Abstract
Herbal supplements are widely used to enhance prostate health. These supplements may contain several types of plant sterols, vitamins, and minerals. By weight, however, plant sterols make up an abundant ingredient component, with saw palmetto extract or its primary component, beta-sitosterol, often comprising the most abundant sterol. Saw palmetto extract/beta-sitosterol has been shown to promote anti-tumorigenic processes in prostate cancer cells and rodent models of prostate cancer. It has also been shown to inhibit the 5α-reductase enzyme, thereby behaving similarly to finasteride and dutasteride, which are widely used to treat prostatic enlargement, or benign prostatic hyperplasia (BPH). The aim of this study is to critically examine in vitro, in vivo, and human clinical studies to assess the safety and clinical utility of herbal supplements containing saw palmetto extract/beta-sitosterol for prostate health. The results of this study suggest multiple mechanisms through which beta-sitosterol represses prostate cancer in vitro and in vivo, particularly through its pro-apoptotic effect on prostate epithelial cells. Multiple studies also show that beta-sitosterol significantly improves lower urinary tract symptoms (LUTS) associated with BPH, but to an extent that is generally less effective than that achieved by pharmaceutical grade alpha-adrenergic receptor antagonists or 5α-reductase inhibitors. This latter finding suggests that supplements containing beta-sitosterol might be most appropriate for younger men with minimal LUTS who don’t wish to embark on a clinical drug regimen for BPH treatment.
Keywords: Beta-sitosterol, saw palmetto, phytosterol, aza-steroid, herbal supplement, prostate cancer, benign prostatic hyperplasia, collagen
Introduction
Serenoa repens, or saw palmetto, is the sole species within the genus Serenoa, which itself is one of many genera within the family Arecaceae, commonly characterized as palms [1]. There are 730 species of American palms whose fruit, bark, sap, flowers, leaves, and roots are used variously as medicine, food, fuel, and other purposes [2,3]. A meta-analysis of American palm ethnomedicine lists 106 species of palm with known medical uses, and, of these, pharmacological analysis has demonstrated a few with proven efficacy for treating infection, diabetes, leishmaniasis, or prostatic hyperplasia [1]. In particular, Serenoa repens has emerged as a natural product for the treatment of prostatic disorders, particularly cancer and hyperplasia.
Herbal supplements containing saw palmetto extracts or its primary phytosterol component, beta-sitosterol, are widely used to enhance prostate health. In the U.S. alone, consumers spent $24,827,065 on supplements containing the phytosterol, beta-sitosterol, in 2020, a 52.3% increase in total sales from the previous year. Beta-sitosterol supplements ranked #18, and saw-palmetto, the primary source of beta-sitosterol, ranked #11, among top-selling herbal supplements in the U.S. The majority of these supplements were marketed for prostate health, followed by cardiovascular health and immune health [4]. Clearly, saw palmetto and beta-sitosterol herbal supplements enjoy wide usage among the American population.
Herbal supplements intended to enhance prostate health may contain several types of sterols, vitamins, and minerals. By weight, however, plant sterols make up an abundant ingredient component, with saw palmetto extract or its primary component, beta-sitosterol, often comprising the most abundant sterol. Publicly available analyses of the plant sterol composition of several prostate herbal supplements using data publicly available from independent laboratories, such as Eurofins and Labcorp (formerly Covance), demonstrates a wide range, by weight, for the most common plant sterols in these supplements - campesterol, stigmasterol, beta-sitosterol, brassicasterol, and human cholesterol (https://www.prostatepillreport.com/prostate-pill-lab-reports.php). Among these, beta-sitosterol was the major plant sterol component, accounting for 42.4%-78.0% of the total plant sterol content (Table 1).
Table 1.
Prostate supplement plant sterol compositions
| Prostagenix, 3 capsules (1.391 g) | Pros-T, 2 softgel (3.18 g) | Prostavar Ultra, 3 capsules (1.168 g) | Pros-terol 3 tablets (1.129 g) | Same composition | Vasotrexx, 3 capsules (0.836 g) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| ProstRX, 3 capsules (1.386 g) | Super Prostate 3X, 3 capsules (1.386 g) | ||||||
| Sterol (mg) | |||||||
| Cholesterol | <0.564 | 3.86 | 1.69 | 1.41 | 2.37 | 2.37 | 3.66 |
| Campesterol | 74.6 | 266 | 201 | 138 | 108 | 108 | 204 |
| Stigmasterol | 8.65 | 179 | 210 | 201 | 73.9 | 73.9 | 169 |
| Beta-sitosterol | 823 | 375 | 374 | 361 | 359 | 359 | 356 |
| Brassicasterol | <0.564 | 17.3 | 2.22 | 11.1 | 2.2 | 2.2 | 3.37 |
| Other | 149 | 44.1 | 42.8 | 44.6 | 34 | 34 | 63.6 |
| Total sterols | 1055.25 | 885.26 | 831.71 | 757.11 | 579.47 | 579.47 | 799.63 |
| % beta-sitosterol | 78.0% | 42.4% | 45.0% | 47.7% | 62.0% | 62.0% | 44.5% |
| Testing lab | Eurofins | Labcorp | Labcorp | Labcorp | Labcorp | Labcorp | Labcorp |
This data suggests that beta-sitosterol is the most prevalent phytosterol in prostate health herbal supplements. This is not by coincidence, as beta-sitosterol has been shown to inhibit the binding of the active form of testosterone, dihydrotestosterone (DHT), to the androgen receptor. As recently reviewed, DHT is the major growth factor of the prostate, and inhibition of DHT activity has been shown to slow the growth of prostate tumors and benign prostatic enlargement [5].
The aim of this study is to critically examine in vitro, in vivo, and human clinical studies to assess the safety and clinical utility of herbal supplements containing saw palmetto extract/beta-sitosterol for prostate health. It will examine the efficacy and mechanism(s) of action of beta-sitosterol for the treatment of prostate cancer and benign prostatic enlargement, with a focus on recent studies that contribute to advancing the scientific understanding of beta-sitosterol activity in the prostate.
Beta-sitosterol for the treatment of prostate cancer (PCa)
Because prostate cancer arises from epithelial malignancy, studies examining the effect(s) of beta-sitosterol on PCa focus on the glandular epithelial component of the prostate gland.
Beta-sitosterol promotes apoptosis of prostate cancer cells in vitro and in vivo
Early studies focused on the effect of beta-sitosterol on cultured prostate cancer cells. Awad et al. reported that PC-3 cells, an androgen-independent prostate cancer cell line isolated from a human prostatic adenocarcinoma metastatic to bone [6], demonstrated decreased invasiveness, motility, and laminin/fibronectin binding when treated with beta-sitosterol compared to cholesterol-treated cells. Moreover, PC-3 xenograft tumors in immunocompromised mice fed beta-sitosterol grew less well and produced fewer lymph node or lung metastases than those in cholesterol- or campesterol-fed mice [7]. Further studies from this group showed that beta-sitosterol induced apoptosis, increased basal prostaglandin levels, and increased reactive oxygen species (ROS) production in PC-3 cells. This study concluded that beta-sitosterol may inhibit tumor growth by stimulating apoptosis and promoting cell cycle arrest through mechanisms involving alterations in ROS and prostaglandin production [8].
