Abstract
Transfer of newly isolated mutations into a fresh background is an essential step of genetic analysis and strain construction. Gene transfer is hampered in Salmonella typhi and in other pathogenic bacteria by the lack of a generalized transduction system. We show here that this problem can be partially circumvented by using electrotransformation as a means for delivering S. typhi DNA into suitable S. typhi or Salmonella typhimurium recipients. Transferred DNA can recombine with the homologous region in the host chromosome. In one application of the method, mutations isolated in S. typhi were genetically mapped in S. typhimurium.
Typhoid fever is a complex systemic infection quite widespread in developing countries (17). Since its causative agent, Salmonella typhi, is a strictly human pathogen, it has been difficult to find an appropriate animal model for virulence studies. This limitation is partially overcome by the use of human cell lines for in vitro studies. Similar to the results obtained for other bacterial pathogens, this approach is beginning to yield information on the genes involved in invasion and other virulence determinants (3, 4, 7).
Analysis of the S. typhi chromosome has revealed some major differences with respect to the closely related bacteria Salmonella typhimurium and Escherichia coli. Most notable is the inversion of large chromosomal segments thought to result from recombination between rRNA loci (11–13). In spite of these differences, the gene order within the inverted regions and elsewhere in the chromosome is virtually the same in S. typhi as in S. typhimurium and the two bacterial serovars share more than 90% homology at the DNA sequence level (5). Indeed, segments of the S. typhimurium chromosome can undergo recombination with the homologous region in the S. typhi chromosome once the natural barrier imposed by the mismatch repair system is eliminated by mutation (mutS) in the recipient strains (22, 23).
A major problem encountered in the genetic analysis of S. typhi is the lack of a convenient gene transfer system and, in particular, of a generalized transducing phage comparable to phage P22 of S. typhimurium. While P22 will deliver DNA into S. typhi, making S. typhimurium-S. typhi crosses possible (22, 23), it is incapable of multiplying inside this host, thus preventing gene transfer in the opposite direction, i.e., from S. typhi to S. typhimurium, or between S. typhi strains. To try to circumvent this problem, we sought to test whether S. typhi genetic material introduced into S. typhimurium cells by electrotransformation could undergo homologous recombination.
Transformation of S. typhimurium with linear DNA.
As a preliminary test of the method we used a cloned DNA fragment from S. typhimurium as input material. The DNA insert of plasmid pCV47 contains the entire S. typhimurium leucine operon plus approximately 13 kb of neighboring DNA (20). This insert is released by BamHI treatment as an 18.5-kb DNA fragment. pCV47 DNA, either cleaved with BamHI or untreated, was used to transform S. typhimurium strains in which the leuA gene was inactivated by a MudJ insertion. Three recipient strains were compared: MA2290, which expresses a functional RecBCD enzyme; MA5133, in which the nuclease activity of RecBCD (Exo V) is inactivated (recD::Tn10dTc); and MA5031, which harbors a recBD deletion but is recombination proficient due to an sbc mutation (sbcE21 [8]). Results shown in Table 1 confirm that, as suggested from previous work with E. coli (18), inactivation of Exo V in S. typhimurium greatly improves the recovery of transformants with linear DNA. Leu+ transformants were found to be kanamycin sensitive and Lac−, consistent with their resulting from recombination events which replace the leuA::MudJ insertion with the wild-type leuA gene. The higher transformation efficiency in the recBD sbcE background is indicative of the “hyper-rec” phenotype of this strain (8, 9).
TABLE 1.
Transformation of S. typhimurium with plasmid DNA
| Straina | rec genotype | No. of transformants/ 109 CFU/μg of DNAb
|
|
|---|---|---|---|
| Circular DNAc | Linear DNAd | ||
| MA2290 | rec+ | 3.1 × 107 | 3.6 × 102 |
| MA5133 | recD | 2.1 × 107 | 2.5 × 103 |
| MA5031 | recBD sbcE | 3.2 × 107 | 2.7 × 105 |
Strains are derivatives of S. typhimurium LT2. Some strains derive from strains described in references 8 and 9; they were constructed by P22-mediated transduction (as previously described [14]). Genotypes are as follows: MA2290, leu-3243 leuA3241::MudJ; MA5133, leu-3243 leuA3241::MudJ recD543::Tn10dTc; MA5031, Δleu-3243 leuA3241::MudJ Δ(argA-recBD)1742 sbcE21 zfe8157::Tn10dTc.
