Abstract
Diagnostics is an important part of medical practice. The information required for diagnosis is typically collected by performing diagnostic tests, some of which include imaging. Magnetic resonance imaging (MRI) is one of the most widely used and effective imaging techniques. To improve the sensitivity and specificity of MRI, contrast agents are used. In this review, the usage of metal–organic frameworks (MOFs) and composite materials based on them as contrast agents for MRI is discussed. MOFs are crystalline porous coordination polymers. Due to their huge design variety and high density of metal ions, they have been studied as a highly promising class of materials for developing MRI contrast agents. This review highlights the most important studies and focuses on the progress of the field over the last five years. The materials are classified based on their design and structural properties into three groups: MRI-active MOFs, composite materials based on MOFs, and MRI-active compounds loaded in MOFs. Moreover, an overview of MOF-based materials for heteronuclear MRI including 129Xe and 19F MRI is given.
Keywords: metal−organic frameworks, magnetic resonance imaging, nanomedicine, theranostics, multimodal imaging
1. Introduction
An accurate diagnosis plays a crucial role in determining a proper course of treatment. It requires collecting information from different sources, including diagnostic tests based on imaging. From this point of view, magnetic resonance imaging (MRI) is one of the most versatile, noninvasive, and nonionizing imaging techniques used in routine clinical examinations providing both anatomical and biochemical information.1 MRI is particularly sensitive in assessing anatomical structures, organs, and soft tissues with a high resolution for the detection and diagnosis of a broad range of pathological conditions. MR images can provide contrast between benign and pathological tissues and may be used to stage cancers as well as to evaluate the response to treatment.2 Although MRI is a very powerful imaging modality, in many cases, the use of contrast probes is mandatory to fully exploit the diagnostic potential of MRI by increasing the specificity and sensitivity of the method.3 To improve the image contrast, different compounds and materials have been examined as contrast agents and from these, few have been approved for a clinical usage.4−7 However, despite their success, there is still room for improvement (in order to maximize the information, which can be retracted) and thus, new compounds and materials are being constantly developed and studied as potential contrast agents.
One of the newer material groups, which have been suggested for applications in MRI, are metal–organic frameworks (MOFs). MOFs are porous crystalline coordination polymers.8−10 They consist of metal ions (or clusters) and bridging ligands. Due to their design variety, tunable properties, and extremely high surface area, they have been investigated in many different applications including gas storage and separation,11−14 catalysis,15,16 sensing,17,18 and also medical areas.19−24 In medicine, due their porosity (and thus a high loading capacity), they have been suggested as highly promising materials for drug delivery applications.19−22 Recently, also their applications in diagnostics have been examined for various imaging modalities, including fluorescence and photoacoustic imaging,25,26 but mainly MRI.27,28 The early stages of MOFs for applications in MRI were reviewed in the beginning of 2018 by Wuttke et al., who reviewed about 25 publications.27 Since then, the field has expanded rapidly resulting in about 161 publications (August 2022, Figure 1). In this review, we highlight the key publications from the past and focus on the latest progress of the field over the last 5 years. Moreover, only publications, which comprise MRI measurements, are included (ca. 55 publications); i.e., reports, which suggest the concept of using MOFs in MRI, but do not show any experimental data, are omitted.
Figure 1.
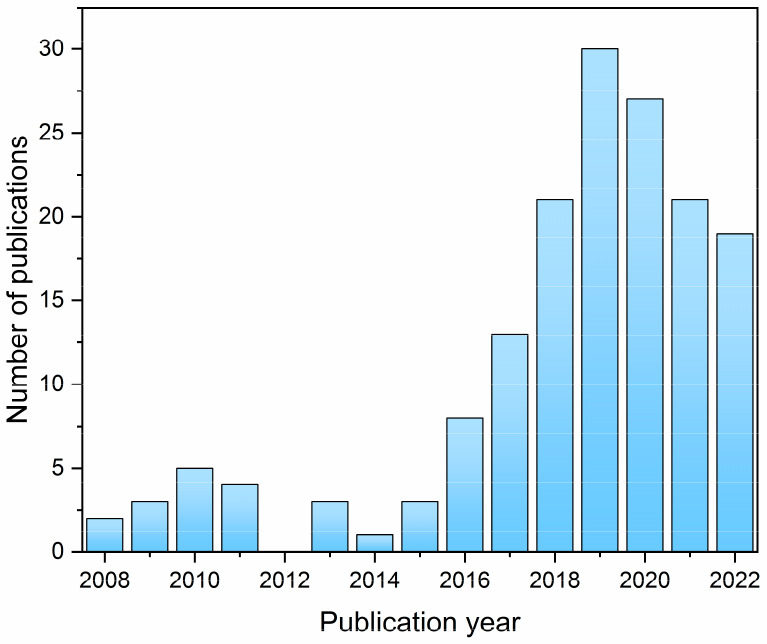
A number of publications per year containing both terms “metal-organic framework” and “magnetic resonance imaging” according to the SciFinder database (August 2022).
2. Basics of MRI
2.1. MR Phenomena
Magnetic resonance (MR) is a method based on the distribution and behavior of magnetic moments of particular isotopes in a magnetic field.29 As the method is nonionizing and provides information about biochemical processes in living tissue, it has become one of the most important noninvasive imaging techniques. Simply put, the principle of the MR method is based on the absorption of energy by nuclei placed in a strong static magnetic field. In general, all isotopes with a nonzero magnetic moment such as hydrogen, fluorine and phosphorus can be employed. However, for routine clinical applications, only 1H nuclei are used because their MR sensitivity is greater than all other nuclei. The distribution of water molecules reflects the structural composition of tissue. As changes in the water properties of tissue also closely reflect pathologic processes, this relationship is an important factor in the high success rate of MR imaging in medical applications.
The frequency of precession of isotopes depends on the magnetic field intensity of external field and on the type of nucleus, which is expressed by gyromagnetic constant γ. The external magnetic field represented by short radiofrequency pulses, the frequency of which corresponds to the Larmor frequency (in the range of 10 MHz–1 GHz), applied perpendicular to the static field, affects the magnetization vector generated by the inserted nuclei. Thus, the system of nuclei starts to absorb the energy of electromagnetic field of the radiofrequency pulses. This phenomenon is called the nuclear magnetic resonance. Depending on the radiofrequency pulse intensity and the duration, resulting magnetic moment can be flipped in any orientation, most often onto a plane that is perpendicular to external static magnetic field. Immediately after the radiofrequency pulse is applied, all excited nuclei are at the same phase and start to return to equilibrium. The return is called the relaxation process, during which the nuclei release absorbed energy in the form of electromagnetic radiation detected in the receiver coils. The induced alternating electromotive force is called the MR signal or the free induction decay signal and contains a signal from each excited nucleus and its amplitude is proportional to the number of nuclei that contribute to its formation.
2.2. MR Relaxation
The speed of relaxation in biological tissues is generally in the range of several milliseconds to a few seconds. As nuclei are parts of molecules, their relaxation processes depend on various factors such as temperature, magnetic field strength, chemical bonds, molecular motions, size of molecule, etc. Relaxation plays a key role in MR imaging because it affects the contrast between tissues; the difference in relaxation times makes it possible to achieve contrast in MR images. There are two independent relaxation processes, each with exponential dependence: longitudinal (T1) relaxation and spin–spin (T2) relaxation. The T1 relaxation can be described as an energy flow between excited spins and their external environment; the predominant mechanism of that is dipole–dipole interactions. The spin–spin relaxation occurs due to an exchange of energy between protons within the excited system; there is no energy change in the system of excited atoms. The T2 relaxation reflects the speed of loss of the measurable macroscopic magnetization in the plane perpendicular to the external static magnetic field. It also indicates the magnetic inhomogeneities inside the excited sample because they can affect the speed of phase coherence lose significantly. In practice, we must take into account the local magnetic field nonuniformities resulting from intrinsic defects in the magnet itself or from susceptibility-induced field distortions produced by the tissue or other materials placed within the field. Therefore, an effective T2* (also called T2 star) relaxation time (always shorter than T2), which covers all sources of field inhomogeneities across a voxel, is often used.
2.3. MR Imaging
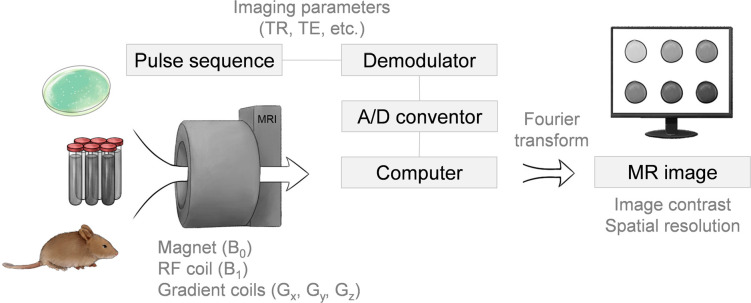
MR images are characterized by signal intensity and contrast, which are affected by the T1 and T2 relaxation as well as proton density. This means that for the same object from the same area, different MR contrasts are produced according to the dominant influence (weighting), i.e., proton density or T1 and T2 (T2*) relaxation. The level of weighting depends on various combinations and the order of radiofrequency (RF) pulses and gradients (small linear magnetic fields superposed to static magnetic field used for slice selection, spatial encoding etc.) that create the MR imaging sequences.30 The simultaneous application of RF pulses and synchronized changes in the gradients lead to signal acquisition from different places in space. There are basically two types of MR imaging sequences: (i) the spin echo forming signal by two radiofrequency pulses (the first a 90° pulse, the second 180° pulse)31,32 and (ii) gradient echo sequence forming signal by one radiofrequency pulse (usually 5–90° pulse) and gradient reversal.33,34 Many parameters characterize the MR imaging sequence. The most important parameters for contrast in MR images are repetition time (TR), echo time (TE), and flip angle of excitation radiofrequency pulse. The sequence parameters should be optimized according to type of tissue and used contrast agents (their relaxation times). It should be noted that in any weighted MR image, there are always contributions from both types of relaxation. A scheme of a typical MRI system with the discussed components is illustrated in Figure 2.
Figure 2.
Scheme of a typical MRI systems. A specimen is placed in a magnetic field (B0), then a radio frequency (RF) coil produces a B1 field that changes the direction of the magnetization in a manner prescribed by the pulse sequence. Spatial localization is generated with the use of the gradient coils. Variation of the imaging parameters such as the repetition time (TR) and echo time (TE) in the pulse sequence provides the basis for different contrast mechanisms.
2.4. General Requirements for MRI Contrast Agents
Contrast agents3−7 are used in clinical practice to improve the quality of images and to enhance detectability of pathological processes and distinguishing pathologies from healthy tissue. In experimental medicine, contrast agents are also used for a visualization of transplanted cells or imaging labels for monitoring drug delivery systems.5,35−39 The main purpose of contrast agents is to change a contrast in the images. In case of magnetic resonance, the change of the contrast enhancement is based on the altering of relaxation times, which goes beyond the intrinsic relaxation behavior of a targeted area such as cells or organs. The majority of MR contrast agents are either paramagnetic gadolinium ion complexes or superparamagnetic magnetite nanoparticles (Figure 3).3−7 These agents shorten both T1 and T2/T2* the relaxation times.40−42 This shortening, which reflects the efficiency of contrast agent, depends on many parameters including the concentration of the contrast agent. To compare the efficacy of contrast agents properly, relaxivity is used, which reflects how the relaxation rates (the inverse of the relaxation times) of the solution change depends on the concentration. Relaxivities r1 and r2, which should be as high as possible, depend on the temperature, field strength and substance in which the contrast agent is dissolved. Typical values for clinically approved contrast agents are up to 10 L· mmol–1·s–1.43 The relaxivities of experimental contrast agents can reach much higher values, especially for r2.44,45 The resulting r2/r1 ratio indicates whether the application of contrast will be more effective as a positive (T1) or negative (T2) contrast agent.46
Figure 3.
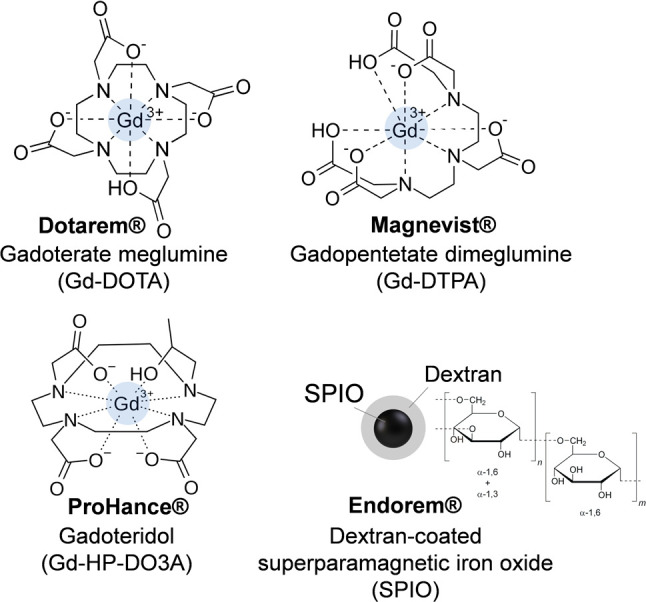
Examples of structures of commercially available contrast agents. DOTA = 2,2′,2″,2‴-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayl)tetraacetic acid; DTPA = 2,2′,2′′,2′′′-{[(carboxymethyl)azanediyl]bis(ethane-2,1-diylnitrilo)}tetraacetic acid; HP-DO3A= 2,2′,2″-[10-(2-hydroxypropyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl]triacetic acid.
Contrast agents are exposed to different conditions in in vivo experiments, so their chemical stability is a very important parameter. In general, maximum stability is required, but in some cases, under certain conditions, such as a change in pH or temperature, a stimuli-triggered degradation of the probe might be desired, for instance, to monitor a drug release.36,47,48
Toxicity is closely related to the chemical stability. In clinical practice, the most common Gd-based extracellular contrast agents are all chelates containing Gd(III) ions. Free gadolinium ions are highly toxic and can cause various negative side effects, such as nephrogenic systemic fibrosis, enzyme inhibition, calcium channel blockade, etc.49−51 Therefore, it is crucial that Gd(III) is tightly bound to the chelate to prevent its toxic effects. However, despite the Gd-chelation, there have been controversies over the agents’ safety.52 These concerns pointed to the importance of reducing the subject’s exposure to the contrast agent and to the importance of evaluating the clearance of administered contrast agents.
Iron-based probes are generally considered nontoxic because iron nanoparticles can be degraded and utilized by cells via the physical pathway of iron metabolism.53 However, some studies have shown that a high iron load in cells is toxic to the cells and can impaired their normal function.54 The agent toxicity is affected by many factors including size, charge and surface chemistry, etc.55−57 The side effects are mainly due to whether the nanoparticles undergo biodegradation in the cellular environment and what cellular reactions the degraded nanoparticles elicit.
In summary, an optimal probe for MR should possess: (i) an adequate solubility or dispersibility in water/body fluids, (ii) high relaxivities r1, r2, (iii) chemical stability, (iv) nontoxicity and reliable pharmacokinetic and pharmacodynamic properties, including biodegradability and circulation time, (v) easily modifiable surface (e.g., for a targeting ligand attachment), and possibly also (vi) a responsivity to stimuli.
3. MOFs as Contrast Agents in MRI
MRI has been considered as the key potential application of MOFs in diagnostics.25−28 Due to the huge design variety of MOFs,8−10 several different strategies for preparing MRI contrast agents based on MOFs have been reported. As the first candidates, MOFs comprising metal ions with suitable MR-properties (Table 1), namely Gd(III), Mn(II) and Fe(III), have been investigated (section 3.1 and Table 2). They offer the advantage that the MOF itself is the active component, which leads to a high efficiency due to the high metal content. Another approach, which have been reported, is an integration of MRI-active metal oxide nanoparticles, such as Fe2O3 and Fe3O4 nanoparticles, into MOFs (section 3.2 and Table 3). Last but not least, also possibilities of including contrast agents based on metal complexes into MOF pores have been proposed (section 3.3). All these three design strategies (Figure 4) are included in this review and discussed in the following chapters. As shown on many examples (Table 2 and 3), MOFs are highly promising materials for developing contrast agents in MRI. However, MOF stability (combined with a possible metal leakage) and toxicity are of a concern. Thus, it is highly important that the material properties such as the material chemical stability (in biological conditions) and toxicity (including the MOF building components) are investigated and reported.