Another study found that beta-sitosterol induced apoptosis in LNCaP cells, cell line derived from a metastatic lymph node lesion of human prostate cancer which is androgen receptor (AR) positive, potentially by activating the sphingomyelin cycle [9,10]. Pradhan et al. reported correlative studies that beta-sitosterol was cytotoxic, and induced apoptosis in PC-3 and DU-145 prostate cancer cells, the latter derived from a prostate cancer metastasis to the brain [11]. The pro-apoptotic activity was correlated with decreased expression of the anti-apoptotic protein, Bcl-2, and increased expression of the pro-apoptotic protein, Bax. Moreover, beta-sitosterol inhibited the migration and proliferation of PC-3 and DU-145 cells associated with up-regulation of E-cadherin expression. Petrangeli et al. reported early changes in membrane composition followed by reduced Akt phosphorylation and increased apoptosis in PC-3 cells treated with LSESr, a lipidosterolic extract of saw palmetto [12]. LSESr, also known as Permixon, is an over-the-counter supplement marketed for the treatment of prostatic hypertrophy with a phytosterol content that is 71.3% beta-sitosterol, 21.0% campesterol, and 8% stigmasterol [13].
Cole et al. pursued studies to determine whether the anti-tumor activities of beta-sitosterol observed for prostate cancer cells cultured in vitro would be replicated in prostate cancer spheroids and xenograft prostate tumors in vivo. Spheroids formed from 22Rv1 prostate cancer cells [14], PSC-100-021 vascular smooth muscle cells and HGF-1 dermal fibroblasts treated with a compound comprising isovanillin, lineolic acid, and beta-sitosterol demonstrated pronounced and selective prostate cancer cell death associated with increased caspase 6 levels, suggestive of apoptosis [15]. Moreover, PC3 cells xenografted sub-cutaneous into nude mice treated with the same compound formed significantly smaller tumors than those treated with vehicle [15]. Though the study did not examine the effects of beta-sitosterol independently of the other components of the compound, nor actually test caspase activation consequent to treatment, the results of the study are consistent with findings that beta-sitosterol may induced prostate cancer cell apoptosis.
Non-apoptotic beta-sitosterol-mediated anti-tumor activities
Pradhan et al. showed that beta-sitosterol down-regulated DNA methyltransferase (DNMT) and histone deacetylase 1/2 (HDAC1/2) protein levels and enzymatic activity, suggesting that it might promote chromatin remodeling, hence, epigenetic up-regulation of DNA expression. This study also associated beta-sitosterol with reduced expression of the inflammatory cytokines IL-6, IL1-β, TNF-α and IL-10, suggesting that it may modulate immune responses [16].
Lomenick et al. identified two proteins, 17β-HSD4 and E-Syt1, that could bind with high affinity beta-sitosterol, but not cholesterol. Because 17β-HSD4 catalyzes the second and third steps of peroxisomal β-oxidation, and that elevated 17β-HSD4 expression and activity as well as increased peroxisomal β-oxidation pathway activity have been found in prostate cancer tissues compared to normal prostate tissue, the authors posit that beta-sitosterol may exert an anti-tumorigenic effect by modulating 17β-HSD4 activity in prostate cancer cells [17]. The precise function of the E-Syt1 protein is unclear but it is located in the endoplasmic reticulum and may be involved in lipid transport, and its interactions with G-proteins are associated with increased prostate cancer cell migration and invasion in vitro [17]. However, neither of these activities enhanced by beta-sitosterol have been definitively shown to provide therapeutic potential for prostate cancer treatment.
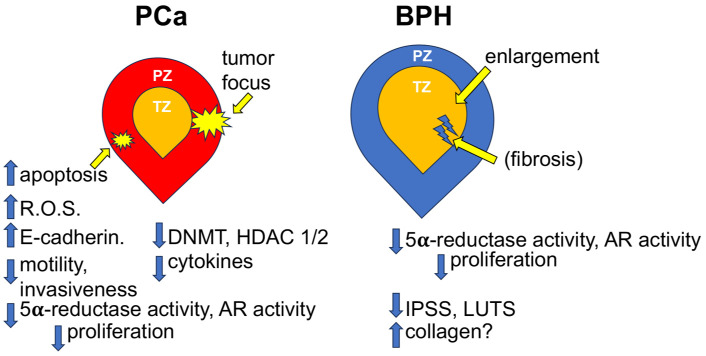
Taken together, these studies suggested potentially multiple roles for beta-sitosterol repression of prostate cancer in vitro and in vivo, particularly with regard to its pro-apoptotic effect on prostate epithelial cells. A summary diagram of the molecular and phenotypic effects of beta-sitosterol on PCa is provided in Figure 1.
Figure 1.
Diagram depicting the molecular and phenotypic effects of beta-sitosterol on prostate cancer (PCa) and benign prostatic hyperplasia (BPH). PZ, peripheral zone of the prostate; TZ, transition zone of the prostate; R.O.S., reactive oxygen species; DNMT, DNA methyltransferase; HDAC, Histone deactylase; AR, androgen receptor; IPSS, International Prostate Symptom Score; LUTS, Lower Urinary Tract Symptoms.
Beta-sitosterol for the treatment of benign prostatic hyperplasia (BPH)
Lower urinary tract dysfunction in men arises from pathobiologies of the epithelial, smooth muscle, and stromal compartments
Lower urinary tract symptoms (LUTS) are a costly and critical medical problem for millions of aging men. This spectrum disorder encompasses symptoms such as weak stream, nocturia, incomplete emptying and intermittent urination, all of which are indicative of lower urinary tract dysfunction (LUTD). If left untreated or treated ineffectively, LUTD can progress to bladder dysfunction, which can lead to urinary retention, recurrent UTI, bladder calculi, and, eventually, renal impairment. LUTD may arise from Benign Prostatic Hyperplasia (BPH) - a proliferative but nonmalignant enlargement of the prostate, and/or excessive and uncontrolled smooth muscle activity. Surgical ablation of prostate tissue and medical approaches including the use of 5α-reductase inhibitors (finasteride, dutasteride) to address epithelial over-proliferation, or α-adrenergic receptor antagonists (doxazosin, tamsulosin) to control smooth muscle activity, are used to improve urinary flow [18,19]. Recent studies have shown that expansion of the stromal component of the prostate concurrent with increased collagenization was recently recognized as a pathobiology also contributing to LUTS [20]. Therefore, the effects of beta-sitosterol on the epithelial and stromal components of prostate tissue will be considered.
Beta-sitosterol inhibits dihydrotestosterone (DHT) binding to the androgen receptor (AR) and reduces epithelial proliferation in vitro and in vivo
The human prostate requires androgenic steroid hormones for proper development, structural integrity, and function [5]. Testosterone, a testicular androgen, is converted to its active form, dihydrotestosterone (DHT), by the 5α reductase enzyme. DHT binds to the androgen receptor (AR), which then dimerizes in the cellular cytoplasm and is transported into the nucleus, where it acts as a transcription factor and binds to androgen response elements in gene promoter or enhancer regions to induce or repress the expression of a multitude of genes [5]. Both prostatic luminal epithelium and stromal fibroblast cells express the AR and are responsive to androgens. Although the precise mechanisms involved have not been completely elucidated, the expression of AR-regulated genes in both the epithelial and stromal compartments in the aging prostate, and the crosstalk between these compartments with inflammatory cells which accumulate in the aging prostate, often results in prostatic enlargement and consequent LUTS.