Electrotransformation was carried out with a Bio-Rad Gene Pulser. Bacteria were made competent by procedures recommended by the manufacturer. Plasmid DNA, purified by equilibrium centrifugation in a CsCl-ethidium bromide gradient (14), was mixed into the bacterial cell suspension (50 μl) in a chilled cuvette (0.2-cm electrode gap). A single pulse of 12.5 kV/cm (2.5 kV, 200 Ω, 25 μF) was applied, and 1 ml of prewarmed SOC medium (2% Bacto Tryptone, 0.5% yeast extract, 10 mM NaCl, 2.5 mM KCl, 10 mM MgCl2, 10 mM MgSO4, 20 mM glucose) was immediately added. The bacteria were transferred to glass tubes and shaken for 1 h at 37°C prior to plating onto selective medium. Data are from one representative experiment. The number of transformants is expressed per microgram of DNA per 109 viable CFU at the end of the recovery period.
Untreated pCV47 DNA (prepared from S. typhimurium; 2.5 ng per transformation). Leu+ transformants were selected on minimal E medium (14). More than 99% of transformants were Ampr and Lac+.
pCV47 DNA cleaved with BamHI (25 ng per transformation). Leu+ transformants were selected as described for circular DNA.
We then evaluated the ability of S. typhimurium to yield Leu+ recombinants when transformed with bulk chromosomal DNA prepared from S. typhimurium or S. typhi. Results in Table 2 show that this is indeed possible provided the nuclease activity of RecBCD of the recipient strain is inactivated. Again, the frequency of recombination is higher in the sbcE21 background. In addition, in the experiments involving S. typhi DNA, the formation of Leu+ recombinants also requires that the recipient strain be defective in mismatch repair (mutS). This is in agreement with the known inhibitory effect of the mismatch repair system on recombination between closely related sequences (22, 23).
TABLE 2.
Transformation of S. typhimurium with chromosomal DNA
| Straina | rec mut genotype | No. of transformants/109 CFUb
|
|
|---|---|---|---|
| S. typhimurium | S. typhi | ||
| MA2290 | rec+ mut+ | 0 | 0 |
| MA5114 | rec+ mutS | 0 | 1 |
| MA5133 | recD mut+ | 21 | 0 |
| MA5116 | recD mutS | 12 | 4 |
| MA5031 | recBD sbcE mut+ | 41 | 1 |
| MA5145 | recBD sbcE mutS | 33 | 17 |
Strains MA5114, MA5116, and MA5145 were derived from S. typhimurium strains MA2290, MA5133 and MA5031, respectively (Table 1), upon introducing the mutS171::Tn10dCm insertion.
Chromosomal DNA (1 to 5 μg), mildly sheared by vortexing for 1 to 2 min, was used for transformation (carried out as described for Table 1). Selection was to prototrophy (Leu+). Data are the averages of three independent determinations.
In a separate experiment, chromosomal DNA from an S. typhi strain carrying a leuA::MudJ insertion (constructed by P22 transduction [22]) was used to transform an S. typhimurium recipient containing an intact leu operon region (MA5100). Kanamycin-resistant (Kanr) recombinants were selected. Although such isolates occurred at a somewhat lower frequency than the Leu+ recombinants described above, the effect of recD and mutS was nearly identical to the data in Table 2 (data not shown). Southern analysis confirmed that the structure of the leu operon region in four independent Kanr transformants was indistinguishable from that of donor DNA (data not shown). Thus, no unusual rearrangements accompanied the acquisition of the MudJ insertion by the recipient strain.
Although the mutS mutation makes genetic exchanges between S. typhi and S. typhimurium possible, the frequencies with which recombinants were recovered in the above experiment are low. We sought to see whether the efficiency of the process could be increased by improving the transformation step. S. typhimurium SL4213 (metA22 metE551 galE496 rpsL120 xyl-104 Δ[Fels2] H1-b H2-e,n,x nml hsdL6 hsdSA29) was previously recognized to be a particularly suitable transformation recipient owing to galE and hsd mutations that favor DNA uptake and lower the restriction barrier, respectively (16). Upon repeating the above experiments with the SL4213 background, we observed a 10-fold increase in the efficiency of recovery of recombinant clones provided that recD and mutS mutations were both present (strain MA5383, see Table 3, footnote a). Such an improvement was not specific to the leu operon region but was also observed in exchanges involving the his operon and the proU operon (data not shown). Unfortunately, limitations in the availability of selectable markers hampered the construction of the triply mutated SL4213 derivative carrying the recBD deletion sbcE21 and mutS::Tn10dCm. We therefore adopted strain MA5383 (Table 3) for the mapping experiments described below.