Table 1. Outer Orbital, Spin, and Calculated Effective Magnetic Moment (μeff) of Selected Metal Ions.
| metal ion | orbital | spin | μeff |
|---|---|---|---|
| Gd(III) | 4f7 | 7/2 | 7.94 |
| Mn(II) | 3d5 | 5/2 | 5.92 |
| Fe(II) | 3d6 | 2 | 4.90 |
| Fe(III) | 3d5 | 5/2 | 5.92 |
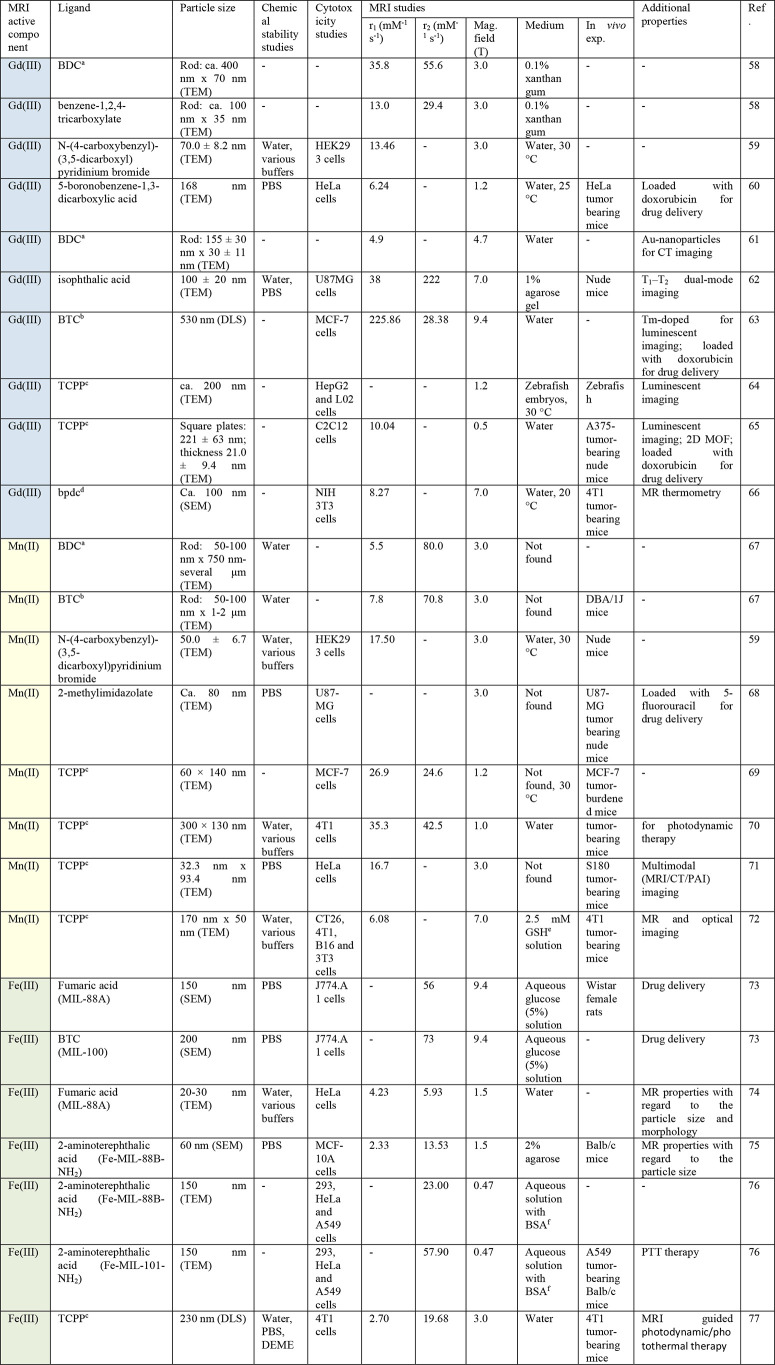
Table 2. Overview of MRI-Active MOFs and Their Properties.
BDC = benzene-1,4-dicarboxylate.
BTC = benzene-1,3,5-tricarboxylate.
TCPP = 5,10,15,20-tetrakis(4-carboxyphenyl)-21H,23H-porphyrin.
bpdc = 2,20-bipyridine-6,60-dicarboxylate.
Glutathione.
Bovine serum albumin.
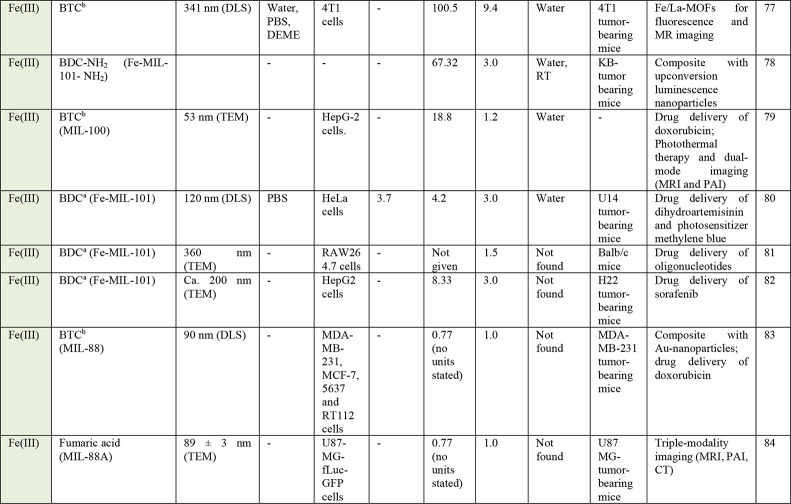
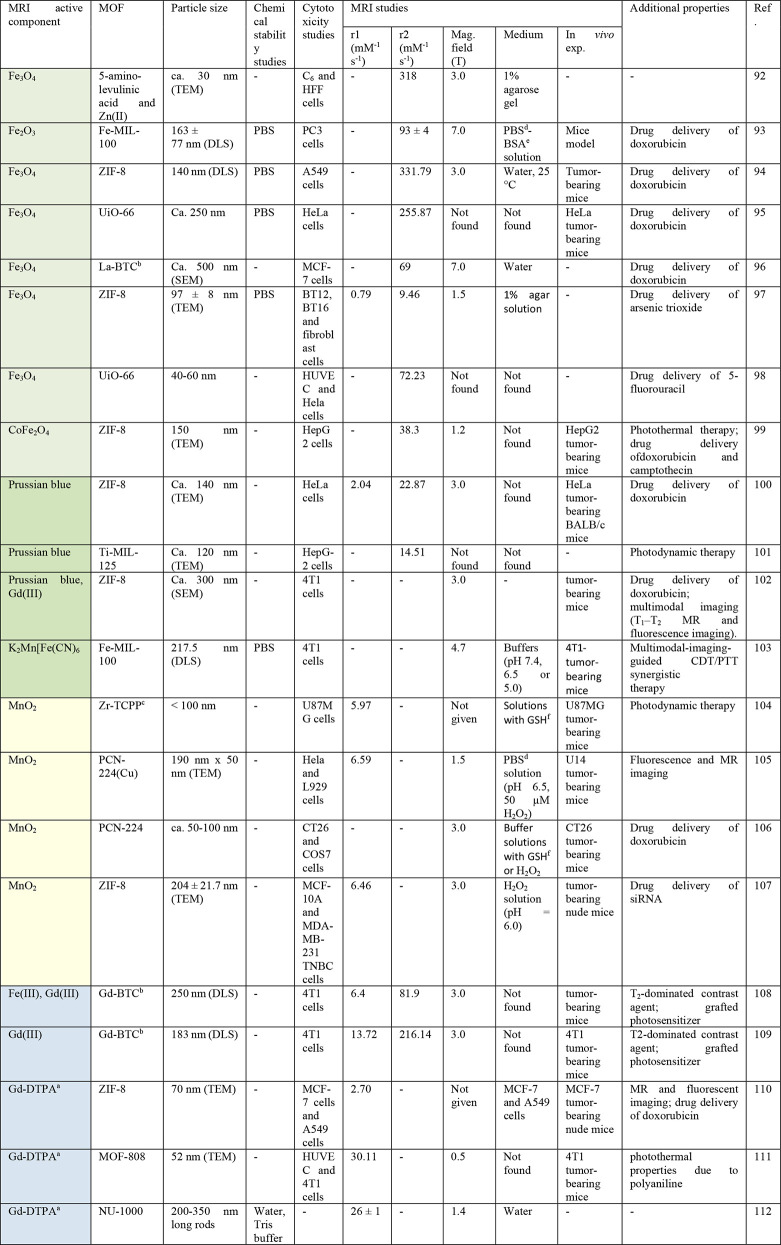
Table 3. Overview of Composites Based on MOFs and MRI-Active Nanoparticles, and Their Properties.
DTPA = diethylenetriamine pentaacetic acid.
BTC = benzene-1,3,5-tricarboxylate.
TCPP = 5,10,15,20-tetrakis(4-carboxyphenyl)-21H,23H-porphyrin.
Phosphate-buffered saline.
Bovine serum albumin.
Glutathione.
Figure 4.
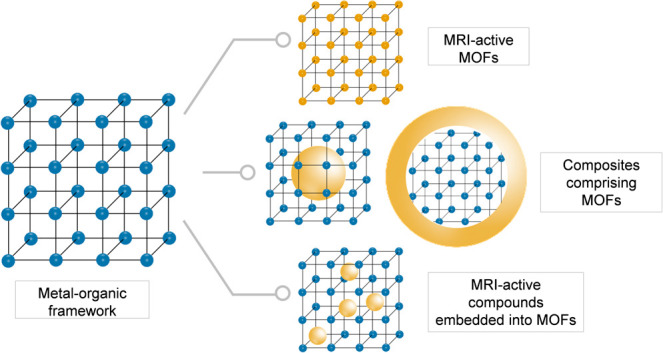
Scheme of different design strategies for preparing MRI-active materials based on MOFs; the MRI-active component is shown in a yellow color.
3.1. MRI-Active MOFs
MOFs are built up from metal ions (or clusters) and bridging organic ligands. Therefore, if metal ions with suitable magnetic properties are used, an MRI-active MOF can be prepared. Due to the magnetic properties (resulting from the number of unpaired electrons), Gd(III)-, Mn(II)- and Fe(III)-based MOFs are the most suitable (Table 1). From these, due to the concerns induced by material (in)stability and possible metal leakage, MOFs comprising Fe(III) ions (i.e., an essential metal element) are considered as the most promising. However, all three groups (Gd-, Mn- and Fe-based MOFs) have been intensively investigated over the past few years (Table 2).
3.1.1. Gd-MOFs
Already in 2006, Lin at al. reported on the first Gd(III)-MOFs as contrast agents for MRI.58 Since then, several other Gd(III)-MOFs comprising mainly carboxylate ligands have been reported (Table 2), including ligands such as benzene-1,4-dicarboxylate58,61 and benzene-1,3,5-tricarboxylate,63 but also N-(4-carboxybenzyl)-(3,5-dicarboxyl)pyridinium bromide59 or 5-boronobenzene-1,3-dicarboxylate.60 Moreover, it has been shown that the particle size85 as well as the particle morphology63 influenced the material relaxivities, and thus these parameters could be used as efficient tools to tailor the material properties for MRI. For instance, relaxation properties of Gd(III)-MOFs comprising either benzene-1,4-dicarboxylate or benzene-1,2,4-tricarboxylate ligands were studied with regard to the crystal size.85 The results clearly indicated a positive correlation between the surface areas of the Gd-MOF nanoparticles with the longitudinal relaxivity in MRI. In particular, Gd-MOF nanoparticles with an average size of 82 nm yielded a high longitudinal relaxivity value of 83.9 mM–1 s–1.
Following a general trend centered around multifunctional materials, the research focus of the field of Gd-MOFs for MRI has slightly shifted over the past few years. Instead of preparing new Gd-MOFs, more attention has been paid to combining known Gd-MOFs with other materials in order to prepare agents for multimodal imaging or theranostic agents (i.e., agents combining therapy and diagnosis). For example, Icten et al. proposed to combine Gd(III)-MOFs (comprising either benzene-1,4-dicarboxylate or benzene-1,3,5-tricarboxylate ligands) with boron-10 isotope to prepare dual agents for MR imaging and neutron capture therapy.86 Boyes et al. combined a Gd(III)-MOF comprising benzene-1,4-dicarboxylate ligands with Au-nanoparticles (Figure 5).61 Au-nanoparticles, due their high atomic number and superior absorption coefficient, have been suggested as contrast agents for CT imaging.87 Thus, if combined with Gd-MOFs, agents for dual imaging can be obtained. The r1 value of the nanocomposite was 4.9 mM–1 s–1 (at 4.7 T). Meanwhile, the nanocomposite also enhanced the contrast of CT imaging, even when the Au concentration was as low as 1.66 mg/mL.
Figure 5.
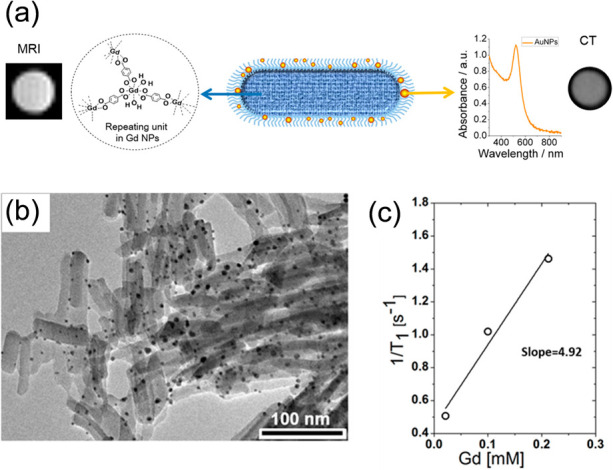
(a) Schematic representation of a Gd-MOF–Au nanostructure proposed as an agent for dual imaging. (b) TEM micrograph of the Gd-MOF–Au nanostructure and its (c) relaxation rate (1/T1) as a function of the Gd-concentration. Adapted with permission from ref (61). Copyright 2015, American Chemical Society.
Xie et al. reported on a Eu/Gd-MOF (comprising isophthalate ligands) as a T1–T2 dual-mode contrast agents.62 To improve the material stability, the particle surface was coated with a layer of silica. The nanoparticles exhibited high longitudinal (38 mM–1 s–1) and transversal (222 mM–1 s–1) relaxivities (at 7.0 T). The authors speculated that such high relaxivity values were due to the rigid confinement of Gd(III)-ions in the nanosystem and slow interexchange of Gd(III) with water molecules. In another work, a Tm/Gd-MOF comprising benzene-1,3,5-tricarboxylate ligands were designed in order to prepare luminescent and MRI active nanoparticles for drug delivery.63 By varying the reaction parameters, different morphologies and sizes were obtained. The particles were loaded with doxorubicin as a model drug, and their surface was modified by mesoporous silica and folic acid. MRI measurements revealed an unusually high longitudinal relaxivity of 225.86 mM–1 s–1 (at 9.4 T). Li et al. suggested a Gd(III)-porphyrin MOF for magnetic resonance and fluorescence imaging due to the gadolinium and porphyrin properties, respectively.64 MOF nanoparticles coated with folic acid featured low biotoxicity, emitted bright red fluorescence and their MR properties were studied on zebrafish embryos and zebrafish. Similarly, Yan et al. also reported on a Gd(III)-porphyrin MOF for MR and fluorescence imaging.65 The prepared nanoparticles were loaded with doxorubicin as a model drug and their MRI properties were investigated both in vitro and in vivo. The r1 relaxivity was determined to be 10.04 mM–1 s–1 (at 0.5 T).