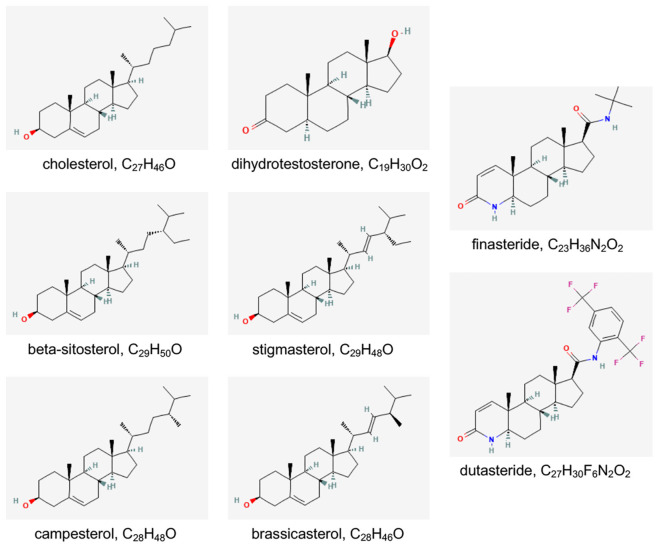
Identification of the role(s) of DHT and the AR in the development and progression of BPH provided rationale for investigators to test and utilize 5α-reductase inhibitors that could promote the reduction of prostatic enlargement and alleviation of LUTS. The mechanism of action of 5α-reductase inhibitors, such as finasteride or dutasteride, is essentially in their name: dutasteride is a Type I/II 5α-reductase enzyme inhibitor, while finasteride is a Type II 5α-reductase enzyme inhibitor. They are competitive inhibitors of 5α-reductase and inhibit the enzyme’s ability to convert testosterone into its active form, dihydrotestosterone (DHT). Chemically, finasteride and dutasteride are steroid derivatives, specifically, aza-steroids, and structurally resemble cholesterol and DHT (Figure 2). Importantly, the plant sterols most commonly included in prostate health supplements - beta-sitosterol, stigmasterol, campesterol, and brassicasterol - also chemically resembles cholesterol, DHT, finasteride, and dutasteride, and, as may be expected, share some molecular and cellular activities with these compounds, include binding to the androgen receptor (AR) in prostate epithelial cells (Figure 2).
Figure 2.
Two-dimensional structure and molecular formula for cholesterol, dihydrotestosterone, beta-sitosterol, finasteride, and dutasteride. Images and data are from PubChem, an open chemistry database at the National Institutes of Health (NIH) (https://pubchem.ncbi.nlm.nih.gov/) [51].
Early studies identified 5α-reductase as a binding partner for components of LSESr, also known as Permixon, a lipidosterolic extract of saw palmetto. The first study, published by Sultan et al. in 1984, reported that this extract was a competitive inhibitor for DHT-binding to the androgen receptor, and inhibited 5α-reductase, the enzyme that converts testosterone to its active form, DHT, in cultured human prostate fibroblasts [21]. That same year, Carilla et al. (with Sultan as a co-author) published that Permixon, a liposterolic extract of saw palmetto, inhibited the binding of R-1881, or synthetic androgen, to the cytosolic androgenic receptor of homogenized rat ventral prostate [22]. As noted above, the major phytosterol component of Permixon is beta-sitosterol [13]. Delos et al. elucidated that LSESr (Permixon) acted as a non-competitive inhibitor of Type I 5α-reductase in DU145 cells in vitro [23], while Bayne et al. showed that this same compound inhibited both Type I and II 5α-reductase isoforms in a co-culture model of primary epithelial and fibroblast cells grown from BPH tissue [24]. Together, these studies provided data that since has been applied to foster the use of saw palmetto extract, particularly the beta-sitosterol component, as a major treatment for BPH and alleviation of consequent lower urinary tract symptoms in men.
Based on these in vitro studies, several studies next examined whether plant phytosterols could inhibit testosterone induced BPH in rat models. Jena et al. prepared methanolic extracts from several Prunus species and identified beta-sitosterol as the primary plant phytosterol in all species. Male Wistar rats were fed various doses of extract, vehicle, or finasteride, and all but the negative control was also given 2 mg/kg testosterone subcutaneously, for 21 days. Upon sacrifice at day 22, body and prostate weights were determined, and histopathological, immunochemical, and biochemical studies were conducted. Rats treated with the Prunus extracts demonstrated substantial reduction of: Prostate and testis weights, prostatic hyperplasia, PCNA expression, and serum creatinine and testosterone levels. They also demonstrated restored antioxidant parameters and decreased inflammation [25]. The Prunus domestica extract, which contained the highest level of beta-sitosterol, produced the most dramatic reduction in BPH measures. Importantly, the anti-BPH effect of the beta-sitosterol-containing extracts was comparable to that of finasteride [25].
It should be noted that these in vivo studies measured the level of serum and, in some cases, prostatic, testosterone in control, phytosterol-treated, and finasteride-treated animals. Interestingly, phytosterol-, but not finasteride-, treatment was associated with decreased testosterone levels. Since both finasteride and phytosterols inhibit 5α-reductase activity, why weren’t testosterone levels similarly affected by these compounds? The answer may lie in the fact that phytosterols, but not finasteride, compete with cholesterol for intestinal absorption, and thereby lower both cholesterol absorption by the intestines and cholesterol concentrations in the plasma [26]. Because plant sterols reduce the amount of cholesterol that can enter the bloodstream, less cholesterol can be taken up by the Leydig cells in the testes; thus, less cholesterol can be converted to testosterone [27].
Buncharoen et al. studied the efficacy of 5α-reductase inhibition by petroleum ether, ethyl acetate, ethanol and aqueous extracts from the stem of Uvaria rufa. Of these, the ethanol acetate extract exhibited the highest potency of inhibition in vitro, and the active compound producing this effect was identified as beta-sitosterol. Male Wistar rats treated with 3 mg/kg testosterone for 30 days to induce BPH, then treated with this extract showed show a significant reduction in prostate weight, prostate index (calculated as the ratio of prostate weight to body weight), and androgen receptor levels in all treated groups when compared to the BPH model group. Histopathological analysis of the prostate glands from these animals showed that the BPH group demonstrated prostatic glandular enlargement and pronounced proliferation of prostate epithelium and polyp formation, all of which were reduced in the extract- and finasteride-treated animals [28].
Sudeep et al. tested the effects of beta-sitosterol enriched saw palmetto (VISPO) and conventional saw palmetto (SPO) oil on testosterone-induced BPH in male Wistar rats. The rats received testosterone (5 mg/kg) with or without SPO and VISPO (200 and 400 mg/kg body weight) or Finasteride (1 mg/kg body weight) for 28 days. VISPO exhibited superior efficacy compared to SPO as evident from the significant decrease in prostate weight to body weight ratio, serum testosterone level and increase in growth inhibition of prostate tissue compared to BPH group (P<0.001). Histological examination of prostate tissue samples showed that VISPO treatment was comparatively better than SPO in improving the hyperplastic patterns and was comparable to finasteride in this regard. Further, VISPO induced apoptosis and reduced the expression of inflammatory markers [29].
The effects of beta-sitosterol are not well defined for the stromal compartment of the rat prostate
The studies by Jena et al. [25], Buncharoen et al. [28], and Sudeep et al. [29] examined rat prostate glandular histopathology, but did not comment on changes in the non-epithelial, or stromal, tissue components of the prostate. Prahalada et al. [30] reported that daily administration of 20, 40, or 80 mg/kg finasteride to male Sprague-Dawley rats over a 1 year period significantly reduced the volumes of both the epithelial and stromal compartments of the prostates of these animals. This group performed a similar study in 1992 where Sprague-Dawley rats were treated with 80 mg/kg finasteride for 15 days and observed significantly decreased intraprostatic DHT and increased testosterone levels [31]. When treatment was extended out to 6 months, the prostate lobes exhibited significantly decreased weight and reduction in the total number of epithelial and stromal cells [31].