TABLE 3.
Mapping S. typhi insertion mutations in S. typhimurium
| Original S. typhi straina | Relevant phenotype | Mud-P22 lysate scoring positiveb | Map position (cs) in S. typhimuriumc | Putative locus affected | Inferred map position (cs) in S. typhid |
|---|---|---|---|---|---|
| TyT1009 | Met− | zgf-3716::MudP | 66–70 | metC | 93–97 |
| TyT1015 | Arg− | cysHIJ1574::MudQ | 64–65 | argA | 99–1 |
| TyT1020 | Ilv− | metE2131::MudP | 85–86 | ilv operon | 88–90 |
| TyT1031 | Leu− | nadC218::MudQ | 1–3 | leu operon | 58–60 |
| TyT1041 | Phe− | purG2149::MudP | 56–59 | pheA | 4–7 |
| NN19 | Chlrf | nadA219::MudP | 17–19 | modCe | 17–19 |
| JJ3 | Chlr | nadA219::MudP | 17–19 | moaAe | 17–19 |
| DD46 | Chlr | zgf-1716::MudQ | 67–69 | uxaC | 95–97 |
Strains are derivatives of S. typhi Ty2. They belong to a collection of MudJ insertion mutants obtained following the protocol of Hughes and Roth (10). Chromosomal DNA from these strains was prepared according to a previously described method (1) and was used to transform cells of strain MA5383 (metA22 metE551 galE496 rpsL120 xyl-104 Δ[Fels2] H1-b H2-e,n,x nml hsdL6 hsdSA29 recD543::Tn10dTc mutS171::Tn10dCm), selecting for Kanr. Insertions were then transferred to strain LT2 by P22-mediated transduction and were mapped, with prototrophy scored as previously described (2). For MudJ insertions conferring phenotypes other than auxotrophies, a Tetr marker was introduced within the MudJ element. This was done by using a P22 lysate prepared from a strain carrying lacZ::Tn10 to transduce the different MudJ-carrying strains, selecting for Tetr. Lac− Tetr transductants were purified and the Tn10 element was mapped on Tets selection medium as previously described (2, 15).
Mud-P22 lysates, enriched for selected regions of the S. typhimurium chromosome, were prepared from a collection of 54 lysogenic strains and were used for transduction as previously described (21). Typically, overlapping lysates would concomitantly score positive in the assay (Fig. 1). Only the lysate producing the strongest signal in each transduction series is shown here.
Centisomes (cs) are from edition VIII of the S. typhimurium genetic map (19). Intervals are those of the two strongest phage signals observed.
Centisomes (cs) were calculated from Table 2 of reference 11, taking 4,780 kb as the size of the S. typhi chromosome.
Assignments were made on the basis of phenotypic characterization of mutants (3) and the relative strengths of the second strongest transduction result in each case.
Chl, chlorate.
Mapping of S. typhi mutations in S. typhimurium.
The possibility of moving mutations isolated in S. typhi to S. typhimurium for mapping analysis was tested with eight independent MudJ insertion mutants. Five such insertions cause amino acid auxotrophies (Met, Arg, Ilv, Leu, Phe) and the remaining three confer chlorate resistance. As schematized in Fig. 1, chromosomal DNA was prepared from each of the eight S. typhi strains and used to transform strain MA5383. Kanr transformants were picked, purified, and used as donors in P22-mediated transductional crosses with wild-type S. typhimurium LT2 as the recipient. Once in the wild-type background, MudJ-associated mutations were mapped by using the “locked-in” Mud-P22 hybrid procedure (21). The auxotrophic mutants allowed the direct selection of prototrophic transductants (Fig. 1). With the chlorate-resistant insertions, a Tn10dTc element was introduced within the lac sequence of MudJ and selection was for the loss of the Tet resistance phenotype (2). The Mud-P22 lysates that scored positive in the transductional screening allowed the positioning of the different MudJ insertions in the S. typhimurium chromosome (Table 3). From these data, the identities of affected loci could be deduced unambiguously for those resulting in an auxotrophy and preliminarily for the others. In the case of the MudJ insertion of strain DD46, the initial identification was recently confirmed by DNA sequence analysis (data not shown). The expected positions of the various loci in the S. typhi chromosome were obtained upon correcting for the known discontinuities between the S. typhimurium and S. typhi physical maps (11).