Gd(III)-based MOFs have been also suggested as contrast agents for magnetic resonance thermometry.66 Magnetic resonance thermometry is a noninvasive method which offers high spatial and temporal resolution for monitoring of temperature, for example, during cancer treatment.88 However, it suffers from low temperature sensitivity and image contrast. Therefore, different compounds have been developed and investigated as agents for improving the contrast. For instance, Zhang et al. prepared a Gd(III) zeolite-like MOF based on 2,20-bipyridine-6,60-dicarboxylate ligands.66 The nanoparticles were shown to be biocompatible and exhibited benchmark performance with respect to in vitro and in vivo MR thermal mapping. Their r1 relaxivity was found to be 8.27 mM–1 s–1 per Gd(III) ion (at 7.0 T). Moreover, a T1-based thermal map (20–50 °C) showed that the Gd-MOF was sufficiently sensitive to quantify temperature changes using T1 effects; even at very low concentrations (as low as 33 mM). Moreover, the high sensitivity was proven also in vivo on an example of thermal mapping of tumor-bearing mice.
3.1.2. Mn-MOFs
Due to suitable magnetic properties, Mn(II)-ions have often been used to prepare contrast agents based on MOFs for MR imaging (Table 2). They can be either integrated as the inorganic unit to build up the MOF, or they can be included as a part of the ligand such as a coordination in porphyrins. Moreover, in the past few years, there has been a significant increase in reports of MOFs based on Mn(III)-ions as GSH-activated T1 contrast agents for cancer diagnosis.72
The first Mn(II)-MOFs as contrast agents for MRI were reported in 2008 by Lin at al.67 They synthesized two Mn(II)-MOFs based on benzene-1,4-dicarboxylate and benezene-1,3,5-tricarboxylate ligands exhibiting r1 of 5.5 and r2 of 80.0 mM–1 s–1, and r1 of 7.8 and r2 of 70.8 mM–1 s–1 (at 3.0 T, per Mn ions), respectively. Since then, several other MOFs based on carboxylate ligands have been developed. For example, Chen et al. reported on a Mn(II)-MOF comprising N-(4-carboxy benzyl)-(3,5-dicarboxyl)pyridinium bromide as a ligand and studied its MR properties in vitro and in vivo.59 MR images of treated mice indicated that kidneys showed remarkably positive signal enhancement after 15 min with intravenous administration of the MOF and the hyperintensity of both kidneys persisted for about 240 min with no obvious tissue damage (Figure 6). The results suggested that the MOF could be used for imaging renal dysfunction, which was rather surprising considering the large particle size 50.0 ± 6.7 nm (as determined by TEM), which usually prevents a renal clearance. The authors suggested that the plausible mechanism could be that these particles disintegrated into smaller sizes through a collision and gradual decomposition over time. In another work, a MOF ZIF-8, which comprises Zn(II) ions and 2-methylimidazolate ligands, was used as a precursor to synthesize a Mn(II)-MOF suitable for visualization by MRI.68 By incubating ZIF-8 with Mn(II)-ions, some of the Zn(II) ions could be postsynthetically exchanged. In the final product, the ratio of Mn to Zn was 1:7. When Mn-ZIF-8 was injected intravenously into the tumor-bearing mice, enhanced T1-weighted MR signals could be observed at the tumor area. The signal intensity of Mn-ZIF-8 increased continuously after intravenous injection and peaked at 12 h.
Figure 6.
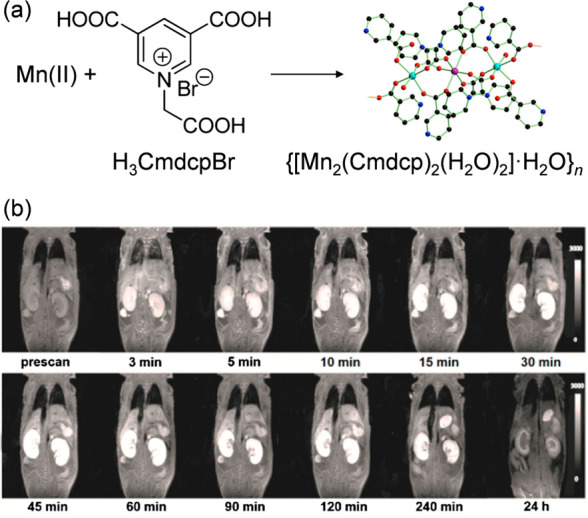
(a) Reaction scheme of the Mn(II)-MOF synthesis and (b) MR signal intensity from a dynamic study of kidneys after intravenous administration of the Mn(II)-MOF. Adapted with permission from ref (59). Copyright 2017, American Chemical Society.
Yin et al. studied a MOF comprising Zr(IV)-ions and Mn-porphyrin ligands as a T1-weighted MR contrast agent.69 The MOF exhibited a high r1 value of 26.9 mM–1 s–1 (at 1.2 T), therefore, it was further studied in vivo in a mouse model. A bright signal was detected in the liver and kidney after 1 h after the injection and decreased after 24 h. The consistent results were observed also in a MCF-7 tumor-bearing mouse model. The signal in both the liver and kidney was enhanced, but in addition to that, also the signal in the tumor was enhanced, suggesting good tumor targeting of the MOF. Moreover, s-nitrosothiol was conjugated to the surfaces of the MOF nanoparticles for heat-sensitive NO generation. In another work, Yang et al. reported on a MOF PCN-222(Mn), comprising Zr(IV) ions and Mn-porphyrin ligands for MRI and for photodynamic therapy.70 Due to the high Mn(II)-concentration through the framework, large open channels and high water affinity in the channels, a high longitudinal relaxivity of 30.3 (at 0.5 T) and 35.3 mM–1 s–1 (at 1.0 T) was measured. Moreover, the intravenous injection of the MOF into a tumor-bearing mice model provided a good T1-weighted contrast of the tumor area. The enhanced brightness observed in these images was maintained for approximately 8 h after injection, indicating that the MOF can provide a long-term enhanced contrast. Cheng et al. designed and studied a MOF based on hafnium clusters and porphyrin ligands functionalized with Mn(II) and coated with folic acid as a theranostic agent suitable for triple-modality imaging (MRI/CT/PAI).71 The r1 relaxivity was 16.75 mM–1 s–1 (at 3.0 T). In vivo MRI experiments carried out on S180 tumor-bearing mice revealed that there was an obvious enhancement in T1-weighted images after the injection in comparison to the preinjection images. The in vivo results further indicated that coating the nanoparticles with folic acid resulted in tumor targeted delivery.
MOFs comprising Mn(III)-ions can be used as responsive systems for antioxidant glutathione (GSH) as demonstrated by Zhang et al.72 They reported on a MOF comprising Mn(III) ions and porphyrin ligands. Interestingly, upon endocytosis by tumor cells, the MOF was decomposed into its building components (i.e., Mn-ions and free porphyrin ligands) and due to the redox reaction between Mn(III) and intracellular GSH, Mn(II)-ions were released. In in vitro experiments, the T1 signal of the MOF before and after adding GSH (2.5 mM) was recorded by MRI. Compared with a control without GSH (r1 of 2.65 mM–1 s–1), the T1-relaxation rate (r1) had a nearly 2.3-fold enhancement in the presence of GSH (r1 of 6.08 mM–1 s–1, at 7.0 T) indicating the potential of MOFs as a GSH-activated T1-contrast agent for cancer diagnosis. The GSH-triggered contrast enhancement was further confirmed also in vivo, suggesting that the nanoparticles could be used not only as contrast agents, but also to monitor MOF disintegration in vivo.
3.1.3. Fe-MOFs
In 2010, Horcajada et al. reported on the first Fe(III)-carboxylate MOFs for MR imaging.73 They demonstrated that the investigated MOFs, namely MIL-88A and MIL-101, had similar transverse relaxivity (r2) as conventional MRI contrast agents based on iron oxides. Since then, Fe(III)-carboxylate MOFs became the most studied class of MOFs for MRI applications (Table 2).
Wuttke et al. studied the MR properties of an Fe(III)-fumarate MOF (MIL-88A) with regard to different particle morphology and size.74 All in all, four different variants were studied. The results showed that both r1 and r2 relaxivities tend to increase with the increase of the particle size because of higher number of paramagnetic Fe-centers in larger particles. Similarly, Khoobi et al. studied the influence of a particle size of Fe-MIL-88B (comprising Fe(III)-cluster and 2-aminoterephthalate ligands) on the MR properties.75 They synthesized the MOF in three different particle sizes (60, 350, and 730 nm) and determined their relaxivity. The studies revealed that by increasing the MOF crystal size, the incremental transverse relaxivity increased and the r2/r1 ratios reached values of 5.80, 42.27, and 127.00. The smallest nanoparticles were also investigated by MRI in vivo.
In another work, the MR properties of Fe-MIL-88B-NH2 were compared with Fe-MIL-101-NH2.76 Particles of both MOFs had an octagonal morphology and uniform size of about 150 nm. The transverse relaxivity (r2) value of Fe-MIL-88B-NH2 was determined to be 23.00 mM–1 s–1 (at 0.47 T), which was approximately 2.5-times less than the relaxivity of Fe-MIL-101-NH2, which was measured to be 57.90 mM–1 s–1 (at 0.47 T, Figure 7). The results suggested that the Fe-MIL-101-NH2 was a better candidate than Fe-MIL-88B-NH2 as a contrast agent in MRI, even though both materials had the same composition, morphology and size. The authors suggested that the difference in the r2 values could be attributed to the different interconnection of the pores resulting in a different diffusivity and exchange of water molecules within the pores. Fe-MIL-101-NH2 was further functionalized with graphene oxide nanosheets and studied as a material for photothermal therapy (PTT).76 Similarly Ren et al. reported on an Fe(III)-MOF based on porphyrin ligands as a promising platform for MR imaging and photodynamic/photothermal therapy.77
Figure 7.
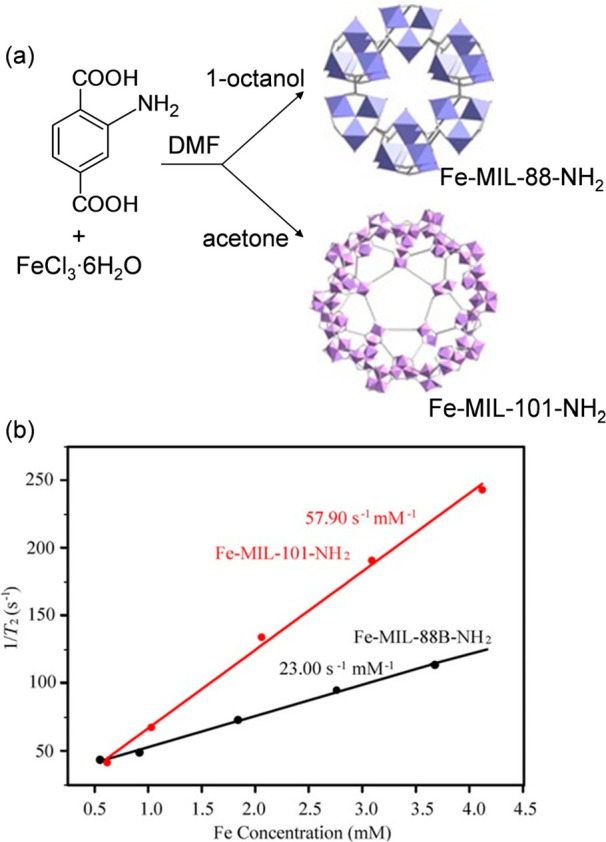
(a) Reaction scheme of the synthesis of Fe-MIL-88-NH2 and Fe-MIL-101-NH2, and (b) relaxation rate (1/T2) versus various Fe(III) molar concentrations for the two MOFs. Adapted with permission from ref (76). Copyright 2017, Wiley-VCH Verlag GmbH and Co. KGaA.
Wang et al. investigate heterometallic Fe/La-MOFs for fluorescence and MR imaging.89 The surface of these particles was coated with a layer of NH2-modified silica and their MR properties were investigated. The T2-weighted MR images showed a clear concentration-dependent contrast enhancement and the relaxivity r2 was determined to be 100.5 mM–1 s–1 (at 9.4 T). The author suggested that this exceptionally high value could be attributed to the seven empty 4f orbitals of La(III), which interact strongly with water molecules. In addition, water molecules are readily accessible to the paramagnetic iron atoms within the Fe/La framework through the polar amino-modified silica shell. Tang et al. reported on core–shell nanoparticles for MR and luminescence imaging prepared by combining upconversion luminescence nanoparticles and Fe-MIL-101-NH2.78 The surface of the particles was modified by a derivative of polyethylene glycol and by folic acid. The T2-relaxation time of water protons was shortened from about 2047 to 5.6 ms and the relaxivity r2 was determined to be 67.32 mM–1 s–1 (at 3.0 T). The particles were tested in vivo for multimodal imaging in KB-tumor bearing mice. After 24 h, the signal intensity at the tumor area decreased by about 35% indicating that the nanoparticles were successfully delivered to the tumors. In another work Wang et al. studied core–shell nanoparticles comprising a polypyrrole core and a Fe-MIL-100 shell.79 Due to the polypyrrole core, the prepared nanoparticles exhibited a strong absorption in the near-infrared region and possessed a good photothermal efficiency. Due to the Fe(III)-MOF shell, the particles could be detected by MRI and their relaxivity value was determined to be r2 = 18.8 mM–1 s–1 (at 1.2 T). Therefore, the nanoparticles were proposed as agents for photothermal therapy and multimodal imaging (MRI and PAI). Moreover, they were also tested for drug delivery of doxorubicin.
Due to the material low toxicity, Fe(III)-MOFs are popularly used in drug delivery systems.90 When combined with MR imaging, theranostics (i.e., agents combining therapy and diagnosis within one system) are prepared. For instance, Fe-MIL-101 was reported as a theranostic agent for MRI and drug delivery of an anticancer drug dihydroartemisinin and photosensitizer methylene blue.80 The MOF nanoparticles were coated with polylactic acid and polyethylene glycol to achieve controllable drug release and good biocompatibility. The transverse relaxivity r2 was determined to be 4.2 mM–1 s–1, while the longitudinal relaxivity r1 was only 3.7 mM–1 s–1 (at 3.0 T), suggesting that the nanoparticles could be used as a T2 contrast agent (Figure 8). In vivo T2 weighted MR images indicated an effective enrichment of the nanoparticles within a tumor area. The MR signal of the tumor areas was much stronger after 9 h and continued up to 24 h postinjection. Qu et al. reported on Fe-MIl-101 for drug delivery of unmethylated cytosine–phosphate–guanine oligonucleotides for enhancing an immune response and MR imaging.81 Both in vitro and in vivo MRI studies were carried out. Similarly, Xu et al. reported on Fe-MIL-101 for drug delivery of sorafenib.82 To enhance the targeting ability, the nanoparticle surface was functionalized with an iRGD peptide. The transverse relaxivity r2 was determined to be 8.33 mM–1 s–1 (at 3.0 T). Kulinowski et al. reported on Fe-MIL-101-NH2 for drug delivery of isoniazid and examined the nanoparticle MR properties on a lung tissue phantom and on rat lungs ex vivo.91
Figure 8.