Justulin et al. found that Wistar rats treated with 25 mg/kg/day doxazosin for 30 days exhibited reduced epithelial proliferation, smaller prostates, and increased area of collagen and reticular fibers in the stromal space [32]. In a later study, this group treated adult male Wistar rats with vehicle or with finasteride + doxazosin for 3 or 30 days and examined the effects of these treatments on prostate tissue morphology, histology, and the expression of several matrix metalloproteases thought to be active in PCa metastasis [33]. Overall, combination treatment at 3 or 30 days resulted in decreased cellular proliferation and increased apoptotic index, largely in the epithelial compartment [33]. Notably, this study found that combination treatment at either 3 or 30 days promoted increased ventral lobe prostate collagen volume and reduced epithelial volume. Both collagen type I and type III fibers were significantly more numerous in the interstitial stromal tissues with combination treatment (P<.005). These studies concluded that changes in the epithelial compartments resulted in altered mechanical demands in the prostate and consequent increased collagen expression and tissue remodeling [32].
Despite the relative paucity of animal studies examining the effects of beta-sitosterol and finasteride on prostatic histology, there is overall agreement that aza-steroids reduced epithelial proliferation and/or the volume of the epithelial component of rat prostates induced to develop BPH. This is likely due to the aza-steroids’ ability to act as competitive inhibitors of 5α-reductase and thereby prevent the enzyme from converting testosterone into its active form, dihydrotestosterone (DHT). There is some evidence that they may induced apoptosis in the epithelial compartment as well. However, these studies are in far less agreement with regard to the effects of beta-sitosterol on the stromal compartment of the prostate. It isn’t clear if this discrepancy may be due to using different rat strains - Wistar vs. Sprague-Dawley - or to other factors that differed between experimental approaches, such as the use of different extracts, whether doxazosin was included in the treatment regimen, the length and dosage of treatment, and other variables.
Results of clinical trials examining the efficacy of phytosterols for the treatment of BPH
Based on the pre-clinical studies described above, the medical world began to assess the efficacy of saw palmetto extracts to improve BPH LUTS. The first large study was conducted in 1993 and published 2 years later by Berge’s et al. in The Lancet [34]. This randomized, double-blind, placebo-controlled multi-center study comprised 200 patients with symptomatic benign prostatic hyperplasia treated with either 20 mg of beta-sitosterol 3X per day or placebo for 6 months. This study found significant improvement in symptoms and urinary flow parameters that demonstrated efficacy of beta-sitosterol in the treatment of benign prostatic hyperplasia. Patients treated with beta-sitosterol demonstrated significantly reduced International Prostate Symptom Scores (IPSS), increased quality of life, decreased residual urinary volume, compared to those treated with placebo. Neither group experienced reduced prostatic volume [34].
Another study published in 2000 by Marks et al. tested a saw palmetto herbal blend lipoidal extract vs. placebo in 45 men with symptomatic BPH [35]. Like the Berges et al. study, no effects on prostate volume were observed in either group. However, prostate epithelial contraction was noted, especially in the transition zone, where percent epithelium decreased from 17.8% at baseline to 10.7% after 6 months of treatment with the saw palmetto herbal blend compared to placebo (P<0.01). This was accompanied by a significant increase, from 25.2% to 40.9%, in atrophic glands after treatment with the saw palmetto herbal blend compared to placebo (P<0.01), like that observed with finasteride treatment. This was accompanied by a notable but not statistically significant increase in stromal tissue. Changes in apoptosis, cellular proliferation, angiogenesis, growth factors or androgen receptor expression were not observed, though this data is not presented [35]. The authors concluded that saw palmetto herbal blend appeared to be a reasonable alternative for men with early, uncomplicated prostatism.
A very large trial, dubbed the PERMAL study, comprised of 811 BPH patients with IPPS≥10 was next published in 2002 by DeBruyn et al. Study participants were recruited in 11 European countries for a 12-month, double-blind randomized trial. 354 patients were randomly assigned to tamsulosin 0.4 mg per day and 350 to Permixon 320 mg per day. At 12 months, IPSS decreased by 4.4 in each group and no differences were observed in either irritative or obstructive symptom improvements. The increase in Qmax was similar in both treatment groups, and PSA remained stable while prostate volume decreased slightly in the Permixon-treated patients. The study concluded that Permixon and tamsulosin were equivalent in the medical treatment of lower urinary tract symptoms in men with BPH, during and up to 12 months of therapy [36].
A follow-up study to the PERMAL study sought to compare the efficacy of the lipido-sterolic extract of Serenoa repens, Permixon, to that of tamsulosin, in the treatment of a subset of PERMAL study participants who suffered from severe LUTS (IPSS>19) consequent to BPH. This was a double-blind, randomized study whereby 65 patients received Permixon 320 mg/day and 59 received tamsulosin 0.4 mg/day over a 12-month period. At 12 months, total IPSS decreased by 7.8 with Permixon and 5.8 with tamsulosin (P<0.051); irritative symptoms significantly improved (P<0.049) with Permixon (2.9 versus 1.9 with tamsulosin). The study concluded that Permixon 320 mg/day was slightly superior to tamsulosin 0.4 mg/day in reducing LUTS in severe BPH patients after 3 months and up to 12 months of treatment [37].
The first large American study was conducted in 2006 and reported in the New England Journal of Medicine [38]. This study conducted a double-blind trial that included 225 men over the age of 49 years who had moderate-to-severe symptoms of BPH. The subjects were treated for 12 months with saw palmetto extract (160 mg twice a day) or placebo. The results of the study showed no significant difference between the saw palmetto and placebo groups in the change in American Urological Association Symptom Index (AUASI) scores, maximal urinary flow rate, prostate size, residual volume after voiding, quality of life, or serum prostate-specific antigen levels. The study concluded that saw palmetto did not improve symptoms or objective measures of BPH. A later study randomized 99 men to receive 3% beta-sitosterol (VISPO), 2% (SPO) or placebo. This study found that men given VISPO experienced the most significant decrease in IPSS (P<0.001) among the 3 groups [39].
The TRIUMPH (TransEuropean Research into the Use of Management Policies for LUTS suggestive of BPH in Primary Healthcare) Study, a large European study, was also published in 2006. This prospective study profiled the use of alpha-adrenergic receptor antagonists (Tamsulosin, Doxazosin, Alfluzosin, Terazosin), 5α-reductase inhibitors (Finasteride), and phytotherapies derived from Serenoa repens and Pygeum africanum (both of which are rich sources of beta-sitosterol) as well as beta-sitosterol used to treat 2351 previously untreated BPH/LUTS patients from 6 European countries over a 12-month period [40]. The results of the study showed that the phytotherapies, although giving statistically significant improvements in symptoms, were less effective than the alpha-adrenergic receptor antagonists or 5α-reductase inhibitors, and improved symptoms for less than half of those treated [40].