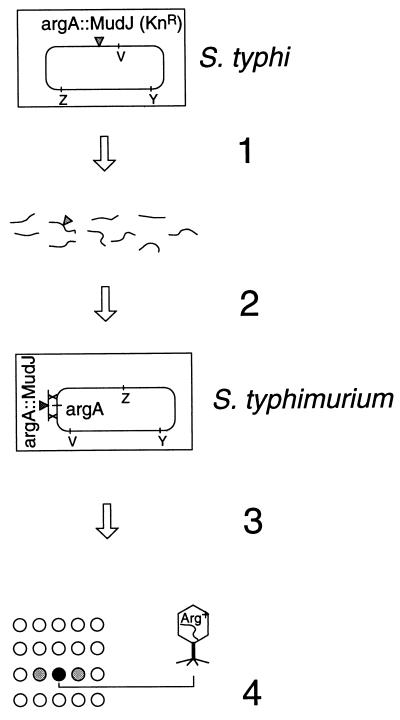
FIG. 1.
Outline of mapping procedure. Step 1. Chromosomal DNA from an S. typhi strain carrying the MudJ insertion to be mapped (for example, the argA locus) is extracted and mildly sheared. Step 2. DNA is used to electrotransform an S. typhimurium recipient in which the recD and mutS genes are inactivated. Selection for Kanr yields recombinants in which the argA locus on the recipient chromosome has been replaced by the argA::MudJ marker. (Although the chromosomal region containing argA is inverted in S. typhimurium relative to S. typhi, the gene order within this region is conserved between the two bacteria.) Step 3. Recombinant bacteria are spread on a minimal plate, and phage P22 transducing lysates individually enriched for various portions of the S. typhimurium chromosome are spotted on the bacterial lawn. Step 4. The lysate enriched for the region carrying the wild-type allele of the MudJ-disrupted locus gives rise to prototrophic transductants (patch of colonies in the spotted area).
In conclusion, the data presented here show that electrotransformation techniques combined with the use of appropriate host strains can partially circumvent the problem resulting from the lack of a suitable transduction system in S. typhi. Although here we used genetic mapping as a test of the method, this is not its only possible application. In a separate line of work, we successfully used this method for moving the MudJ insertions which confer chlorate resistance (affecting bacterial replication in epithelial cells) from the mutagenized background in which they were originally isolated into a “fresh” S. typhi background (3). Such backcrosses were crucial for unambiguously correlating individual mutations with their respective phenotypes. Surprisingly, in these S. typhi-S. typhi exchanges, recD mutational inactivation was no longer a prerequisite for the recovery of transformants. Similar findings were recently made with E. coli and were ascribed to a transient inhibition of Exo V following electroshock conditions (6).
Acknowledgments
We are indebted to Nello Bossi for discussions and comments on the manuscript and J. R. Roth for strain SL4213 and strain TT16808, which carries the mutS171::Tn10dCm allele used here. We also thank reviewers for constructive criticism.
This work was supported by the ECOS-CONICYT France-Chile cooperation program (grant no. C94B06), by FONDECYT grants 1960255 and 2970029, and by the Centre National de la Recherche Scientifique.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Short protocols in molecular biology. 2nd ed. New York, N.Y: John Wiley & Sons, Inc.; 1992. [Google Scholar]
- 2.Benson N R, Goldman B S. Rapid mapping in Salmonella typhimurium with Mud-P22 prophages. J Bacteriol. 1992;174:1673–1681. doi: 10.1128/jb.174.5.1673-1681.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Contreras I, Toro C S, Troncoso G, Mora G C. Salmonella typhi mutants defective in anaerobic respiration are impaired in their ability to replicate within epithelial cells. Microbiology. 1997;143:2665–2672. doi: 10.1099/00221287-143-8-2665. [DOI] [PubMed] [Google Scholar]
- 4.Contreras I, Obreque V H, Blanco L P, Toro C S, Mora G C. Anaerobically induced Salmonella typhi genes are involved in entry and proliferation within human-derived cell lines. Southeast Asian J Trop Med Public Health. 1995;26:110–117. [Google Scholar]
- 5.Crosa J H, Brenner D J, Ewing W H, Falkow S. Molecular relationships among the Salmonellae. J Bacteriol. 1973;115:307–315. doi: 10.1128/jb.115.1.307-315.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El Karoui, M., and A. Gruss. Submitted for publication.