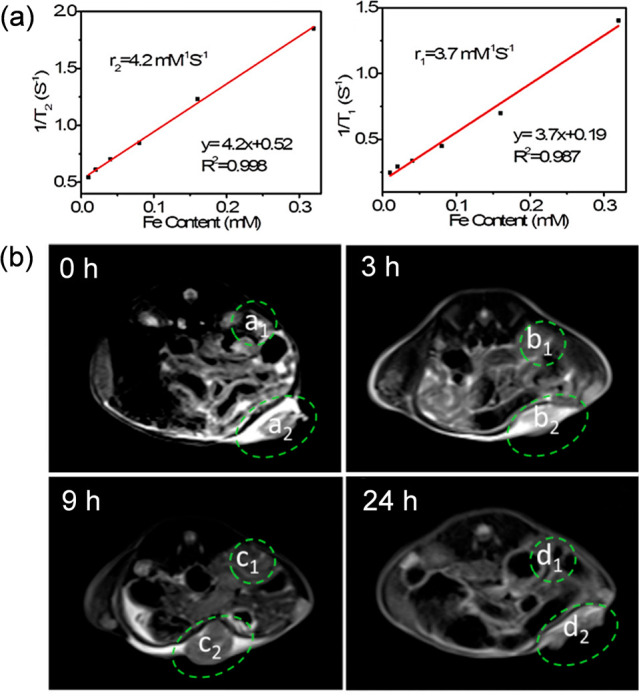
(a) Transverse (1/T2) (left) and longitudinal (1/T1) relaxation rate of suspensions of Fe-MIL-101 plotted versus the Fe-content and (b) in vivo MR images of a mouse bearing implanted U14 cancer after the intravenous injection of MOFs (10 μg Fe per g) at 0 h, 3, 9, and 24 h (a1, b1, c1, and d1 represent the normal tissues areas; a2, b2, c2, and d2 represent the tumor areas). Adapted with permission from ref (80). Copyright 2019, American Chemical Society.
Fe(III)-MOFs can be also combined with gold nanoparticles to prepare theranostics for photothermal therapy. Whereas the Fe(III)-MOF is used for drug loading and MR imaging, Au-nanoparticles, when irradiated with a laser, can be used as agents for photothermal therapy and heat induced drug release. Tian et al. combined Au-nanoparticles with a MOF based on Fe(III) ions and benzene-1,3,5-tricarboxylate ligands (MIL-88).83 The r2 relaxivity of the agent was determined to be 0.77 and its feasibility in in vivo MR imaging was investigated. The particles were injected into MDA-MB-231 tumor-bearing mice and time-dependent T2 MR imaging was carried out. The signal intensity increased 1 h postinjection and continued to rise over the next 24 h. In another work, the authors combined Au-nanoparticles and MIL-88A to prepare core–shell nanoparticles for multimodal imaging.84 The Au-core possessed CT enhancement and PAI optical properties, while the MOF shell exhibited a T2-weighted MR property. The surface of the nanoparticles was modified by poly(ethylene glycol)-carboxylic acid to improve the dispersibility of the particles. In order to investigate the nanoparticles as a platform for MRI of tumors, T2-weighted images were obtained from mice with U87MG tumors. A remarkable darkening effect was observed in the tumors of injected mice after 12 h suggesting a high passive uptake of the nanoparticles by tumors.
3.2. Composites Based on MOFs and MRI-Active Nanoparticles
Besides MOFs based on MRI active metal ions described in the previous section, a strategy of including MRI-active nanoparticles into MOFs has been extensively studied over the past few years (Table 3). For the purpose of this review, we divided these materials into five groups (Figure 9): (i) iron oxide nanoparticles in MOF matrices, (ii) core–shell iron oxide-MOF particles, (iii) core–shell Prussian blue-MOF nanoparticles, (iv) manganese oxide MOF nanoparticles, and (v) Gd-MOFs with a core–shell structure. By introducing MRI active nanoparticles within a MOF, the variety of MOFs, which can be used is enlarged, because these MOFs do not have to comprise MRI-active metal ions. In such approach, the MRI active component is responsible for the imaging and the porous MOF can be used for loading other active species including drug molecules. Such materials could be useful for multitargeted medical applications in both diagnosis and therapy.
Figure 9.
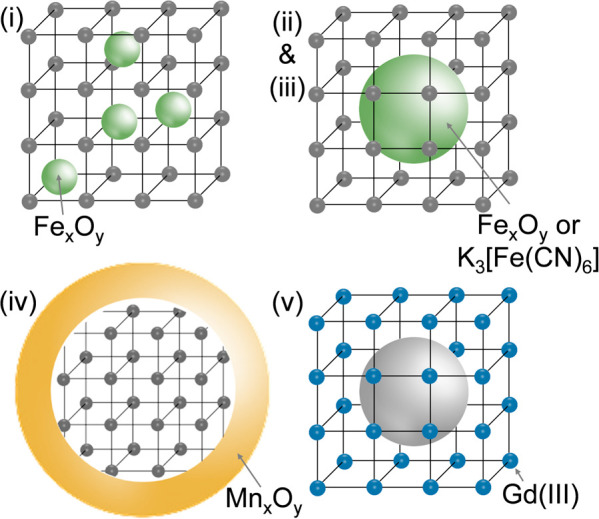
Different structures of composites based on MOFs studied as potential MRI contrast agents: (i) iron oxide nanoparticles in MOF matrices, (ii) core–shell iron oxide-MOF particles, (iii) core–shell Prussian blue-MOF nanoparticles, (iv) manganese oxide MOF nanoparticles, and (v) Gd-MOFs with a core–shell structure.
3.2.1. Iron Oxide Nanoparticles in MOF Matrices
Iron oxide nanoparticles can be embedded into a MOF matrix either during the synthesis or postsynthetically. For instance, Khoobi et al. first prepared Fe3O4 nanoparticles, which were then integrated within a MOF constructed from 5-aminolevulinic acid and zinc(II) ions during the MOF synthesis.92 The resulted particles were about 30 nm large and exhibited a high transverse relaxivity of 318 mM–1 s–1 (at 3.0 T). In another work, Steuneu et al. synthesized separately MOF nanoparticles of Fe-MIL-100 and citrate coated Fe2O3 nanoparticles (7 ± 3 nm large).93 By combining aqueous solutions of the MOF and Fe2O3 nanoparticles at a pH value, in which both materials exhibited opposite surface charges, an efficient coupling of the agents with a fine control over the MOF/Fe2O3 ratio was achieved. At 10% w/w loading of Fe2O3, the composite had a high value r2 of 93 mM–1 s–1 (at 7.0 T), which was similar to the clinically approved contrast agents. Since only small amounts of Fe2O3 were needed to facilitate the efficient imaging performance, the MOFs retained their porosity after conjugation, allowing the authors to load the pores with an anticancer drug doxorubicin. Furthermore, the application of these materials as MRI contrast agents was demonstrated in vivo. Their high T2*-effect led to a homogeneous decrease in the liver and spleen signal (Figure 10), generating a 52% decrease in signal-to-noise ratio and suggesting rapid internalization of the MOF/Fe2O3 composites.
Figure 10.
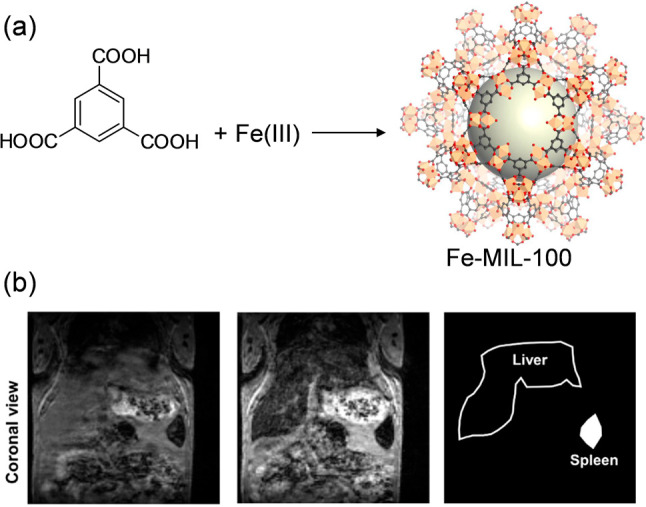
(a) Reaction scheme of the synthesis of Fe-MIL-100 and (b) 3D T2*-weighted gradient echo images of the mouse abdomen before and after administration of the Fe-MIL-100/Fe2O3 material showing a decrease in the liver and spleen signal upon the nanoparticle administration. Adapted with permission from ref (93). Copyright 2017, Elsevier.
3.2.2. Core–Shell Iron Oxide-MOF Particles
To synthesized core–shell iron oxide-MOF particles, first, the magnetic core is prepared and then the MOF shell is grown around it.113 The MOF growth can be done as a one-step process94,97 or stepwise by a layer-by-layer growth.95,96,98,99
Chen et al. reported on ZIF-8 with a core formed by carbon-encapsulated superparamagnetic Fe3O4 nanoparticles.94 While the carbon dots could serve as agents for fluorescent imaging, the Fe3O4 nanoparticles were included to enable the detection by MRI. The star relaxivity (r2*) of the nanoparticles was determined to be 331.79 mM–1 s–1 (at 3.0 T) and the cellular uptake of the nanoparticles was studied in A549 cells by MRI. Compared with untreated cells, darker signal intensity was observed after the treatment of the cancer cells with the nanoparticles indicating that the nanoparticles could be endocytosed by the cells effectively. Moreover, in vivo MRI studies on tumor-bearing mice were carried out. The T2*-signal in liver became darker suggesting that the liver was the major organ for the metabolism and clearance of the nanocarriers.
Fu et al. reported on a Fe3O4–UiO-66 platform for delivery of doxorubicin.95 They synthesized Fe3O4-clusters with a diameter of 150 nm and coated them with UiO-66. By varying the reaction conditions, particles with three different thickness of the MOF shell −5, 25, and 50 nm, were prepared. T2-weighted MR images of Fe3O4–UiO-66 showed an expected concentration-dependent darkening effect with a high transverse relaxivity (r2) of 255.87 mM–1 s–1, and the r2 values decreased as the thickness of the UiO-66 shell increased due to the reduced ratio of Fe3O4 to UiO-66 in the composite. The feasibility of the Fe3O4–UiO-66 nanoparticles for in vivo MRI was tested on HeLa tumor-bearing mice. A remarkable darkening effect was observed in the tumor area just 1 h postinjection and the MR image became even darker at 9 h postinjection. In another work, a Fe3O4–La-MOF comprising benzene-1,3,5-tricarboxylate ligands was synthesized by the layer-by-layer method and used for drug delivery of doxorubicin.96 In the synthesis, graphene oxide (GO) was added to form MOF/GO (10%) layers. Composites comprising 10 and 20 layers were prepared. With an increase of the number of layers, the overall particle size increased to 300 nm for 10 layers and to 500 nm for the particles with 20 layers. The relaxivities r2 for the nanoparticles with 10 and 20 layers were determined to be 35 mM–1 s–1 and 69 mM–1 s–1 (at 7.0 T), respectively. The higher r2 for the nanoparticles with 20 layers was explained by the higher content of GO in these particles. The high content of hydrophilic groups on the GO resulted in enhanced accessibility of water to the magnetic core.
The average diameter of the majority of reported Fe3O4-MOF core–shell nanoparticles is over 200 nm,113 which is not optimal for drug delivery applications in cancer treatment, because it has been suggested that only small nanoparticles (below 150 nm) can be accumulated in tumors via the enhanced permeability and retention effect.114 Therefore, we recently reported on the synthesis of Fe3O4-MOF core–shell nanoparticles below 100 nm large (97 ± 8 nm as determined by TEM).97 To prepare such small nanoparticles, it was necessary to synthesize very small Fe3O4-clusters (here, only 35.1 ± 4.5 nm large), which were then coated with a MOF, in this work with ZIF-8. The nanocomposite with a r2/r1 ratio of 12.39 was loaded with arsenic trioxide (a promising anticancer drug115) and studied as a potential theragnostic agent. Similarly, Yang et al. also reported on core–shell Fe3O4-MOF nanoparticles, which were only 40–60 nm large (as determined by SEM).98 The nanoparticles based on Fe3O4 and a MOF UiO-66 were proposed for MRI and drug delivery applications. To gain a control over the drug release, the particle surface was functionalized by pillararene-based pseudorotaxanes as tightness-adjustable nanovalves. The particles were loaded with 5-fluoruracil as a model drug and their MR properties were investigated.
Yin et al. reported on CoFe2O4-ZIF-8 nanoparticles for MRI and photothermal therapy.99 A mesoporous CoFe2O4-core was included to act as a T2-weighted MRI agent, PTT agent and a platform for loading doxorubicin. To prevent premature drug release, the core was coated by polydopamine which also facilitated the coating with ZIF-8. The ZIF-8 shell served for loading camptothecin (CPT) and enabled a pH-responsive drug release.
3.2.3. Prussian Blue Nanoparticle in MOF Matrices
Prussian blue, K3[Fe(CN)6], consists of Fe ions connected by CN– anions. Due to its unique Fe(II)–C≡N–Fe(III) structure [Fe(II): low spin, S = 0; Fe(III): high spin, S = 5/2), Prussian blue nanoparticles can serve both T1 and T2 MRI contrast agents.116 Recently, nanoparticles of Prussian blue has been combined with MOFs to prepare theranostic agents. For instance, Chen et al. coated Prussian blue nanoparticles with a ZIF-8 shell and used the nanocomposite for delivery of doxorubicin.100In vitro, the r1 and r2 values were measured to be 2.04 mM–1 s–1 and 22.87 mM–1 s–1 (at 3.0 T), respectively. Subsequently, in vivo MR images were also conducted. Compared with the preinjected images, both T1-weighted and T2*-weighted images of a tumor site exhibited enhanced MR signal at 24 h postinjection indicating an accumulation of the nanocomposite in the tumor. The MR signal of liver became darker suggesting that liver was the major organ for the metabolism and clearance of the nanoparticles. In another work, nanoparticles of Prussian blue were coated with a MOF Ti-MIL-125.101 While the core serves as a contrast agent for MRI, the outer shell of Ti-MIL-125 can serve as photosensitive reagent for photodynamic therapy (PDT). The particles exhibited an r2 value of 14.51 mM–1 s–1, which indicated that they could be used as potential T2 MRI contrast agents. Wang et al. reported on Prussian blue nanoparticles doped with Gd(III) and Tm(III), and subsequently coated with a MOF ZIF-8 and polydopamine to prepare composite nanoparticles suitable for drug delivery of doxorubicin and multimodal imaging (T1–T2 dual-mode MR and fluorescence imaging).102 A quantitative in vivo analysis in a mouse model confirmed that after the injection of the nanoparticles, T1-weighted images became brighter, while the T2-weighted images were darker.
By combining Prussian blue nanoparticles with MOFs, the drug loading capacity is enlarged. However, the nanoparticles of Prussian blue themselves are also porous and can be used for drug loading. For example, Tian et al. loaded nanoparticles of Prussian blue with sorafenib and investigated their MRI properties.117 Moreover, analogues of Prussian blue can also be used in a combination with MOFs for applications in MRI. For instance, nanoparticles of K2Mn[Fe(CN)6] coated with a MOF Fe-MIL-100 were studied as agents for multimodal imaging and synergistic therapy.103 The authors showed that in a mildly acidic tumor microenvironment, Mn(II) ions were released which resulted in an “ON” state of both T1-weighted magnetic resonance imaging and photoacoustic signals.
3.2.4. Manganese Oxide MOF Nanoparticles
In comparison to the design of iron oxide-MOF composites, in which Fe3O4 usually forms the core, manganese oxide, namely MnO2, is usually used as a shell to coat MOF nanoparticles. However, also examples of Mn3O4 nanoparticles embedded into MOFs are known. For example, Kefayad et al. reported on manganese oxide (Mn3O4) nanoparticles conjugated with poly(acrylic acid) incorporated into a MOF ZIF-8 in order to prepare a pH-sensitive drug delivery system suitable for MR imaging.118 The r1 relaxivity of the nanoparticles was measured to be 3.3 mM–1 s–1. In another work, Zhang et al. reported on MnFe2O4-MOF core–shell nanoparticles.119 The MnFe2O4 nanoparticles were included because of their catalase-like and glutathione peroxidase-like activities. As a MOF, porphyrin-based MOF, which can act as a photosensitizer, was selected. Moreover, the MnFe2O4-MOF showed relaxivity r1 of 2.94 mM–1 s–1 and r2 of 51.53 mM–1 s–1 due to the paramagnetic manganese and iron ions. To evaluate the potential of the nanoparticles as contrast agents in vivo, T1 weighted MRI imaging of tumor bearing mice was performed. A positive contrast in the tumor area could be detected at 24 h after the injection indicating the nanoparticles could be used as contrast agents in MRI.