Several studies also examined the efficacy of Serenoa repens extract for reduction of BPH symptoms compared to the α-adrenergic receptor antagonist, Tamsulosin. In 2007, Hizl and Uygur published a study of 60 men randomized into 3 arms of 20 men each treated with either 320 mg per day Serenoa repens extract, Tamsulosin at 0.4 mg per day, or with a combination of Serenoa repens extract and Tamsulosin. The trial intended to discover whether the combined treatment with Tamsulosin, an alpha-adrenergic receptor inhibitor, and Serenoa repens extract, with 5α-reductase inhibitor activity, would provide superior improvement in BPH symptoms and physiological measures compared to those treated with either compound alone. After 6 months of treatment, all patients demonstrated similar decreases International Prostate Symptom Scores (IPSS), increases in maximal urinary flow rate, and other measures, that were not statistically significantly different between groups. The study concluded that that treatment of BPH with both Serenoa repens and Tamsulosin alone seemed to be equally effective, and combined therapy did not provide extra benefits [41]. An Italian study also tested which of various combinations of Serenoa repens extract, lycopene, selenium, and tamsulosin therapy were most effective for reducing BPH symptoms among a group of 224 patients ranging in age from 55-80 years. This randomized study found that 71.6% of men treated concurrently with all 4 therapeutic agents experienced IPSS reduction of ≥3 points compared to 53% of men treated with Serenoa repens extract, lycopene, selenium or 51.3% of men treated with Tamsulosin [42].
A network meta-analysis of data from 2115 publications, including some of those referenced here, compared the efficacy of hexanic extract of SeR (HESr) versus non-HESr (nHESr) versus placebo versus alpha-blockers (ABs) in patients affected by LUTS secondary to BPH. The study constructed ‘rankograms’ that calculated the cumulative ranking probability for all treatments in the network. Based on this analysis, terazosin showed the best probability and highest score (99.6%), followed by alfuzosin, tamsulosin, silodosin, HESr, and nHESr with scores of 53.7%, 42.3%, 68.5%, 36.7%, and 47.3%, respectively [43]. With regard to the Serenoa repens extracts, the study concluded that they showed some efficacy, at least after a 12-month period of usage, and that they might be appropriate for patients who wanted to avoid the side effects of alpha-blockers and did not require rapid symptom relief [43]. An observational study with a final cohort of 1,838 men classified as previously treated patients (PTPs) or previously untreated patients (PUPs) suffering from LUTS compared the outcomes of treatment with alpha-blockers, alpha-blockers + phytotherapy, and phytotherapy alone among several other forms of pharmacotherapy. Although ‘phytotherapy’ is oddly not defined in the study, one can assume much of those included comprised Serenoa repens extracts containing beta-sitosterol as that is the most popular form of phytotherapy for LUTS. The results of this study largely agreed with others cited here and showed that an IPSS reduction of ≥3 points in 74.6% of patients given alpha-blockers alone, 75.0% of patients given alpha-blockers + phytotherapy, and 46.1% of patients given phytotherapy alone in the PUP cohort, and 41.4% of patients given alpha-blockers alone, 50.0% of patients given alpha-blockers + phytotherapy, and 42.9% of patients given phytotherapy alone in the PTP cohort. Thus, phytotherapy does show some efficacy for relief from LUTS, though not at the same level as that of alpha-blockers alone and does not improve this relief when combined with alpha-blocker treatment [44].
Effects of aza-steroids on human prostate histology
An early study by Marks et al. [35] examined prostate biopsies taken from men before and after 6 months treatment with finasteride. They noted that the post-treatment biopsies of the transition zone, through which the urethra travels, demonstrated a significant decrease in percent epithelium and a significant increase in the stroma-to-epithelial ratio compared to pre-treatment biopsies. These changes were not observed in the post-treatment biopsies from the peripheral zone compared to pre-treatment biopsies. They also noted that the stroma of the post, but not pre-treatment biopsies stained blue after Massons trichrome staining [35]. The blue staining is indicative of high levels of the protein collagen. Collagen is a trimeric structural protein and major component of the extracellular matrix. Fibroblasts secrete collagen in response to many stimuli, which includes inflammatory mediators secreted by senescent or immune cells in the aging tissue microenvironment or by traumatized tissue [45]. In some cases, fibroblasts exposed to these stimuli become ‘activated’ or fully phenoconvert to myofibroblasts and secrete higher-than-normal amounts of collagen in an uncontrolled manner. This process can produce tissue fibrosis, where normal tissues become replaced by fibrous connective tissue, resulting in organ dysfunction [20]. The observance of higher collagen content in post- compared to pre-treatment biopsy tissues in the Marks et al. study is suggestive that finasteride may have provoked a fibrotic response in the transition zone of the prostate.
Vacherot et al. performed various histological examinations on 10 normal prostate tissues free of histological evidence of BPH and 20 BPH prostate tissues, 10 from men who had never received medical therapy, and 10 from men treated for 3 months prior to tissue collection with LSESr (Permixon), a lipidosterolic extract of saw palmetto [12,46]. This study, like that of Marks et al., observed significantly increased expansion of the stromal compartment in BPH specimens. This study also noted that tissues from LSESr-treated patients demonstrated significantly reduced proliferation (7.7- and 4.9-fold in epithelium and stroma, respectively) and increased apoptosis (5.4- and 8.8-fold, respectively) relative to LSESr-untreated BPH patients [46]. Thus, the saw palmetto extract appeared to exert similar effects of reducing cellular proliferation and increasing cellular apoptosis in both the epithelial and stromal compartments of the prostate. Interestingly, the extract produced more pronounced effects in the epithelial rather than stromal compartments.
A more recent study by Macoska et al. of transition zone biopsies from the Medical Therapy of Prostatic Symptoms (MTOPS) trial [47,48] reported that collagen content was significantly higher in the transition zone of the prostate for men whose BPH progressed though treated with finasteride and doxazosin, and was associated with expansion and collagenization of stromal tissue [49]. Although the effects of doxazosin on these histological changes should not be discounted, comparison with the Marks et al. study [35] suggests that the stromal compartment expansion and collagenization may be ascribed to treatment with aza-steroids, e.g., finasteride and phytosterols.
A summary diagram of the molecular and phenotypic effects of beta-sitosterol on BPH is provided in Figure 1.
Discussion
Studies focused on the use of plant sterols, particularly beta-sitosterol, exhibit some dichotomy with respect to whether the study is concerned with prostate cancer or benign prostatic enlargement. Those concerned with whether beta-sitosterol is effective for the treatment of prostate cancer emphasize whether it can induce apoptosis, thus kill prostate cancer cells, or reduce the expression or activity of particular proteins and signaling pathways such that prostate cancer cell proliferation or prostate tumor progression is impeded. Overall, these studies do show that beta-sitosterol induces anti-cancer activities, but not at a level that competes with current standard of care using other clinically tested therapeutic approaches. For this reason, herbal supplements derived from saw palmetto and containing beta-sitosterol and other plant sterols are not considered efficacious for the treatment of prostate cancer.
Rather, most of the enthusiasm for beta-sitosterol treatment for prostatic disease has been focused on its’ utility for treating benign prostatic hyperplasia. These studies analogize the chemical structures of plant sterols with those of the 5α-reductase inhibitors, finasteride and dutasteride, and have largely shown that plant sterols can interact with the 5α-reductase enzyme and essentially prevent it from binding to testosterone and converting it to DHT, the form in which it can bind to the androgen receptor. Moreover, phytosterols, but not finasteride, compete with cholesterol for intestinal absorption, and thereby lower both cholesterol absorption by the intestines and cholesterol concentrations in the plasma [26]. Because plant sterols reduce the amount of cholesterol that can enter the bloodstream, less cholesterol can be taken up by the Leydig cells in the testes; thus, less cholesterol can be converted to testosterone [27]. Therefore, plant sterols exhibit dual activities that lower the availability of DHT to prostatic epithelial cells: 1) They inhibit the conversion of testosterone to DHT, and 2) They reduce the pool of cholesterol available to the Leydig cells, hence, less cholesterol is converted to testosterone for conversion to DHT.
