments. Published 2014. https://picscheme.org/docview/3443 Accessed May 23, 2023.
- 19.Greenhalgh L.L., Passos M.M.B.D., Agrizzi A.L., Monteiro M.S.S.B. Compounded medications for cardiovascular use in neonatology: an integrative review. Rev Paul Pediatr. 2022 Sep 9;41 doi: 10.1590/1984-0462/2023/41/2021167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruggiero A., Ariano A., Triarico S., Capozza M.A., Ferrara P., Attinà G. Neonatal pharmacology and clinical implications. Drugs Context. 2019;8 doi: 10.7573/dic.212608. Published 2019 Oct 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glass B.D., Haywood A. Stability considerations in liquid dosage forms extemporaneously prepared from commercially available products. J Pharm Pharm Sci. 2006;9(3):398–426. [PubMed] [Google Scholar]
- 22.Valeur K.S., Holst H., Allegaert K. Excipients in neonatal medicinal products: never prescribed, commonly administered. Pharmaceut Med. 2018;32(4):251–258. doi: 10.1007/s40290-018-0243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pabari R.M., McDermott C., Barlow J., Ramtoola Z. Stability of an alternative extemporaneous captopril fast-dispersing tablet formulation versus an extemporaneous oral liquid formulation. Clin Ther. 2012;34(11):2221–2229. doi: 10.1016/j.clinthera.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Foley L, Toney J, Barlow JW, O'Connor M, Fitzgerald-Hughes D, Ramtoola Z. Investigation of the physical, chemical and microbiological stability of losartan potassium 5 mg/mL extemporaneous Oral liquid suspension. Molecules. n.d. [DOI] [PMC free article] [PubMed]
- 25.Crommelin D.J., Bouwman-Boer Y. Pharmacy preparations: Back in the limelight? Pharmacists make up your mind! Int J Pharm. 2016;514(1):11–14. doi: 10.1016/j.ijpharm.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 26.Carvalho M. UCL School of Pharmacy; London, UK: 2012. Extemporaneously Compounded Oral Medicines in European Hospital Pharmacies.https://discovery.ucl.ac.uk/id/eprint/1396480/1/PhD%20THESIS%20Maria%20Carvalho.pdf [Doctoral Dissertation] Accessed May 25 2023. [Google Scholar]
- 27.Kiseļova O., Mauriņa B., Šidlovska V., Zvejnieks J. The extent of extemporaneous preparation and regulatory framework of extemporaneous compounding in Latvia. Medicina (Kaunas) 2019 Aug 26;55(9):531. doi: 10.3390/medicina55090531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.AlKhatib H.S., Jalouqa S., Maraqa N., Ratka A., Elayeh E., Al Muhaissen S. Prevalence, determinants, and characteristics of extemporaneous compounding in Jordanian pharmacies. BMC Health Serv Res. 2019;19(1):816. doi: 10.1186/s12913-019-4684-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuliani S.H., Putri D.C.A., Virginia D.M., Gani M.R., Riswanto F.D.O. Prevalence, risk, and challenges of extemporaneous preparation for pediatric patients in developing nations: a review. Pharmaceutics. 2023 Mar 4;15(3):840. doi: 10.3390/pharmaceutics15030840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gargiulo D.A., Chemal C., Joda L., et al. Extemporaneous compounding in veterinary practice: a New Zealand perspective. N Z Vet J. 2013 Nov;61(6):311–315. doi: 10.1080/00480169.2013.773853. [DOI] [PubMed] [Google Scholar]
- 31.Gross Z. Why specials manufacturing units are needed now as much as they ever were. Pharm J. 2005;275(7380):743–746. [Google Scholar]
- 32.Aquilina A. The extemporaneous compounding of paediatric medicines at mater Dei hospital. J Malta Coll Pharm Pract. 2013;19:28–30. [Google Scholar]
- 33.Zaid A.N., Al-Ramahi R., Shahed Q., Saleh B., Elaraj J. Determinants and frequency of pharmaceutical compounding in pharmacy practice in Palestine. Int J Pharm Pract. 2012;20(1):9–14. doi: 10.1111/j.2042-7174.2011.00157.x. [DOI] [PubMed] [Google Scholar]
- 34.Buurma H., de Smet P.A., van den Hoff O.P., Sysling H., Storimans M., Egberts A.C. Frequency, nature and determinants of pharmacy compounded medicines in Dutch community pharmacies. Pharm World Sci. 2003;25(6):280–287. doi: 10.1023/b:phar.0000006521.41736.db. [DOI] [PubMed] [Google Scholar]
- 35.http://www.extemp.ie/ Accessed on May 29, 2023.
- 36.Smith T., Kokotajlo S., Lise J., Passerrello N., Weilnau J., Cash J. Advocacy Committee for the Pediatric Pharmacy Advocacy Group. Standardization of concentrations for extemporaneously compounded Oral liquid medications. J Pediatr Pharmacol Ther. 2019;Jul-Aug;24(4):327-329 doi: 10.5863/1551-6776-24.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Assefa D., Paulos G., Kebebe D., et al. Investigating the knowledge, perception, and practice of healthcare practitioners toward rational use of compounded medications and its contribution to antimicrobial resistance: a cross-sectional study. BMC Health Serv Res. 2022 Feb 23;22(1):243. doi: 10.1186/s12913-022-07649-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beer N., Hegger I., Kaae S., et al. Scenarios for 3D printing of personalized medicines - a case study. Explor Res Clin Soc Pharm. 2021 Sep 25;4 doi: 10.1016/j.rcsop.2021.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Research data is available upon request.