MnO2, which is often used as a shell in MOF-composites, is stable at physiological conditions with only a weak T1 weighted MR imaging ability. However, when MnO2 encounters glutathione (GSH), Mn(IV)-ions are gradually reduced to Mn(II), which enhances the T1-MR contrast. Therefore, MnO2 has been suggested as a GSH sensing platform detectable by MRI.120 Yin et al. reported on a Zr(IV)-porphyrin MOF coated with MnO2 to realize an oxidation of GSH by MnO2 for enhanced photodynamic therapy.121 MOF particles were first coated with poly(allylamine hydrochloride), then a uniform MnO2 layer was coated on the surface by a redox reaction between KMnO4 and poly(allylamine hydrochloride). The MRI properties were investigated in the presence of GSH and without. In a solution with GSH, the T1 relaxation rate r1 was 6.59 mM–1 s–1, which was 7.7-fold higher than that without GSH. The nanoparticles were further tested in vivo in MR imaging of tumor-bearing mice. The images of mice were recorded after local injection of the nanoparticles into tumor tissues and the muscle on the opposite side. A strong T1-MR signal was observed at the tumor area because of the reduction of MnO2 to Mn(II) by GSH in tumor cells. In comparison, the muscle section showed a less T1-signal after the injection. Simultaneously, strong T1-signals were observed in the kidney due to the rapid renal excretion of Mn(II)-ions. Similarly, also Chen et al, reported on a Zr(IV)-porphyrin MOF coated with MnO2 as agents for photodynamic therapy (Figure 11).104 The relaxivity of the nanoparticles was determined to be 5.97 mM–1 s–1 in the presence of GSH. Moreover, it was shown that not only MRI-properties, but also fluorescence and photodynamic activities could be turned on by GSH. In another work, a Zr(IV)-MOF based on porphyrin ligands coated with a MnO2 shell was studied as an agent for bimodal imaging (fluorescence and MR imaging).122 The authors suggested that due to the responsiveness of the MnO2 layer to H+ and H2O2, O2 can be produced, which can enhance O2-mediated singlet oxygen (1O2) generation for photodynamic therapy. Moreover, during the redox reaction, Mn(II)-ions are released, which can act as contrast agents in MRI. As expected, the T1-weighted images of the nanoparticles in H2O2 solution (pH = 5.5) were much brighter than those in neutral aqueous solution and the r1 relaxivity in a presence of H2O2 was determined to be 4.51 mM–1 s–1, while in a neutral aqueous solution, it was only 0.03 mM–1 s–1. Similarly, Yang et al. studied nanoparticles of Zr(IV)-MOF comprising porphyrin ligands with coordinated Cu(II)-ions coated by a MnO2 shell as agents for fluorescence and MR imaging.105 The r1 relaxivity at pH 6.5 in the presence of H2O2 was determined to be 6.59 mM–1 s–1 (at 1.5 T). In another work, porphyrin-based MOF nanoparticles coated with a MnO2 shell were used for drug delivery of doxorubicin and T1-MRI.106 MR imaging of nanoparticles treated with GSH or incubated in buffer solutions with different pH values was performed. As expected, it was found that the addition of GSH significantly enhanced the signal, which confirmed the effective degradation of MnO2 into paramagnetic Mn(II).
Figure 11.
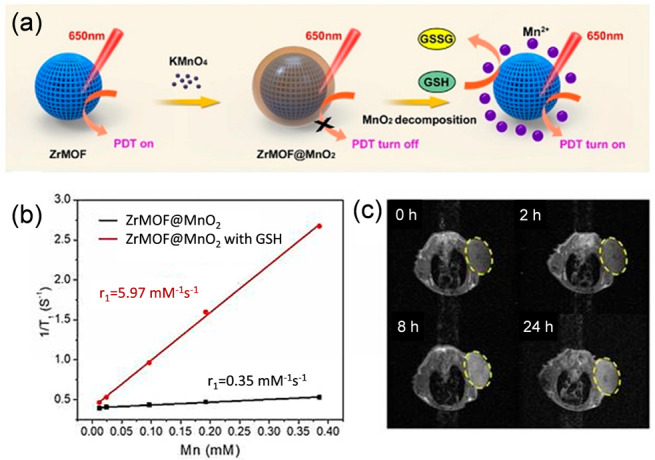
(a) Scheme of the synthesis of GSH-responsive Zr-MOF@MnO2 hybrid nanoparticles for MRI-guided enhanced tumor therapy, (b) longitudinal (1/T1) relaxation rate of suspensions of the particles without and in the presence of GSH plotted versus the Mn-content, and (c) T1-weighted images of a mouse at different times after intravenous injection with PEGylated Zr-MOF@MnO2 hybrid nanoparticles. Adapted with permission from ref (104). Copyright 2019, Theranostics.
Shen et al. reported on a MOF ZIF-8 coated with MnO2 for delivery of siRNA against pyruvate kinase muscle isozyme M2.107 siRNA was loaded in situ during the MOF synthesis. After that, the particles were coated with MnO2 and finally, molecules of folic acid as targeting ligands were attached to the particle surface. The r1 relaxivity of such particles was measured to be 6.46 mM–1 s–1 (at 3.0 T). This result was confirmed by in vivo experiment in mice receiving intravenous injection of the nanoparticles where the signal intensity in T1-weighted MR images rapidly increased at the tumor area at 24 h postinjection and reached a peak at 48 h postinjection.
3.2.5. Gd-MOFs with a Core–Shell Structure
Fan et al. reported on a composite comprising a core based of self-assembly of doxorubicin and Fe(III) ions.108 In a subsequent step, the core was coated by a Gd(III)-MOF based on benzene-1,3,5-tricarboxylate ligands, followed by grafting photosensitizer indocyanine green onto the surface, to enable multimodal imaging. In the design, the Gd-MOF shell did not act only as an MRI contrast agent, but also provided a protection for the core, and thus a control for the drug release. The relaxivity of the nanoparticles was determined to be r1 of 6.4 mM–1 s–1 and r2 of 81.9 mM–1 s–1 (at 3.0 T) with a high r2/r1 ratio (>12) indicating that the nanocomposite was a T2-dominated contrast agent. In another work, Fan et al. combined a Gd(III)-MOF with polydopamine.109 First, Gd-ions and polydopamine were combined to prepare Gd-doped polydopamine nanoparticles. Then, a widely used PDT photosensitizer chlorin e6 (Ce6) was loaded into the nanoparticles, followed by coating the nanoparticles with a Gd(III)-MOF comprising benzene-1,3,5-tricarboxylate ligands via a stepwise assembly process (Figure 12). After 5 reaction steps, the size of the nanoparticles gradually increased from 128 to 183 nm (determined by DLS). The relaxivity was determined to be r1 of 13.72 mM–1 s–1 and r2 of 216.14 mM–1 s–1 (at 3.0 T). Due to the high r2/r1 ratio (>15), the nanoparticles could be assigned as a T2-dominated contrast agent. Zhang et al. reported on integrating Gd-doped silica nanoparticles within a MOF ZIF-8, which was loaded with chlorin e6 and doxorubicin (anticancer drug) in order to prepare an agent for drug delivery, and bimodal MR and fluorescent imaging.106 Moreover, the particle surface was functionalized with folic acid to enable tumor targeted delivery. The relaxivity of such particles was determined to be r1 of 2.70 mM–1 s–1. Further, the particles were administrated via intraperitoneal injection into MCF-7 tumor-bearing nude mice. After the injection, a strong MRI signal was observed at the tumor area, indicating the ability of the magnetic resonance contrast-strengthening effect of the nanocomposites.
Figure 12.
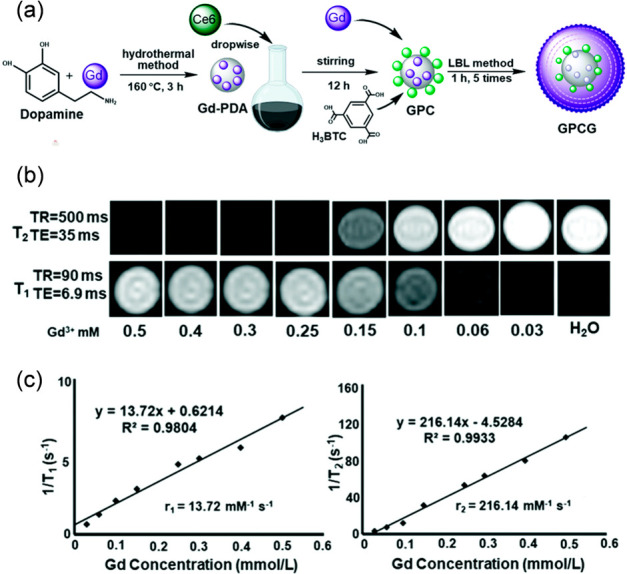
(a) Schematic synthesis of Gd-doped polydopamine Gd-MOF nanoparticles, (b) their T1 and T2 MR images at different concentrations, and (c) their relaxation rates (1/T1,2) versus different Gd-concentrations. Adapted with permission from ref (109). Copyright 2021, Royal Society of Chemistry.
3.3. Gadolinium Complexes Embedded into MOFs
An insufficient stability, and thus uncontrolled metal leakage, of Gd-MOFs is a concern regarding their biomedical application as contrast agents in MRI. Therefore, as an alternative, introducing stable gadolinium complexes within the pores of MOFs has been suggested. For instance, Yang et al. reported on incorporating Gd(III)-complexes (Gd-DTPA, DTPA = diethylenetriamine pentaacetic acid) within the pores of MOF-808.111 The MOF nanoparticles were first immersed in a DTPA sodium salt aqueous solution to graft DTPA molecules onto MOF-808, and then submerged into a Gd(NO3)3 solution leading to chelation of Gd(III) by DTPA within the MOF. The surface of the particles was further modified with polyaniline. The geometric restriction of polyaniline on the surface largely ensured that Gd-DTPA remained inside the MOF pores. At the same time, the photothermal properties of polyaniline also provided a possibility for photothermal therapy. In another work, Meade et al. reported on inserting Gd-complexes (Gd-DTPA) into pores of different MOFs in order to investigate the influence of the framework structure and composition on relaxivity.112 The authors postsynthetically incorporate Gd-DTPA into Zr-MOFs (NU-1000 and NU-901) using solvent-assisted ligand incorporation. The impact of a particle size (nanosized vs microsized) and the MOF type on proton relaxivity was investigated. The Gd-functionalized nanoparticles of NU-1000 displayed the highest loading of the Gd(III) complex (1.9 ± 0.1 complexes per node) and exhibited the most enhanced proton relaxivity (r1 of 26 ± 1 mM–1 s–1 at 1.4 T).
4. MOFs in Heteronuclear MRI
1H MRI is by far the most common technique due to high 1H abundance and high sensitivity compared to other nuclei. However, also other nuclei can be detected, including 19F and 129Xe.
4.1. MOFs and Hyperpolarized 129Xe MRI
In 129Xe MRI, hyperpolarized 129Xe is usually used, because it can boost the signal sensitivity to over 10 000-fold compared with conventional MRI.123 Hyperpolarization is a process, which results in the nuclear spin polarization of a material in a magnetic field far beyond thermal equilibrium conditions determined by the Boltzmann distribution;124 meaning that less of the spin states cancel each other resulting in a higher sensitivity. The process of hyperpolarization is usually performed using spin-exchange optical pumping using circularly polarized light.124 However, the polarized light cannot directly transfer angular momentum to the gas nuclei, thus, an alkali metal atom such as rubidium is used as an intermediary. Subsequently, when 129Xe nuclei collide with Rb, the polarization is transferred from the Rb valence electron to the nuclear spin of the noble gas atom.
Due to the sensitivity enhancement, hyperpolarized 129Xe MRI can be used for diagnosis of the respiratory system diseases.124 However, a detection of specific compounds in blood remains challenging due to the weak 129Xe signal in an aqueous solution. Here nanoparticles could play an important role as carriers of xenon. From this point of view, highly porous materials like MOFs seem to be perfect candidates to fulfill the task. If xenon is loaded inside MOF pores, its chemical shift is clearly distinguishable from that of free 129Xe in water, due to the surface and pore environment of the MOF, and thus such xenon nanocarriers can be detected. For instance, Zhou et al. studied the chemical shift of xenon when entrapped in pores of ZIF-8.125 Due to the hydrophobic pore environment, which offers specific interaction with the xenon atom, a significant chemical shift near 84 ppm was detected, which is ∼109 ppm apart from that of free 129Xe in aqueous solution (near 193 ppm). Moreover, the signal intensity of hyperpolarized 129Xe entrapped in MOF pores, corresponding to integral, was four times stronger than that of free 129Xe. In a follow-up work the authors studied the influence of a structure of MOF pores on MR properties of hyperpolarized 129Xe trapped inside these pores.126 A class of MOFs formed by similar octahedral Zn–O–C clusters and benzenecarboxylate ligands, namely IRMOF-1, IRMOF-8, and IRMOF-10 with pore diameters 7.93, 9.17, and 12.15 Ă, were selected. As expected by the authors, the 129Xe atom in each MOF produced an MR signal at its unique chemical shift - IRMOF-1 at 48 ppm, IRMOF-8 at 17 ppm, and IRMOF-10 at 26 ppm (Figure 13), and these irradiation differences were large enough to excite the signal from only one MOF under its particular frequency. The corresponding ultrasensitive MRI also showed a concentration-dependent intensity. Hence, the exploited MOF nanoparticles could be used as ultrasensitive MRI stains with diverse colors, making it possible to analyze complex samples qualitatively and quantitatively in the future.
Figure 13.
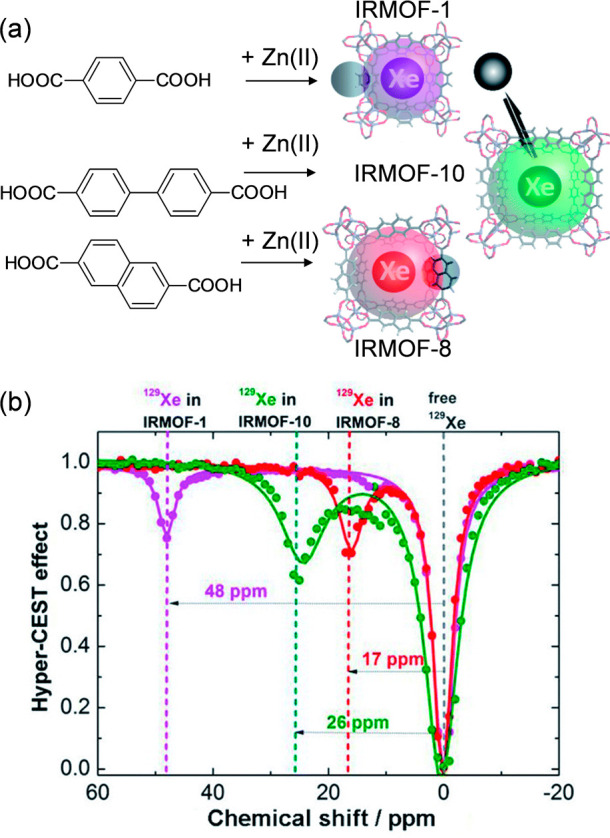
(a) Reaction scheme of the synthesis of IRMOF-1, IRMOF-10, and IRMOF-8 and (b) their frequency-dependent saturation spectra. To eliminate the influence of solvent, chemical shifts were referenced to the dissolved free 129Xe atom. Adapted with permission from ref (126). Copyright 2021, Royal Society of Chemistry.