Although the studies discussed in this review uniformly show that plant sterols can reduce DHT levels in rodent and human prostatic epithelium, they exhibit weaker efficacy than flutamide and dutasteride. Hence, on the spectrum of aza-steroid utility as 5α-reductase inhibitors, plant sterols rank below that of both finasteride and dutasteride. This is confirmed in the TRUMPH study, which showed that phytotherapies, although giving statistically significant improvements in LUTS, were less effective in this regard than the alpha-adrenergic receptor antagonists or 5α-reductase inhibitors, and improved symptoms for less than half of those treated [40]. Also, the Russo et al. meta-analysis study concluded that, although phytotherapy did show some efficacy for relief from LUTS, it was not at the same level as that of alpha-blockers alone and did not improve this relief when combined with alpha-blocker treatment [43].
Perhaps a bit of a conundrum is encountered when analyzing the effects of aza-steroids on the stromal compartment of the prostate. Most of these studies in rodents and humans focused on finasteride rather than plant sterols, and often included alpha-adrenergic receptor antagonists such as doxazosin, which makes it difficult to pin down which therapeutic contributed to observed changes in prostatic stroma. The study by Prahalada et al. [30] reported that finasteride-treated rats exhibited reduction in the total number of epithelial and stromal cells [31]. Justulin et al. found in two separate rodent studies that treatment with doxazosin alone [32] or doxazosin + finasteride [33] combination treatment resulted in decreased cellular proliferation and increased apoptotic index, largely in the epithelial compartment. However, the combination treatment promoted a reduction of epithelial volume and increase in ventral lobe prostate collagen with significantly increased collagen type I and type III fibers. Studies focused on histological changes in human prostate after treatment with finasteride reported concurrent reduction of the epithelial compartment and increased collagen content, consistent with a fibrotic response, in the transition zone [35]. Vacherot et al., reported that prostate tissues from men given Permixon demonstrated significantly increased expansion of the stromal compartment in BPH specimens. This study also reported that Permixon significantly reduced proliferation and increased apoptosis, though these effects were more produced in the epithelial than stromal compartments [46]. Thus, though not well studied, it appears that aza-steroids, including plant sterols, may alter the stromal compartment of the prostate, though it isn’t clear whether this alteration is towards increased or decreased volume, or includes changes in collagen content.
One of the limitations of this review is the lack of complete datasets, e.g., the lack of in vitro, in vivo, and human clinical trials/human tissue histology data useful for examining the effects of beta-sitosterol on the development or treatment of prostate cancer and BPH. Prostate cancer studies were focused on the use of cell lines in vitro and spheroids and xenografts prostate tumors in vivo to examine the pro-apoptotic or other tumor reduction effects of beta-sitosterol. There is no literature regarding changes in human prostate tumor histology subsequent to the use of supplements containing beta-sitosterol. The literature is much more abundant for in vitro, in vivo, and human clinical trial/human tissue histology data for the use of beta-sitosterol to treat BPH. However, there is an overwhelming emphasis on histological analysis of the prostatic epithelium and little on that of the prostatic stroma in human studies. This may have a historical rationale as the importance of changes in the prostatic stroma contributing to LUTD and LUTS have only recently been elucidated [50]. Another limitation is that different methods for the extraction of phytosterols from saw palmetto were used in the studies examined, and that some studies used purified beta-sitosterol versus saw palmetto extract. This casts some doubt on how accurately one can compare the studies to elucidate particular phenomena, e.g., effects of beta-sitosterol on prostatic epithelial proliferation or programmed cell death.
Conclusion
The results of this study suggest multiple roles for beta-sitosterol-mediated repression of prostate cancer in vitro and in vivo, particularly regarding its pro-apoptotic effect on prostate epithelial cells. However, supplements containing beta-sitosterol for prostate health are overwhelmingly taken to relieve LUTS associated with BPH. These supplements have not been associated with toxicities or adverse events among the millions of men who take them daily. Moreover, there is no doubt that these supplements can be beneficial for relieving LUTS, as many of the studies described above affirm. Even so, these studies also affirm that plant sterols exhibit reduced activity in this regard compared to that of finasteride and dutasteride. Taken together, these data have led many investigators and clinicians to conclude that supplements containing plant sterols, among which beta-sitosterol is the most abundant, might be most appropriate for younger men with minimal symptoms who don’t wish to embark on a clinical drug regimen for BPH treatment. This scientifically sound conclusion is supported by the studies reviewed here and describes a clear and appropriate utility for the use of plant sterols, particularly beta-sitosterol, in the treatment of LUTS.
Disclosure of conflict of interest
None.
References
- 1.Sosnowska J, Balslev H. American palm ethnomedicine: a meta-analysis. J Ethnobiol Ethnomed. 2009;5:43. doi: 10.1186/1746-4269-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dransfield J, Uhl NW, Asmussen CB, Baker WJ, Harley MM, Lewis CE. Genera palmarum - the evolution and classification of the palms. London, UK: Royal Botanic Gardens, Kew; 2008. p. 732. [Google Scholar]
- 3.Balslev H, Barfod A. Ecuadorean palms---an overview. Opera Bot. 1987;92:17–35. [Google Scholar]
- 4.Smith T, Eckl V, Reynolds CM. Herbal supplement sales in US increase by record-breaking 17.3% in 2020. HerbalGram. 2021;131:52–65. [Google Scholar]
- 5.Vickman RE, Franco OE, Moline DC, Vander Griend DJ, Thumbikat P, Hayward SW. The role of the androgen receptor in prostate development and benign prostatic hyperplasia: a review. Asian J Urol. 2020;7:191–202. doi: 10.1016/j.ajur.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3) Invest Urol. 1979;17:16–23. [PubMed] [Google Scholar]