- 7.Elsinghorst E, Baron L, Kopecko D. Penetration of human intestinal epithelial cells by Salmonella: molecular cloning and expression of Salmonella typhi invasion determinants in Escherichia coli. Proc Natl Acad Sci USA. 1989;86:5173–5177. doi: 10.1073/pnas.86.13.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figueroa-Bossi N, Coissac E, Netter P, Bossi L. Unsuspected prophage-like elements in Salmonella typhimurium. Mol Microbiol. 1997;25:161–173. doi: 10.1046/j.1365-2958.1997.4451807.x. [DOI] [PubMed] [Google Scholar]
- 9.Garí E, Figueroa-Bossi N, Blanc-Potard A-B, Spirito F, Schmid M B, Bossi L. A class of gyrase mutants of Salmonella typhimurium show quinolone-like lethality and require rec functions for viability. Mol Microbiol. 1996;21:111–122. doi: 10.1046/j.1365-2958.1996.6221338.x. [DOI] [PubMed] [Google Scholar]
- 10.Hughes K T, Roth J R. Transitory cis complementation: a method for providing transposition functions to defective transposons. Genetics. 1988;119:9–12. doi: 10.1093/genetics/119.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu S L, Sanderson K E. Genomic cleavage map of Salmonella typhi Ty2. J Bacteriol. 1995;177:5099–5107. doi: 10.1128/jb.177.17.5099-5107.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S L, Sanderson K E. Rearrangements in the genome of the bacterium Salmonella typhi. Proc Natl Acad Sci USA. 1995;92:1018–1022. doi: 10.1073/pnas.92.4.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S L, Sanderson K E. Highly plastic chromosomal organization in Salmonella typhi. Proc Natl Acad Sci USA. 1996;93:10303–10308. doi: 10.1073/pnas.93.19.10303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maloy S R. Experimental techniques in bacterial genetics. Boston, Mass: Jones and Bartlett Publishers; 1990. [Google Scholar]
- 15.Maloy S R, Nunn W. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981;145:1110–1112. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Callaghan D O, Charbit A. High efficiency transformation of Salmonella typhimurium and Salmonella typhi by electroporation. Mol Gen Genet. 1990;223:156–158. doi: 10.1007/BF00315809. [DOI] [PubMed] [Google Scholar]
- 17.Pang T, Bhutta Z A, Finlay B B, Altwegg M. Typhoid fever and other salmonellosis: a continuing challenge. Trends Microbiol. 1995;3:253–255. doi: 10.1016/s0966-842x(00)88937-4. [DOI] [PubMed] [Google Scholar]
- 18.Russel C B, Thaler D S, Dahlquist F W. Chromosomal transformation of Escherichia coli recD strains with linearized plasmids. J Bacteriol. 1989;171:2609–2613. doi: 10.1128/jb.171.5.2609-2613.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanderson K E, Hessel A, Rudd K E. Genetic map of Salmonella typhimurium, edition VIII. Microbiol Rev. 1995;59:241–303. doi: 10.1128/mr.59.2.241-303.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Squires C H, DeFelice M, Lago C T, Calvo J M. ilvHI locus of Salmonella typhimurium. J Bacteriol. 1983;154:1054–1063. doi: 10.1128/jb.154.3.1054-1063.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Youderian P, Sugiono P, Brewer K L, Higgins N P, Elliot T. Packaging specific segments of the Salmonella chromosome with locked-in Mud-P22 prophages. Genetics. 1988;118:581–592. doi: 10.1093/genetics/118.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zahrt T C, Mora G C, Maloy S. Inactivation of mismatch repair overcomes the barrier to transduction between Salmonella typhimurium and Salmonella typhi. J Bacteriol. 1994;176:1527–1529. doi: 10.1128/jb.176.5.1527-1529.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zahrt T C, Maloy S. Barriers to recombination between closely related bacteria: MutS and RecBCD inhibit recombination between Salmonella typhimurium and Salmonella typhi. Proc Natl Acad Sci USA. 1997;94:9786–9791. doi: 10.1073/pnas.94.18.9786. [DOI] [PMC free article] [PubMed] [Google Scholar]