4.2. MOFs and 19F MRI
One of the major drawbacks of traditional 1H MRI is the high natural background of 1H atoms impeding the accurate visualization of the contrast agent distribution. To address this problem, utilizing MRI based on a detection of fluorine has been proposed.12719F MRI “hot-spot” visualization of fluorinated tracers is an auspicious specific diagnostic method enabling very high contrast in MRI images due to the nearly zero fluorine background in the body. Furthermore, the resonance frequency of 19F is very close to that of 1H, allowing visualization of fluorinated tracers by commercial MRI scanners with only minor adjustments of the hardware and software. To employ 19F MRI in practice, fluorine probes are needed. These must contain a high content of fluorine atoms, which are chemically equivalent, have suitable relaxation times, adequate solubility in water, and an easily modifiable structure for targeting and biocompatibility/degradability.127 To prepare such agents, recently also MOF-based materials have been investigated.128−130
Wang et al. reported on a pH-responsive fluorinated ZIF for in vitro and in vivo19F MRI.128 ZIF-8 comprises Zn(II) ions and 2-methylimidazolate ligands and is known to be pH-responsive (stable at neutral and slightly basic conditions, but rather unstable in acidic conditions131,132). In this work, some of the ligands were exchanged to 4-(trifluoromethyl)imidazole to provide the fluorine moieties for the detection by 19F MRI. As expected, when the 19F nuclei were part of the framework, the peak intensity of fluorine signal ppm was very weak. However, the 19F MRI signal intensity could be turned-on by lowering the pH value to 5.5, which was attributed to the known disassembly of the nanoparticles at acidic conditions, and thus the ligand release. The half peak width of the fluorine probe was narrow indicating suitable T2 relaxation time which allows various MRI applications. Similarly, Yang et al. reported on a pH- and GSH-responsive 1H/19F bimodal MRI contrast agent, which was constructed by incorporating MnOx into Zr-based MOF nanoparticles comprising tetrafluoroterephthalate ligands.130 Under an acidic environment, the nanoparticles disassembled releasing MnOx and free fluorinated ligands. Due to the released of the ligands, 19F MRI signal was enhanced. Meanwhile, GSH, which is overexpressed in a tumor microenvironment, reduced MnOx to Mn(II) ions, and thus, the T1-weighted MR imaging capability was improved.
5. Conclusions and Outlook
Considering the growing number of publications on MOFs for applications in MRI reported every year, there is no doubt that MOFs are extremely promising materials for developing novel contrast agents for MR imaging. Due to the versatile design possibilities of MOFs, materials with desired, precisely defined properties can be prepared. This offers opportunities for preparing not only agents for MRI, but also multifunctional responsive agents enabling multimodal imaging or combining imaging and drug delivery, i.e., theranostic agents, which are generally very challenging to synthesize but in great demand.
Although we have already learnt a lot about MOFs for applications in MRI over the past few years, there are still some issues that should be considered and addressed in order to facilitate the translation of such agents from “bench to bedside”. One of the most crucial points is bringing a clarity and standards to the material characterization. In order to evaluate and compare different materials, reported analytical data should be complete, including all measurement details. In the case of MR measurements, the following parameters must be given: solvent (water, buffer, gel, etc.), concentration, temperature, and also the field strength, relaxation times, and other relevant parameters (e.g., bandwidth, repetition time, echo time, temporal/spatial resolution, etc.). If relaxivities are determined, it should be clearly stated, whether they were calculated with respect to the molar concentration of the nanoparticles or of the metal ions. Moreover, it has been shown many times that the particle size and morphology also influenced the material MR properties significantly; therefore, the materials properties must be properly analyzed, and the corresponding analytical data must be provided as well as studies reporting on the material stability (in biological conditions) and toxicity (including the MOF building components). Last but not least, MRI in vitro studies should be carried out in simulated conditions, which are as close as possible to in vivo conditions.
Developing MRI contrast agents is a big endeavor. To maximize its efficiency, resources and success rate, a close collaboration between material scientists and clinicians is essential as well as employing innovative approaches and theoretical modeling, side by side with systematic investigations. Considering the MOFs unique features, which are not achievable with other materials, we have no doubts that the future of the research of MOFs for applications in MRI is bright and that great results can be expected.
Acknowledgments
HB acknowledges the support from the SPP 1928, COORNETs supported by the German Research Foundation. DJ acknowledges the support from the MH CR-DRO (Institute for Clinical and Experimental Medicine IKEM, IN00023001) and from the project National Institute for Research of Metabolic and Cardiovascular Diseases (Programme EXCELES, Project No. LX22NPO5104) funded by the European Union - Next Generation EU.
The authors declare no competing financial interest.
References
- Herzog H., Shah N. J.. Chapter 9: Introduction and Historical Overview. Hybrid MR-PET Imaging: Systems, Methods and Applications; The Royal Society of Chemistry, 2018; pp 203–213. [Google Scholar]
- Padhani A. R.; Liu G.; Koh D. M.; Chenevert T. L.; Thoeny H. C.; Takahara T.; Dzik-Jurasz A.; Ross B. D.; Van Cauteren M.; Collins D.; Hammoud D. A.; Rustin G. J.; Taouli B.; Choyke P. L. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 2009, 11, 102–125. 10.1593/neo.81328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner E. J.; Datta A.; Jocher C. J.; Raymond K. N. High-Relaxivity MRI Contrast Agents: Where Coordination Chemistry Meets Medical Imaging. Angew. Chem., Int. Ed. 2008, 47, 8568–8580. 10.1002/anie.200800212. [DOI] [PubMed] [Google Scholar]
- Ni D.; Bu W.; Ehlerding E. B.; Cai W.; Shi J. Engineering of inorganic nanoparticles as magnetic resonance imaging contrast agents. Chem. Soc. Rev. 2017, 46, 7438–7468. 10.1039/C7CS00316A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahsner J.; Gale E. M.; Rodríguez-Rodríguez A.; Caravan P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. 10.1021/acs.chemrev.8b00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y. D.; Paudel R.; Liu J.; Ma C.; Zhang Z. S.; Zhou S. K. MRI contrast agents: Classification and application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326. 10.3892/ijmm.2016.2744. [DOI] [PubMed] [Google Scholar]
- Jeong Y.; Hwang H. S.; Na K. Theranostics and contrast agents for magnetic resonance imaging. Biomater. Res. 2018, 22, 20. 10.1186/s40824-018-0130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa H.; Cordova K. E.; O’Keeffe M.; Yaghi O. M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. 10.1126/science.1230444. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Chen Z.; Liu X.; Hanna S. L.; Wang X.; Taheri-Ledari R.; Maleki A.; Li P.; Farha O. K. A historical overview of the activation and porosity of metal–organic frameworks. Chem. Soc. Rev. 2020, 49, 7406–7427. 10.1039/D0CS00997K. [DOI] [PubMed] [Google Scholar]
- Kirchon A.; Feng L.; Drake H. F.; Joseph E. A.; Zhou H.-C. From fundamentals to applications: a toolbox for robust and multifunctional MOF materials. Chem. Soc. Rev. 2018, 47, 8611–8638. 10.1039/C8CS00688A. [DOI] [PubMed] [Google Scholar]
- Li H.; Wang K.; Sun Y.; Lollar C. T.; Li J.; Zhou H.-C. Recent advances in gas storage and separation using metal–organic frameworks. Mater. Today 2018, 21, 108–121. 10.1016/j.mattod.2017.07.006. [DOI] [Google Scholar]
- Goh S. H.; Lau H. S.; Yong W. F. Metal–Organic Frameworks (MOFs)-Based Mixed Matrix Membranes (MMMs) for Gas Separation: A Review on Advanced Materials in Harsh Environmental Applications. Small 2022, 18, 2107536. 10.1002/smll.202107536. [DOI] [PubMed] [Google Scholar]
- Adil K.; Belmabkhout Y.; Pillai R. S.; Cadiau A.; Bhatt P. M.; Assen A. H.; Maurin G.; Eddaoudi M. Gas/vapour separation using ultra-microporous metal–organic frameworks: insights into the structure/separation relationship. Chem. Soc. Rev. 2017, 46, 3402–3430. 10.1039/C7CS00153C. [DOI] [PubMed] [Google Scholar]
- Qian Q.; Asinger P. A.; Lee M. J.; Han G.; Mizrahi Rodriguez K.; Lin S.; Benedetti F. M.; Wu A. X.; Chi W. S.; Smith Z. P. MOF-Based Membranes for Gas Separations. Chem. Rev. 2020, 120, 8161–8266. 10.1021/acs.chemrev.0c00119. [DOI] [PubMed] [Google Scholar]
- Guo J.; Qin Y.; Zhu Y.; Zhang X.; Long C.; Zhao M.; Tang Z. Metal–organic frameworks as catalytic selectivity regulators for organic transformations. Chem. Soc. Rev. 2021, 50, 5366–5396. 10.1039/D0CS01538E. [DOI] [PubMed] [Google Scholar]
- Bavykina A.; Kolobov N.; Khan I. S.; Bau J. A.; Ramirez A.; Gascon J. Metal–Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020, 120, 8468–8535. 10.1021/acs.chemrev.9b00685. [DOI] [PubMed] [Google Scholar]
- Koo W.-T.; Jang J.-S.; Kim I.-D. Metal-Organic Frameworks for Chemiresistive Sensors. Chem. 2019, 5, 1938–1963. 10.1016/j.chempr.2019.04.013. [DOI] [Google Scholar]
- Li H.-Y.; Zhao S.-N.; Zang S.-Q.; Li J. Functional metal–organic frameworks as effective sensors of gases and volatile compounds. Chem. Soc. Rev. 2020, 49, 6364–6401. 10.1039/C9CS00778D. [DOI] [PubMed] [Google Scholar]
- Rojas S.; Arenas-Vivo A.; Horcajada P. Metal-organic frameworks: A novel platform for combined advanced therapies. Coord. Chem. Rev. 2019, 388, 202–226. 10.1016/j.ccr.2019.02.032. [DOI] [Google Scholar]
- Osterrieth J. W. M.; Fairen-Jimenez D. Metal–Organic Framework Composites for Theragnostics and Drug Delivery Applications. Biotechnol. J. 2021, 16, 2000005. 10.1002/biot.202000005. [DOI] [PubMed] [Google Scholar]
- Yang J.; Yang Y.-W. Metal–Organic Frameworks for Biomedical Applications. Small 2020, 16, 1906846. 10.1002/smll.201906846. [DOI] [PubMed] [Google Scholar]
- Cai W.; Chu C.-C.; Liu G.; Wang Y.-X. J. Metal–Organic Framework-Based Nanomedicine Platforms for Drug Delivery and Molecular Imaging. Small 2015, 11, 4806–4822. 10.1002/smll.201500802. [DOI] [PubMed] [Google Scholar]
- Mendes R. F.; Figueira F.; Leite J. P.; Gales L.; Almeida Paz F. A. Metal–organic frameworks: a future toolbox for biomedicine?. Chem. Soc. Rev. 2020, 49, 9121–9153. 10.1039/D0CS00883D. [DOI] [PubMed] [Google Scholar]
- Liang W.; Wied P.; Carraro F.; Sumby C. J.; Nidetzky B.; Tsung C.-K.; Falcaro P.; Doonan C. J. Metal–Organic Framework-Based Enzyme Biocomposites. Chem. Rev. 2021, 121, 1077–1129. 10.1021/acs.chemrev.0c01029. [DOI] [PubMed] [Google Scholar]
- Demir Duman F.; Forgan R. S. Applications of nanoscale metal–organic frameworks as imaging agents in biology and medicine. J. Mater. Chem. B 2021, 9, 3423–3449. 10.1039/D1TB00358E. [DOI] [PubMed] [Google Scholar]
- Wang H.-S. Metal–organic frameworks for biosensing and bioimaging applications. Coord. Chem. Rev. 2017, 349, 139–155. 10.1016/j.ccr.2017.08.015. [DOI] [Google Scholar]
- Peller M.; Böll K.; Zimpel A.; Wuttke S. Metal–organic framework nanoparticles for magnetic resonance imaging. Inorg. Chem. Front. 2018, 5, 1760–1779. 10.1039/C8QI00149A. [DOI] [Google Scholar]
- Peller M.; Lanza A.; Wuttke S. MRI-Active Metal-Organic Frameworks: Concepts for the Translation from Lab to Clinic. Adv. Therap. 2021, 4, 2100067. 10.1002/adtp.202100067. [DOI] [Google Scholar]
- Worthoff W. A., Yun S. D., Shah N. J.. Chapter 1: Introduction to Magnetic Resonance Imaging. Hybrid MR-PET Imaging: Systems, Methods and Applications; The Royal Society of Chemistry, 2018; pp 1–44. [Google Scholar]
- Bernstein M. A., King K. F., Zhou X. J.. Handbook of MRI Pulse Sequences; Elsevier: Oxford, 2004; pp 267–297. [Google Scholar]
- Hahn E. L. Spin Echoes. Phys. Rev. 1950, 80, 580–594. 10.1103/PhysRev.80.580. [DOI] [Google Scholar]
- Hennig J.; Friedburg H. Clinical applications and methodological developments of the RARE technique. Magn. Reson. Imaging 1988, 6, 391–395. 10.1016/0730-725X(88)90475-4. [DOI] [PubMed] [Google Scholar]
- Frahm J.; Haase A.; Matthaei D. Rapid NMR imaging of dynamic processes using the FLASH technique. Magn. Reson. Med. 1986, 3, 321–327. 10.1002/mrm.1910030217. [DOI] [PubMed] [Google Scholar]
- Elster A. D. Gradient-echo MR imaging: techniques and acronyms. Radiology 1993, 186, 1–8. 10.1148/radiology.186.1.8416546. [DOI] [PubMed] [Google Scholar]
- Taylor A.; Wilson K. M.; Murray P.; Fernig D. G.; Lévy R. Long-term tracking of cells using inorganic nanoparticles as contrast agents: are we there yet?. Chem. Soc. Rev. 2012, 41, 2707–2717. 10.1039/c2cs35031a. [DOI] [PubMed] [Google Scholar]
- Kolouchova K.; Jirak D.; Groborz O.; Sedlacek O.; Ziolkowska N.; Vit M.; Sticova E.; Galisova A.; Svec P.; Trousil J.; Hajek M.; Hruby M. Implant-forming polymeric 19F MRI-tracer with tunable dissolution. J. Controlled Release 2020, 327, 50–60. 10.1016/j.jconrel.2020.07.026. [DOI] [PubMed] [Google Scholar]