- 7.Awad AB, Fink CS, Williams H, Kim U. In vitro and in vivo (SCID mice) effects of phytosterols on the growth and dissemination of human prostate cancer PC-3 cells. Eur J Cancer Prev. 2001;10:507–13. doi: 10.1097/00008469-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Awad AB, Burr AT, Fink CS. Effect of resveratrol and beta-sitosterol in combination on reactive oxygen species and prostaglandin release by PC-3 cells. Prostaglandins Leukot Essent Fatty Acids. 2005;72:219–26. doi: 10.1016/j.plefa.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Horoszewicz JS, Leong SS, Chu TM, Wajsman ZL, Friedman M, Papsidero L, Kim U, Chai LS, Kakati S, Arya SK, Sandberg AA. The LNCaP cell line--a new model for studies on human prostatic carcinoma. Prog Clin Biol Res. 1980;37:115–32. [PubMed] [Google Scholar]
- 10.von Holtz RL, Fink CS, Awad AB. beta-sitosterol activates the sphingomyelin cycle and induces apoptosis in LNCaP human prostate cancer cells. Nutr Cancer. 1998;32:8–12. doi: 10.1080/01635589809514709. [DOI] [PubMed] [Google Scholar]
- 11.Stone KR, Mickey DD, Wunderli H, Mickey GH, Paulson DF. Isolation of a human prostate carcinoma cell line (DU 145) Int J Cancer. 1978;21:274–81. doi: 10.1002/ijc.2910210305. [DOI] [PubMed] [Google Scholar]
- 12.Petrangeli E, Lenti L, Buchetti B, Chinzari P, Sale P, Salvatori L, Ravenna L, Lococo E, Morgante E, Russo A, Frati L, Di Silverio F, Russo MA. Lipido-sterolic extract of Serenoa repens (LSESr, Permixon) treatment affects human prostate cancer cell membrane organization. J Cell Physiol. 2009;219:69–76. doi: 10.1002/jcp.21648. [DOI] [PubMed] [Google Scholar]
- 13.Penugonda K, Lindshield BL. Fatty acid and phytosterol content of commercial saw palmetto supplements. Nutrients. 2013;5:3617–33. doi: 10.3390/nu5093617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sramkoski RM, Pretlow TG 2nd, Giaconia JM, Pretlow TP, Schwartz S, Sy MS, Marengo SR, Rhim JS, Zhang D, Jacobberger JW. A new human prostate carcinoma cell line, 22Rv1. In Vitro Cell Dev Biol Anim. 1999;35:403–9. doi: 10.1007/s11626-999-0115-4. [DOI] [PubMed] [Google Scholar]
- 15.Cole C, Burgoyne T, Lee A, Stehno-Bittel L, Zaid G. Arum palaestinum with isovanillin, linolenic acid and beta-sitosterol inhibits prostate cancer spheroids and reduces the growth rate of prostate tumors in mice. BMC Complement Altern Med. 2015;15:264. doi: 10.1186/s12906-015-0774-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pradhan N, Parbin S, Kausar C, Kar S, Mawatwal S, Das L, Deb M, Sengupta D, Dhiman R, Patra SK. Paederia foetida induces anticancer activity by modulating chromatin modification enzymes and altering pro-inflammatory cytokine gene expression in human prostate cancer cells. Food Chem Toxicol. 2019;130:161–173. doi: 10.1016/j.fct.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Lomenick B, Shi H, Huang J, Chen C. Identification and characterization of beta-sitosterol target proteins. Bioorg Med Chem Lett. 2015;25:4976–4979. doi: 10.1016/j.bmcl.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laborde EE, McVary KT. Medical management of lower urinary tract symptoms. Rev Urol. 2009;11(Suppl 1):S19–25. [PMC free article] [PubMed] [Google Scholar]
- 19.Strope SA, Yang L, Nepple KG, Andriole GL, Owens PL. Population based comparative effectiveness of transurethral resection of the prostate and laser therapy for benign prostatic hyperplasia. J Urol. 2012;187:1341–5. doi: 10.1016/j.juro.2011.11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Nieves JA, Macoska JA. Prostatic fibrosis, lower urinary tract symptoms, and BPH. Nat Rev Urol. 2013;10:546–50. doi: 10.1038/nrurol.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sultan C, Terraza A, Devillier C, Carilla E, Briley M, Loire C, Descomps B. Inhibition of androgen metabolism and binding by a liposterolic extract of “Serenoa repens B” in human foreskin fibroblasts. J Steroid Biochem. 1984;20:515–9. doi: 10.1016/0022-4731(84)90264-4. [DOI] [PubMed] [Google Scholar]
- 22.Carilla E, Briley M, Fauran F, Sultan C, Duvilliers C. Binding of Permixon, a new treatment for prostatic benign hyperplasia, to the cytosolic androgen receptor in the rat prostate. J Steroid Biochem. 1984;20:521–3. doi: 10.1016/0022-4731(84)90265-6. [DOI] [PubMed] [Google Scholar]
- 23.Delos S, Iehle C, Martin PM, Raynaud JP. Inhibition of the activity of ‘basic’ 5 alpha-reductase (type 1) detected in DU 145 cells and expressed in insect cells. J Steroid Biochem Mol Biol. 1994;48:347–52. doi: 10.1016/0960-0760(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 24.Bayne CW, Donnelly F, Ross M, Habib FK. Serenoa repens (Permixon): a 5alpha-reductase types I and II inhibitor-new evidence in a coculture model of BPH. Prostate. 1999;40:232–41. doi: 10.1002/(sici)1097-0045(19990901)40:4<232::aid-pros4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Jena AK, Vasisht K, Sharma N, Kaur R, Dhingra MS, Karan M. Amelioration of testosterone induced benign prostatic hyperplasia by prunus species. J Ethnopharmacol. 2016;190:33–45. doi: 10.1016/j.jep.2016.05.052. [DOI] [PubMed] [Google Scholar]
- 26.Lees AM, Mok HY, Lees RS, McCluskey MA, Grundy SM. Plant sterols as cholesterol-lowering agents: clinical trials in patients with hypercholesterolemia and studies of sterol balance. Atherosclerosis. 1977;28:325–38. doi: 10.1016/0021-9150(77)90180-0. [DOI] [PubMed] [Google Scholar]
- 27.Sedes L, Thirouard L, Maqdasy S, Garcia M, Caira F, Lobaccaro JA, Beaudoin C, Volle DH. Cholesterol: a gatekeeper of male fertility? Front Endocrinol (Lausanne) 2018;9:369. doi: 10.3389/fendo.2018.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buncharoen W, Saenphet K, Saenphet S, Thitaram C. Uvaria rufa Blume attenuates benign prostatic hyperplasia via inhibiting 5alpha-reductase and enhancing antioxidant status. J Ethnopharmacol. 2016;194:483–494. doi: 10.1016/j.jep.2016.10.036. [DOI] [PubMed] [Google Scholar]
- 29.Sudeep HV, Venkatakrishna K, Amrutharaj B, Anitha, Shyamprasad K. A phytosterol-enriched saw palmetto supercritical CO(2) extract ameliorates testosterone-induced benign prostatic hyperplasia by regulating the inflammatory and apoptotic proteins in a rat model. BMC Complement Altern Med. 2019;19:270. doi: 10.1186/s12906-019-2697-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prahalada SR, Keenan KP, Hertzog PR, Gordon LR, Peter CP, Soper KA, van Zwieten MJ, Bokelman DL. Qualitative and quantitative evaluation of prostatic histomorphology in rats following chronic treatment with finasteride, a 5-alpha reductase inhibitor. Urology. 1994;43:680–5. doi: 10.1016/0090-4295(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 31.Prahalada S, Rhodes L, Grossman SJ, Heggan D, Keenan KP, Cukierski MA, Hoe CM, Berman C, van Zwieten MJ. Morphological and hormonal changes in the ventral and dorsolateral prostatic lobes of rats treated with finasteride, a 5-alpha reductase inhibitor. Prostate. 1998;35:157–64. doi: 10.1002/(sici)1097-0045(19980515)35:3<157::aid-pros1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 32.Justulin LA Jr, Delella FK, Felisbino SL. Doxazosin reduces cell proliferation and increases collagen fibers in rat prostatic lobes. Cell Tissue Res. 2008;332:171–83. doi: 10.1007/s00441-007-0559-3. [DOI] [PubMed] [Google Scholar]
- 33.Justulin LA Jr, Acquaro C, Carvalho RF, Silva MD, Felisbino SL. Combined effect of the finasteride and doxazosin on rat ventral prostate morphology and physiology. Int J Androl. 2010;33:489–99. doi: 10.1111/j.1365-2605.2009.00963.x. [DOI] [PubMed] [Google Scholar]
- 34.Berges RR, Windeler J, Trampisch HJ, Senge T. Randomised, placebo-controlled, double-blind clinical trial of beta-sitosterol in patients with benign prostatic hyperplasia. Beta-sitosterol Study Group. Lancet. 1995;345:1529–32. doi: 10.1016/s0140-6736(95)91085-9. [DOI] [PubMed] [Google Scholar]
- 35.Marks LS, Partin AW, Epstein JI, Tyler VE, Simon I, Macairan ML, Chan TL, Dorey FJ, Garris JB, Veltri RW, Santos PB, Stonebrook KA, deKernion JB. Effects of a saw palmetto herbal blend in men with symptomatic benign prostatic hyperplasia. J Urol. 2000;163:1451–6. [PubMed] [Google Scholar]
- 36.Debruyne F, Koch G, Boyle P, Da Silva FC, Gillenwater JG, Hamdy FC, Perrin P, Teillac P, Vela-Navarrete R, Raynaud JP. Comparison of a phytotherapeutic agent (Permixon) with an alpha-blocker (Tamsulosin) in the treatment of benign prostatic hyperplasia: a 1-year randomized international study. Eur Urol. 2002;41:497–506. discussion 506-507. [PubMed] [Google Scholar]
- 37.Debruyne F, Boyle P, Calais Da Silva F, Gillenwater JG, Hamdy FC, Perrin P, Teillac P, Vela-Navarrete R, Raynaud JP, Schulman CC. Evaluation of the clinical benefit of permixon and tamsulosin in severe BPH patients-PERMAL study subset analysis. Eur Urol. 2004;45:773–779. doi: 10.1016/j.eururo.2004.01.015. disucssion 779-780. [DOI] [PubMed] [Google Scholar]
- 38.Bent S, Kane C, Shinohara K, Neuhaus J, Hudes ES, Goldberg H, Avins AL. Saw palmetto for benign prostatic hyperplasia. N Engl J Med. 2006;354:557–66. doi: 10.1056/NEJMoa053085. [DOI] [PubMed] [Google Scholar]
- 39.Sudeep HV, Thomas JV, Shyamprasad K. A double blind, placebo-controlled randomized comparative study on the efficacy of phytosterol-enriched and conventional saw palmetto oil in mitigating benign prostate hyperplasia and androgen deficiency. BMC Urol. 2020;20:86. doi: 10.1186/s12894-020-00648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hutchison A, Farmer R, Verhamme K, Berges R, Navarrete RV. The efficacy of drugs for the treatment of LUTS/BPH, a study in 6 European countries. Eur Urol. 2007;51:207–15. doi: 10.1016/j.eururo.2006.06.012. discussion 215-6. [DOI] [PubMed] [Google Scholar]
- 41.Hizli F, Uygur MC. A prospective study of the efficacy of Serenoa repens, tamsulosin, and Serenoa repens plus tamsulosin treatment for patients with benign prostate hyperplasia. Int Urol Nephrol. 2007;39:879–86. doi: 10.1007/s11255-006-9106-5. [DOI] [PubMed] [Google Scholar]
- 42.Morgia G, Russo GI, Voce S, Palmieri F, Gentile M, Giannantoni A, Blefari F, Carini M, Minervini A, Ginepri A, Salvia G, Vespasiani G, Santelli G, Cimino S, Allegro R, Collura Z, Fragala E, Arnone S, Pareo RM. Serenoa repens, lycopene and selenium versus tamsulosin for the treatment of LUTS/BPH. An Italian multicenter double-blinded randomized study between single or combination therapy (PROCOMB trial) Prostate. 2014;74:1471–80. doi: 10.1002/pros.22866. [DOI] [PubMed] [Google Scholar]
- 43.Russo GI, Scandura C, Di Mauro M, Cacciamani G, Albersen M, Hatzichristodoulou G, Fode M, Capogrosso P, Cimino S, Marcelissen T, Cornu JN, Gacci M, Minervini A, Cocci A European Association of Urology Young Academic Urologists (EAU-YAU) Men’s Health and Functional Urology Working Groups. Clinical efficacy of serenoa repens versus placebo versus alpha-blockers for the treatment of lower urinary tract symptoms/benign prostatic enlargement: a systematic review and network meta-analysis of randomized placebo-controlled clinical trials. Eur Urol Focus. 2021;7:420–431. doi: 10.1016/j.euf.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Tubaro A, Speakman M, de la Taille A, Martinez-Pineiro L, Berges R, Patel A, Bjartell A, Caris C, Witjes W. A European registry evaluating symptomatic effectiveness of pharmacologically treated patients with lower urinary tract symptoms due to benign prostatic enlargement: lessons learned. J Urol. 2021;205:1145–1152. doi: 10.1097/JU.0000000000001506. [DOI] [PubMed] [Google Scholar]
- 45.Begley LA, Kasina S, MacDonald J, Macoska JA. The inflammatory microenvironment of the aging prostate facilitates cellular proliferation and hypertrophy. Cytokine. 2008;43:194–9. doi: 10.1016/j.cyto.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vacherot F, Azzouz M, Gil-Diez-De-Medina S, Colombel M, De La Taille A, Lefrere Belda MA, Abbou CC, Raynaud JP, Chopin DK. Induction of apoptosis and inhibition of cell proliferation by the lipido-sterolic extract of Serenoa repens (LSESr, Permixon in benign prostatic hyperplasia) Prostate. 2000;45:259–66. doi: 10.1002/1097-0045(20001101)45:3<259::aid-pros9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 47.Bautista OM, Kusek JW, Nyberg LM, McConnell JD, Bain RP, Miller G, Crawford ED, Kaplan SA, Sihelnik SA, Brawer MK, Lepor H. Study design of the medical therapy of prostatic symptoms (MTOPS) trial. Control Clin Trials. 2003;24:224–43. doi: 10.1016/s0197-2456(02)00263-5. [DOI] [PubMed] [Google Scholar]
- 48.McConnell JD, Roehrborn CG, Bautista OM, Andriole GL Jr, Dixon CM, Kusek JW, Lepor H, McVary KT, Nyberg LM Jr, Clarke HS, Crawford ED, Diokno A, Foley JP, Foster HE, Jacobs SC, Kaplan SA, Kreder KJ, Lieber MM, Lucia MS, Miller GJ, Menon M, Milam DF, Ramsdell JW, Schenkman NS, Slawin KM, Smith JA Medical Therapy of Prostatic Symptoms (MTOPS) Research Group. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387–98. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 49.Macoska JA, Uchtmann K, Leverson G, McVary KT, Ricke WA. Prostate transition zone fibrosis in men who who failed doxazosin, finasteride, or combination therapy in the medical therapy of prostatic symptoms (MTOPS). Submitted. 2019 [Google Scholar]
- 50.Ma J, Gharaee-Kermani M, Kunju L, Hollingsworth JM, Adler J, Arruda EM, Macoska JA. Prostatic fibrosis is associated with lower urinary tract symptoms. J Urol. 2012;188:1375–81. doi: 10.1016/j.juro.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, Li Q, Shoemaker BA, Thiessen PA, Yu B, Zaslavsky L, Zhang J, Bolton EE. PubChem 2023 update. Nucleic Acids Res. 2023;51:D1373–D1380. doi: 10.1093/nar/gkac956. [DOI] [PMC free article] [PubMed] [Google Scholar]