- Gálisová A.; Herynek V.; Swider E.; Sticová E.; Pátiková A.; Kosinová L.; Kříž J.; Hájek M.; Srinivas M.; Jirak D. A Trimodal Imaging Platform for Tracking Viable Transplanted Pancreatic Islets In Vivo: F-19 MR, Fluorescence, and Bioluminescence Imaging. Mol. Imaging Biol. 2019, 21, 454–464. 10.1007/s11307-018-1270-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirak D.; Kriz J.; Strzelecki M.; Yang J.; Hasilo C.; White D. J.; Foster P. J. Monitoring the survival of islet transplants by MRI using a novel technique for their automated detection and quantification. Magn. Reson. Mater. Phys. Biol. Med. 2009, 22, 257–265. 10.1007/s10334-009-0172-4. [DOI] [PubMed] [Google Scholar]
- Reeßing F.; Stuart M. C. A.; Samplonius D. F.; Diercky R. A. J. O.; Feringa B. L.; Helfrich W.; Szymanski W. A light-responsive liposomal agent for MRI contrast enhancement and monitoring of cargo delivery. Chem. Commun. 2019, 55, 10784–10787. 10.1039/C9CC05516A. [DOI] [PubMed] [Google Scholar]
- Caravan P.; Ellison J. J.; McMurry T. J.; Lauffer R. B. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem. Rev. 1999, 99, 2293–2352. 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- Weinmann H. J.; Ebert W.; Misselwitz B.; Schmitt-Willich H. Tissue-specific MR contrast agents. Eur. J. Radiol. 2003, 46, 33–44. 10.1016/S0720-048X(02)00332-7. [DOI] [PubMed] [Google Scholar]
- Bottrill M.; Kwok L.; Long N. J. Lanthanides in magnetic resonance imaging. Chem. Soc. Rev. 2006, 35, 557–571. 10.1039/b516376p. [DOI] [PubMed] [Google Scholar]
- Rohrer M.; Bauer H.; Mintorovitch J.; Requardt M.; Weinmann H.-J. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest. Radiol. 2005, 40, 715–724. 10.1097/01.rli.0000184756.66360.d3. [DOI] [PubMed] [Google Scholar]
- Antonelli A.; Sfara C.; Battistelli S.; Canonico B.; Arcangeletti M.; Manuali E.; Salamida S.; Papa S.; Magnani M. New Strategies to Prolong the In Vivo Life Span of Iron-Based Contrast Agents for MRI. PLoS One 2013, 8, e78542. 10.1371/journal.pone.0078542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coroiu I. Relaxivities of different superparamagnetic particles for application in NMR tomography. J. Magn. Magn. Mater. 1999, 201, 449–452. 10.1016/S0304-8853(99)00025-6. [DOI] [Google Scholar]
- Huh Y.-M.; Jun Y.-W.; Song H.-T.; Kim S.; Choi J.-S.; Lee J.-H.; Yoon S.; Kim K.-S.; Shin J.-S.; Suh J.-S.; Cheon J. In Vivo Magnetic Resonance Detection of Cancer by Using Multifunctional Magnetic Nanocrystals. J. Am. Chem. Soc. 2005, 127, 12387–12391. 10.1021/ja052337c. [DOI] [PubMed] [Google Scholar]
- Nounou M. M.; El-Khordagui L. K.; Khalafallah N. A.; Khalil S. A. In vitro release of hydrophilic and hydrophobic drugs from liposomal dispersions and gels. Acta Pharma 2006, 56, 311–324. [PubMed] [Google Scholar]
- Washington C. Drug release from microdisperse systems: a critical review. Int. J. Pharm. 1990, 58, 1–12. 10.1016/0378-5173(90)90280-H. [DOI] [Google Scholar]
- Desreux J. F., Gilsoul D.. Chemical synthesis of paramagnetic complexes. Trends in contrast media; Springer Verlag: Heidelberg, 1999; pp 161–169. [Google Scholar]
- Idee J.-M.; Port M.; Raynal I.; Schaefer M.; Greneur S. L.; Corot C. Clinical and biological consequences of transmetallation induced by contrast agents for magnetic resonance imaging: a review. Fundam. Clin. Pharmacol. 2006, 20, 563–576. 10.1111/j.1472-8206.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- Morcos S. K. Nephrogenic systemic fibrosis following the administration of extracellular gadolinium based contrast agents: is the stability of the contrast agent molecule an important factor in the pathogenesis of this condition?. Br. J. Radiol. 2007, 80, 73–76. 10.1259/bjr/17111243. [DOI] [PubMed] [Google Scholar]
- Marckmann P.; Skov L.; Rossen K.; Thomsen H. S. Clinical manifestation of gadodiamide-related nephrogenic systemic fibrosis. Clin. Nephrol. 2008, 69, 161–168. 10.5414/CNP69161. [DOI] [PubMed] [Google Scholar]
- Bulte J. W.; Kraitchman D. L. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004, 17, 484–499. 10.1002/nbm.924. [DOI] [PubMed] [Google Scholar]
- Kedziorek D. A.; Muja N.; Walczak P.; Ruiz-Cabello J.; Gilad A. A.; Jie C. C.; Bulte J. W. Gene expression profiling reveals early cellular responses to intracellular magnetic labeling with superparamagnetic iron oxide nanoparticles. Magn. Reson. Med. 2010, 63, 1031–1043. 10.1002/mrm.22290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinski N.; Colvin V.; Drezek R. Cytotoxicity of nanoparticles. Small 2008, 4, 26–49. 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- Jiráková K.; Moskvin M.; Machová U. L.; Rössner P.; Elzeinová F.; Chudíčková M.; Jirák D.; Ziolkowska N.; Horák D.; Kubinová Š.; Jendelová P. The negative effect of magnetic nanoparticles with ascorbic acid on peritoneal macrophages. Neurochem. Res. 2020, 45, 159–170. 10.1007/s11064-019-02790-9. [DOI] [PubMed] [Google Scholar]
- Li L.; Jiang W.; Luo K.; Song H.; Lan F.; Wu Y.; Gu Z. Superparamagnetic Iron Oxide Nanoparticles as MRI contrast agents for Non-invasive Stem Cell Labeling and Tracking. Theranostics 2013, 3, 595–615. 10.7150/thno.5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieter W. J.; Taylor K. M. L.; An H.; Lin W.; Lin W. Nanoscale Metal–Organic Frameworks as Potential Multimodal Contrast Enhancing Agents. J. Am. Chem. Soc. 2006, 128, 9024–9025. 10.1021/ja0627444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L.; Sun Z.-Y.; Cheng K.; Liu S.-W.; Pang J.-X.; Xia L.-M.; Chen W.-H.; Cheng Z.; Chen J.-X. Zwitterionic Manganese and Gadolinium Metal–Organic Frameworks as Efficient Contrast Agents for in Vivo Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2017, 9, 41378–41386. 10.1021/acsami.7b09608. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Shang Y.; Li Y.-H.; Sun S.-K.; Yin X.-B. Smart Metal–Organic Framework-Based Nanoplatforms for Imaging-Guided Precise Chemotherapy. ACS Appl. Mater. Interfaces 2019, 11, 1886–1895. 10.1021/acsami.8b19048. [DOI] [PubMed] [Google Scholar]
- Tian C.; Zhu L.; Lin F.; Boyes S. G. Poly(acrylic acid) Bridged Gadolinium Metal–Organic Framework–Gold Nanoparticle Composites as Contrast Agents for Computed Tomography and Magnetic Resonance Bimodal Imaging. ACS Appl. Mater. Interfaces 2015, 7, 17765–17775. 10.1021/acsami.5b03998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. D.; Chen H.; Tang W.; Lee D.; Xie J. Gd and Eu Co-Doped Nanoscale Metal–Organic Framework as a T1–T2 Dual-Modal Contrast Agent for Magnetic Resonance Imaging. Tomography 2016, 2, 179–187. 10.18383/j.tom.2016.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Zhang C.; Xu C.; Lin C.; Sun K.; Wang J.; Chen X.; Li L.; Whittaker A. K.; Xu H.-B. Controlled synthesis of up-conversion luminescent Gd/Tm-MOFs for pH-responsive drug delivery and UCL/MRI dual-modal imaging. Dalton Trans. 2018, 47, 11253–11263. 10.1039/C8DT02436G. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Liu W.; Shang Y.; Cao P.; Cui J.; Li Z.; Yin X.; Li Y. Folic acid-nanoscale gadolinium-porphyrin metal-organic frameworks: Fluorescence and magnetic resonance dual-modality imaging and photodynamic therapy in hepatocellular carcinoma. Int. J. Nanomedicine 2019, 14, 57–74. 10.2147/IJN.S177880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J.; Xue Y.; Lei B.; Xu L.; Sun M.; Li N.; Zhao H.; Wang M.; Luo M.; Zhang C.; Huang B.; Du Y.; Yan C.-H. Multimodal channel cancer chemotherapy by 2D functional gadolinium metal–organic framework. Natl. Sci. Rev. 2021, 8, nwaa221. 10.1093/nsr/nwaa221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.-Y.; Wang Z.-Y.; Gao J.; Wang K.; Gianolio E.; Aime S.; Shi W.; Zhou Z.; Cheng P.; Zaworotko M. J. A Gadolinium(III) Zeolite-like Metal-Organic Framework-Based Magnetic Resonance Thermometer. Chem. 2019, 5, 1609–1618. 10.1016/j.chempr.2019.04.010. [DOI] [Google Scholar]
- Taylor K. M. L.; Rieter W. J.; Lin W. Manganese-Based Nanoscale Metal–Organic Frameworks for Magnetic Resonance Imaging. J. Am. Chem. Soc. 2008, 130, 14358–14359. 10.1021/ja803777x. [DOI] [PubMed] [Google Scholar]
- Pan Y.-B.; Wang S.; He X.; Tang W.; Wang J.; Shao A.; Zhang J. A combination of glioma in vivo imaging and in vivo drug delivery by metal–organic framework based composite nanoparticles. J. Mater. Chem. B 2019, 7, 7683–7689. 10.1039/C9TB01651A. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Tian X.-T.; Shang Y.; Li Y.-H.; Yin X.-B. Theranostic Mn-Porphyrin Metal–Organic Frameworks for Magnetic Resonance Imaging-Guided Nitric Oxide and Photothermal Synergistic Therapy. ACS Appl. Mater. Interfaces 2018, 10, 28390–28398. 10.1021/acsami.8b09680. [DOI] [PubMed] [Google Scholar]
- He M.; Chen Y.; Tao C.; Tian Q.; An L.; Lin J.; Tian Q.; Yang H.; Yang S. Mn–Porphyrin-Based Metal–Organic Framework with High Longitudinal Relaxivity for Magnetic Resonance Imaging Guidance and Oxygen Self-Supplementing Photodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 41946–41956. 10.1021/acsami.9b15083. [DOI] [PubMed] [Google Scholar]
- Bao J.; Zu X.; Wang X.; Li J.; Fan D.; Shi Y.; Xia Q.; Cheng J. Multifunctional Hf/Mn-TCPP Metal-Organic Framework Nanoparticles for Triple-Modality Imaging-Guided PTT/RT Synergistic Cancer Therapy. Int. J. Nanomedicine 2020, 15, 7687–7702. 10.2147/IJN.S267321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S.-S.; Cheng Q.; Zeng X.; Zhang X.-Z. A Mn(III)-Sealed Metal–Organic Framework Nanosystem for Redox-Unlocked Tumor Theranostics. ACS Nano 2019, 13, 6561–6571. 10.1021/acsnano.9b00300. [DOI] [PubMed] [Google Scholar]
- Horcajada P.; Chalati T.; Serre C.; Gillet B.; Serbie C.; Baati T.; Eubank J. F.; Heurtaux D.; Clayette P.; Kreuz C.; Chang J.-S.; Hwang Y. K.; Marsaud V.; Bories P.-N.; Cynober L.; Gil S.; Férey G.; Couvreur P.; Gref R. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178. 10.1038/nmat2608. [DOI] [PubMed] [Google Scholar]
- Hirschle P.; Hirschle C.; Böll K.; Döblinger M.; Höhn M.; Tuffnell J. M.; Ashling C. W.; Keen D. A.; Bennett T. D.; Rädler J. O.; Wagner E.; Peller M.; Lächelt U.; Wuttke S. Tuning the Morphological Appearance of Iron(III) Fumarate: Impact on Material Characteristics and Biocompatibility. Chem. Mater. 2020, 32, 2253–2263. 10.1021/acs.chemmater.9b03662. [DOI] [Google Scholar]
- Dehghani S.; Alam N. R.; Shahriarian S.; Mortezazadeh T.; Haghgoo S.; Golmohamadpour A.; Majidi B.; Khoobi M. The effect of size and aspect ratio of Fe-MIL-88B-NH2 metal-organic frameworks on their relaxivity and contrast enhancement properties in MRI: in vitro and in vivo studies. J. Nanopart. Res. 2018, 20, 278. 10.1007/s11051-018-4376-2. [DOI] [Google Scholar]
- Meng J.; Chen X.; Tian Y.; Li Z.; Zheng Q. Nanoscale Metal–Organic Frameworks Decorated with Graphene Oxide for Magnetic Resonance Imaging Guided Photothermal Therapy. Chem. Eur. J. 2017, 23, 17521–17530. 10.1002/chem.201702573. [DOI] [PubMed] [Google Scholar]
- Zhu W.; Liu Y.; Yang Z.; Zhang L.; Xiao L.; Liu P.; Wang J.; Yi C.; Xu Z.; Ren J. Albumin/sulfonamide stabilized iron porphyrin metal organic framework nanocomposites: targeting tumor hypoxia by carbonic anhydrase IX inhibition and T1–T2 dual mode MRI guided photodynamic/photothermal therapy. J. Mater. Chem. B 2018, 6, 265–276. 10.1039/C7TB02818K. [DOI] [PubMed] [Google Scholar]
- Li Y.; Tang J.; He L.; Liu Y.; Liu Y.; Chen C.; Tang Z. Core–Shell Upconversion Nanoparticle@Metal–Organic Framework Nanoprobes for Luminescent/Magnetic Dual-Mode Targeted Imaging. Adv. Mater. 2015, 27, 4075–4080. 10.1002/adma.201501779. [DOI] [PubMed] [Google Scholar]
- Chen X.; Zhang M.; Li S.; Li L.; Zhang L.; Wang T.; Yu M.; Mou Z.; Wang C. Facile synthesis of polypyrrole@metal–organic framework core–shell nanocomposites for dual-mode imaging and synergistic chemo-photothermal therapy of cancer cells. J. Mater. Chem. B 2017, 5, 1772–1778. 10.1039/C6TB03218D. [DOI] [PubMed] [Google Scholar]
- Wang C.; Jia X.; Zhen W.; Zhang M.; Jiang X. Small-Sized MOF-Constructed Multifunctional Diagnosis and Therapy Platform for Tumor. ACS Biomater. Sci. Eng. 2019, 5, 4435–4441. 10.1021/acsbiomaterials.9b00813. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Liu C.; Wang F.; Liu Z.; Ren J.; Qu X. Metal–organic-framework-supported immunostimulatory oligonucleotides for enhanced immune response and imaging. Chem. Commun. 2017, 53, 1840–1843. 10.1039/C6CC09280B. [DOI] [PubMed] [Google Scholar]
- Liu X.; Zhu X.; Qi X.; Meng X.; Xu K. Co-Administration of iRGD with Sorafenib-Loaded Iron-Based Metal-Organic Framework as a Targeted Ferroptosis Agent for Liver Cancer Therapy. Int. J. Nanomedicine 2021, 16, 1037–1050. 10.2147/IJN.S292528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang W.; Peng L.; Guo P.; Hui H.; Yang X.; Tian J. Metal–Organic Frameworks as a Theranostic Nanoplatform for Combinatorial Chemophotothermal Therapy Adapted to Different Administration. ACS Biomater. Sci. Eng. 2020, 6, 1008–1016. 10.1021/acsbiomaterials.9b01075. [DOI] [PubMed] [Google Scholar]
- Shang W.; Zeng C.; Du Y.; Hui H.; Liang X.; Chi C.; Wang K.; Wang Z.; Tian J. Core–Shell Gold Nanorod@Metal–Organic Framework Nanoprobes for Multimodality Diagnosis of Glioma. Adv. Mater. 2017, 29, 1604381. 10.1002/adma.201604381. [DOI] [PubMed] [Google Scholar]
- Hatakeyama W.; Sanchez T. J.; Rowe M. D.; Serkova N. J.; Liberatore M. W.; Boyes S. G. Synthesis of Gadolinium Nanoscale Metal–Organic Framework with Hydrotropes: Manipulation of Particle Size and Magnetic Resonance Imaging Capability. ACS Appl. Mater. Interfaces 2011, 3, 1502–1510. 10.1021/am200075q. [DOI] [PubMed] [Google Scholar]
- Icten O. Preparation of Gadolinium-Based Metal-Organic Frameworks and the Modification with Boron-10 Isotope: A Potential Dual Agent for MRI and Neutron Capture Therapy Applications. ChemistrySelect 2021, 6, 1900–1910. 10.1002/slct.202100438. [DOI] [Google Scholar]
- Xi D.; Dong S.; Meng X.; Lu Q.; Meng L.; Ye J. Gold nanoparticles as computerized tomography (CT) contrast agents. RSC Adv. 2012, 2, 12515–12524. 10.1039/c2ra21263c. [DOI] [Google Scholar]
- Odéen H.; Parker D. L. Magnetic resonance thermometry and its biological applications - Physical principles and practical considerations. Prog. Nucl. Magn. Reson. Spectrosc. 2019, 110, 34–61. 10.1016/j.pnmrs.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.; Chi B.; Xu C.; Zhang C.; Tian F.; Xu Z.; Li L.; Whittaker A. K.; Wang J. Multifunctional drug carrier on the basis of 3d–4f Fe/La-MOFs for drug delivery and dual-mode imaging. J. Mater. Chem. B 2019, 7, 6612–6622. 10.1039/C9TB01509D. [DOI] [PubMed] [Google Scholar]
- Tamames-Tabar C.; Cunha D.; Imbuluzqueta E.; Ragon F.; Serre C.; Blanco-Prieto M. J.; Horcajada P. Cytotoxicity of nanoscaled metal–organic frameworks. J. Mater. Chem. B 2014, 2, 262–271. 10.1039/C3TB20832J. [DOI] [PubMed] [Google Scholar]
- Wyszogrodzka-Gawel G.; Dorozynski P.; Giovagnoli S.; Strzempek W.; Pesta E.; Weglarz W. P.; Gil B.; Menaszek E.; Kulinowski P. An Inhalable Theranostic System for Local Tuberculosis Treatment Containing an Isoniazid Loaded Metal Organic Framework Fe-MIL-101-NH2-From Raw MOF to Drug Delivery System. Pharmaceutics 2019, 11, 687. 10.3390/pharmaceutics11120687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimpour A.; Alam N. R.; Abdolmaleki P.; Hajipour-Vrdom B.; Tirgar F.; Ebrahimi T.; Khoobi M. Magnetic Metal–Organic Framework Based on Zinc and 5-Aminolevulinic Acid: MR Imaging and Brain Tumor Therapy. J. Inorg. Organomet. Polym. 2021, 31, 1208–1216. 10.1007/s10904-020-01782-5. [DOI] [Google Scholar]
- Sene S.; Marcos-Almaraz M. T.; Menguy N.; Scola J.; Volatron J.; Rouland R.; Greneche J.-M.; Miraux S.; Menet C.; Guillou N.; Gazeau F.; Serre C.; Horcjada P.; Steunou N. Maghemite-nanoMIL-100(Fe) Bimodal Nanovector as a Platform for Image-Guided Therapy. Chem. 2017, 3, 303–322. 10.1016/j.chempr.2017.06.007. [DOI] [Google Scholar]
- He M.; Zhou J.; Chen J.; Zheng F.; Wang D.; Shi R.; Guo Z.; Wang H.; Chen Q. Fe3O4@carbon@zeolitic imidazolate framework-8 nanoparticles as multifunctional pH-responsive drug delivery vehicles for tumor therapy in vivo. J. Mater. Chem. B 2015, 3, 9033–9042. 10.1039/C5TB01830G. [DOI] [PubMed] [Google Scholar]
- Zhao H.-X.; Zou Q.; Sun S.-K.; Yu C.; Zhang X.; Li R.-J.; Fu Y.-Y. Theranostic metal–organic framework core–shell composites for magnetic resonance imaging and drug delivery. Chem. Sci. 2016, 7, 5294–5301. 10.1039/C6SC01359G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C.; Zhang C.; Wang Y.; Li L.; Li L.; Whittaker A. K. Controllable synthesis of a novel magnetic core–shell nanoparticle for dual-modal imaging and pH-responsive drug delivery. Nanotechnology 2017, 28, 495101. 10.1088/1361-6528/aa929b. [DOI] [PubMed] [Google Scholar]
- Ettlinger R.; Moreno N.; Ziółkowska N.; Ullrich A.; Krug von Nidda H.-A.; Jirak D.; Kerl K.; Bunzen H. In Vitro Studies of Fe3O4-ZIF-8 Core–Shell Nanoparticles Designed as Potential Theragnostics. Part. Part. Syst. Charact. 2020, 37, 2000185. 10.1002/ppsc.202000185. [DOI] [Google Scholar]
- Wu M.-X.; Gao J.; Wang F.; Yang J.; Song N.; Jin X.; Mi P.; Tian J.; Luo J.; Liang F.; Yang Y.-W. Multistimuli Responsive Core–Shell Nanoplatform Constructed from Fe3O4@MOF Equipped with Pillar[6]arene Nanovalves. Small 2018, 14, 1704440. 10.1002/smll.201704440. [DOI] [PubMed] [Google Scholar]
- Yang J.-C.; Chen Y.; Li Y.-H.; Yin X.-B. Magnetic Resonance Imaging-Guided Multi-Drug Chemotherapy and Photothermal Synergistic Therapy with pH and NIR-Stimulation Release. ACS Appl. Mater. Interfaces 2017, 9, 22278–22288. 10.1021/acsami.7b06105. [DOI] [PubMed] [Google Scholar]
- Wang D.; Zhou J.; Shi R.; Wu H.; Chen R.; Duan B.; Xia G.; Xu P.; Wang H.; Zhou S.; Wang C.; Wang H.; Guo Z.; Chen Q. Biodegradable Core-shell Dual-Metal-Organic-Frameworks Nanotheranostic Agent for Multiple Imaging Guided Combination Cancer Therapy. Theranostics 2017, 7, 4605–4617. 10.7150/thno.20363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X.; Ji G.; Cui R.; Liu Z. Controlled synthesis of MOFs@MOFs core-shell structure for photodynamic therapy and magnetic resonance imaging. Mater. Lett. 2019, 237, 197–199. 10.1016/j.matlet.2018.11.097. [DOI] [Google Scholar]
- Xu M.; Chi B.; Han Z.; He Y.; Tian F.; Xu Z.; Li L.; Wang J. Controllable synthesis of rare earth (Gd3+,Tm3+) doped Prussian blue for multimode imaging guided synergistic treatment. Dalton Trans. 2020, 49, 12327–12337. 10.1039/D0DT02152K. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Li Z.-H.; Pan P.; Hu J.-J.; Cheng S.-X.; Zhang X.-Z. Tumor-Microenvironment-Triggered Ion Exchange of a Metal-Organic Framework Hybrid for Multimodal Imaging and Synergistic Therapy of Tumors. Adv. Mater. 2020, 32, 2001452. 10.1002/adma.202001452. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Gong C. S.; Lin L.; Zhou Z.; Liu Y.; Yang Z.; Shen Z.; Yu G.; Wang Z.; Wang S.; Ma Y.; Fan W.; He L.; Niu G.; Dai Y.; Chen X. Core-shell metal-organic frameworks with fluorescence switch to trigger an enhanced photodynamic therapy. Theranostics 2019, 9, 2791–2799. 10.7150/thno.34740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Liu B.; Sun Q.; Dong S.; Kuang Y.; Dong Y.; He F.; Gai S.; Yang P. Fusiform-Like Copper(II)-Based Metal–Organic Framework through Relief Hypoxia and GSH-Depletion Co-Enhanced Starvation and Chemodynamic Synergetic Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 17254–17267. 10.1021/acsami.0c01539. [DOI] [PubMed] [Google Scholar]
- Li S.-Y.; Zhao L.-P.; Zheng R.-R.; Fan G.-L.; Liu L.-S.; Zhou X.; Chen X.-T.; Qiu X.-Z.; Yu X.-Y.; Cheng H. Tumor Microenvironment Adaptable Nanoplatform for O2 Self-Sufficient Chemo/Photodynamic Combination Therapy. Part. Part. Syst. Charact. 2020, 37, 1900496. 10.1002/ppsc.201900496. [DOI] [Google Scholar]
- Huang S.; Zhu W.; Zhang F.; Chen G.; Kou X.; Yang X.; Ouyang G.; Shen J. Silencing of Pyruvate Kinase M2 via a Metal–Organic Framework Based Theranostic Gene Nanomedicine for Triple-Negative Breast Cancer Therapy. ACS Appl. Mater. Interfaces 2021, 13, 56972–56987. 10.1021/acsami.1c18053. [DOI] [PubMed] [Google Scholar]
- Zhu Y.; Xin N.; Qiao Z.; Chen S.; Zeng L.; Zhang Y.; Wie D.; Sun J.; Fan H. Bioactive MOFs Based Theranostic Agent for Highly Effective Combination of Multimodal Imaging and Chemo-Phototherapy. Adv. Healthcare Mater. 2020, 9, 2000205. 10.1002/adhm.202000205. [DOI] [PubMed] [Google Scholar]
- Pu Y.; Zhu Y.; Qiao Z.; Xin N.; Chen S.; Sun J.; Jin R.; Nie Y.; Fan H. A Gd-doped polydopamine (PDA)-based theranostic nanoplatform as a strong MR/PA dual-modal imaging agent for PTT/PDT synergistic therapy. J. Mater. Chem. B 2021, 9, 1846–1857. 10.1039/D0TB02725A. [DOI] [PubMed] [Google Scholar]
- Qin Y.-T.; Peng H.; He X.-W.; Li W.-Y.; Zhang Y.-K. pH-Responsive Polymer-Stabilized ZIF-8 Nanocomposites for Fluorescence and Magnetic Resonance Dual-Modal Imaging-Guided Chemo-/Photodynamic Combinational Cancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 34268–34281. 10.1021/acsami.9b12641. [DOI] [PubMed] [Google Scholar]
- Jia M.; Yang X.; Chen Y.; He M.; Zhou W.; Lin J.; An L.; Yang S. Grafting of Gd-DTPA onto MOF-808 to enhance MRI performance for guiding photothermal therapy. J. Mater. Chem. B 2021, 9, 8631–8638. 10.1039/D1TB01596F. [DOI] [PubMed] [Google Scholar]
- McLeod S. M.; Robison L.; Parigi G.; Olszewski A.; Drout R. J.; Gong X.; Islamoglu T.; Luchinat C.; Farha O. K.; Meade T. J. Maximizing Magnetic Resonance Contrast in Gd(III) Nanoconjugates: Investigation of Proton Relaxation in Zirconium Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2020, 12, 41157–41166. 10.1021/acsami.0c13571. [DOI] [PubMed] [Google Scholar]
- Aghayi-Anaraki M.; Safarifard V. Fe3O4@MOF Magnetic Nanocomposites: Synthesis and Applications. Eur. J. Inorg. Chem. 2020, 20, 1916–1937. 10.1002/ejic.202000012. [DOI] [Google Scholar]
- Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 2001, 41, 189–207. 10.1016/S0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- Sönksen M.; Kerl K.; Bunzen H. Current status and future prospects of nanomedicine for arsenic trioxide delivery to solid tumors. Med. Res. Rev. 2022, 42, 374–398. 10.1002/med.21844. [DOI] [PubMed] [Google Scholar]
- Shokouhimehr M.; Soehnlen E. S.; Hao J.; Griswold M.; Flask C.; Fan X.; Basilion J. P.; Basu S.; Huang S. D. Dual purpose Prussian blue nanoparticles for cellular imaging and drug delivery: a new generation of T1-weighted MRI contrast and small molecule delivery agents. J. Mater. Chem. 2010, 20, 5251–5259. 10.1039/b923184f. [DOI] [Google Scholar]
- Zhou T.; Liang X.; Wang P.; Hu Y.; Qi Y.; Jin Y.; Du Y.; Fang C.; Tian J. A Hepatocellular Carcinoma Targeting Nanostrategy with Hypoxia-Ameliorating and Photothermal Abilities that, Combined with Immunotherapy, Inhibits Metastasis and Recurrence. ACS Nano 2020, 14, 12679–12696. 10.1021/acsnano.0c01453. [DOI] [PubMed] [Google Scholar]
- Samiei Foroushani M.; Zahmatkeshan A.; Arkaban H.; Karimi Shervedani R.; Kefayat A. A drug delivery system based on nanocomposites constructed from metal-organic frameworks and Mn3O4 nanoparticles: Preparation and physicochemical characterization for BT-474 and MCF-7 cancer cells. Colloids Surf. B: Biointerfaces 2021, 202, 111712. 10.1016/j.colsurfb.2021.111712. [DOI] [PubMed] [Google Scholar]
- Yin S.-Y.; Song G.; Yang Y.; Zhao Y.; Wang P.; Zhu L.-M.; Yin X.; Zhang X.-B. Persistent Regulation of Tumor Microenvironment via Circulating Catalysis of MnFe2O4@Metal–Organic Frameworks for Enhanced Photodynamic Therapy. Adv. Funct. Mater. 2019, 29, 1901417. 10.1002/adfm.201901417. [DOI] [Google Scholar]
- Cai X.; Zhu Q.; Zeng Y.; Zeng Q.; Chen X.; Zhan Y. Manganese Oxide Nanoparticles As MRI Contrast Agents In Tumor Multimodal Imaging And Therapy. Int. J. Nanomedicine 2019, 14, 8321–8344. 10.2147/IJN.S218085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X.-T.; Cao P.-P.; Zhang H.; Li Y.-H.; Yin X.-B. GSH-activated MRI-guided enhanced photodynamic- and chemo-combination therapy with a MnO2-coated porphyrin metal organic framework. Chem. Commun. 2019, 55, 6241–6244. 10.1039/C9CC01957J. [DOI] [PubMed] [Google Scholar]
- Zhang D.; Ye Z.; Wei L.; Luo H.; Xiao L. Cell Membrane-Coated Porphyrin Metal–Organic Frameworks for Cancer Cell Targeting and O2-Evolving Photodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 39594–39602. 10.1021/acsami.9b14084. [DOI] [PubMed] [Google Scholar]
- Fan B.; Xu S.; Wei Y.; Liu Z. Progresses of hyperpolarized 129Xe NMR application in porous materials and catalysis. Magn. Reson. Lett. 2021, 1, 11–27. 10.1016/j.mrl.2021.100005. [DOI] [Google Scholar]
- Roos J. E.; McAdams H. P.; Kaushik S. S.; Driehuys B. Hyperpolarized Gas MR Imaging: Technique and Applications. Magn. Reson. Imaging Clin. N. Am. 2015, 23, 217–229. 10.1016/j.mric.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q.; Bie B.; Guo Q.; Zhou X.; et al. Hyperpolarized Xe NMR signal advancement by metal-organic framework entrapment in aqueous solution. Proc. Natl. Acad. Sci. U.S.A. 2020, 117, 17558–17563. 10.1073/pnas.2004121117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Zhang Y.; Wang B.; Guo Q.; Yuan Y.; Jiang W.; Shi L.; Yang M.; Chen S.; Lou X.; Zhou X. Coloring ultrasensitive MRI with tunable metal–organic frameworks. Chem. Sci. 2021, 12, 4300–4308. 10.1039/D0SC06969H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirak D.; Galisova A.; Kolouchova K.; Babuka D.; Hruby M. Fluorine polymer probes for magnetic resonance imaging: quo vadis?. J. Magn. Magn. Mater. 2019, 32, 173–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C.; Xu S.; Arshad A.; Wang L. A pH-responsive nanoprobe for turn-on 19F-magnetic resonance imaging. Chem. Commun. 2018, 54, 9853–9856. 10.1039/C8CC06129G. [DOI] [PubMed] [Google Scholar]
- Zhou H.; Qi M.; Shao J.; Li X.; Zhou Z.; Yang S.; Yang H. Tumor micro-environment sensitive 19F-magnetic resonance imaging in vivo. J. Magn. Magn. Mater. 2021, 518, 167436. 10.1016/j.jmmm.2020.167436. [DOI] [Google Scholar]
- Zhou H.; Qi M.; Shao J.; Wang F.; Li X.; Zhou Z.; Yang S.; Yang H. Manganese oxide/Metal-Organic Frameworks-Based Nanocomposites for Tumr Micro-environment Sensitive 1H/19F Dual-mode Magnetic Resonance Imaging in Vivo. J. Organomet. Chem. 2021, 933, 121652. 10.1016/j.jorganchem.2020.121652. [DOI] [Google Scholar]
- Velásquez-Hernández M. de J.; Ricco R.; Carraro F.; Limpoco F. T.; Linares-Moreau M.; Leitner E.; Wiltsche H.; Rattenberger J.; Schröttner H.; Frühwirt P.; Stadler E. M.; Gescheidt G.; Amenitsch H.; Doonan C. J.; Falcaro P. Degradation of ZIF-8 in phosphate buffered saline media. CrystEngComm 2019, 21, 4538–4544. 10.1039/C9CE00757A. [DOI] [Google Scholar]
- Bunzen H. Chemical Stability of Metal-organic Frameworks for Applications in Drug Delivery. ChemNanoMat 2021, 7, 998–1007. 10.1002/cnma.202100226. [DOI] [Google Scholar]