Abstract
Background
Aloin has cardioprotective effects, however, its cardioprotective role in sepsis remains unclear. This study aimed to analyze whether aloin could prevent sepsis‐related myocardial damage and explore the underlying mechanisms by examining the expression of long‐noncoding RNA (lncRNA) SNHG1 and microRNA‐21 (miR‐21).
Methods
The interaction of SNHG1 with miR‐21 was identified by dual‐luciferase reporter assay. The levels of SNHG1 and miR‐21 were measured by real‐time quantitative PCR. The cardioprotective function of aloin was assessed in a sepsis animal model, which was induced by cecal ligation and puncture, and in a myocardial injury cell model in H9C2 cells stimulated by lipopolysaccharide. Myocardial injury biomarker levels and hemodynamic indicators in mice model were measured to evaluate cardiac function. The viability of H9C2 cells was assessed by cell counting kit‐8 assay. Inflammatory cytokine levels were examined by an ELISA method.
Results
Decreased SNHG1 and increased miR‐21 were found in sepsis patients with cardiac dysfunction, and they were negatively correlated. Aloin significantly attenuated myocardial damage and inflammatory responses of mice model, and increased the viability and suppressed inflammation in H9C2 cell model. In addition, SNHG1 expression was upregulated and miR‐21 expression was downregulated by aloin in both mice and cell models. Moreover, in mice and cell models, SNHG1/miR‐21 axis affected sepsis‐related myocardial damage, and mediated the cardioprotective effects of aloin.
Conclusion
Our findings indicated that aloin exerts protective effects in sepsis‐related myocardial damage through regulating cardiac cell viability and inflammatory responses via regulating the SNHG1/miR‐21 axis.
Keywords: Aloin, inflammation, LncRNA SNHG1, miR‐21, myocardial damage, sepsis
(A,B) The myocardial damage markers cTnI and BNP were increased by CLP in mice, but was reduced by aloin treatment in CLP‐treated mice model. (C,D) CLP treatment had inhibitory effects on LVEF and +dp/dtmax, and had promotion effects on –dp/dtmax, but aloin alleviated these effects. (E,F) CLP treatment increased the levels of IL‐1β and TNF‐α in mice, and the effects of CLP on mice were alleviated by aloin. **p < 0.01, ***p < 0.001 versus Sham group; ## p < 0.01, ### p < 0.001 versus CLP group. BNP, brain natriuretic peptide; CLP, cecal ligation and puncture; cTnI, cardiac troponin I; IL‐1β, interleukin 1β; LVEF, left ventricular ejection fraction; TNF‐α, tumor necrosis factor alpha.
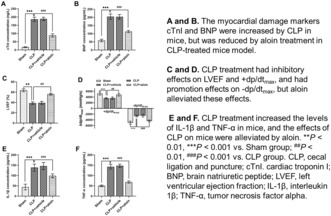
1. INTRODUCTION
Sepsis is defined as a severe and potentially fatal organ dysfunction caused by an inadequate or dysregulated host response to infection, 1 with high morbidity, mortality and treatment costs. The heart is known to be one of the most vulnerable target organs in sepsis. It was first reported in 1951 by Waisbren that sepsis can lead to myocardial depression, characterized by impaired systolic function, cardiac enlargement and decreased ejection fraction. 2 An important feature of sepsis is an excessive inflammatory response, and a particularly intense inflammatory response leads to a disruption of the homeostasis of the cardiovascular system. 3 Unfortunately, there are still few effective treatments for sepsis‐related myocardial damage.
Aloin (also known as barbaloin), one of the major bioactive ingredients obtained from Aloe vera (as a Chinese herbal medicine), 4 has been shown to possess important anti‐inflammatory, 5 , 6 anti‐tumor 7 and cardioprotective functions. 8 , 9 Additionally, a study by Lee et al. has reported the ameliorative effect of aloin on sepsis induced renal injury. 10 However, whether aloin also exerts protective effects against myocardial damage induced by sepsis still unclear. Therefore, in view of the significant cardioprotective effects of aloin, this study aimed to explore whether aloin can alleviate sepsis‐related myocardial damage. In addition, this study also focused on the mechanism of aloin function.
An increasing number of studies have pointed out that drug may play biological effects by regulating the non‐coding RNAs system. 11 Non‐coding RNAs are RNAs that lack the potential to encode proteins, such as microRNAs (miRNAs) and long non‐coding RNAs (lncRNAs), which can be involved in multiple biological processes and regulate physiological and developmental processes. It has been reported that miR‐21 mediates the improvement of osteopenic disorders by aloin. 12 miR‐21 shows significantly upregulated levels in sepsis and is implicated in the dysfunction of various organs induced by sepsis, including cardiac dysfunction, 13 , 14 , 15 becoming an area of intensive investigation in sepsis related diseases. LncRNA SNHG1 is one of the ceRNAs found as miR‐21, which can competitively bind to miR‐21 to cause decrease in its levels. 16 , 17 Recently, a study has pointed out that SNHG1 has an important role in relieving sepsis‐related myocardial injury, 18 and the cardioprotective function of SNHG1 has attracted great interest. 19 , 20 Therefore, we speculated that SNHG1/miR‐21 axis might be related with sepsis‐related myocardial damage, and aloin may play a role in sepsis‐related myocardial damage by the SNHG1/miR‐21 axis.
Therefore, on the basis of confirming the relationship between SNHG1 and miR‐21, this study explored the role of aloin and the SNHG1/miR‐21 axis in sepsis‐related myocardial damage, and revealed the molecular mechanism of aloin function by analyzing the relationship between the axis and aloin. This study may provide evidence for a promising natural drug for the prevention and treatment of sepsis‐related myocardial damage.
2. METHODS AND MATERIALS
2.1. Serum samples from sepsis patients
Blood samples from 48 patients with sepsis admitted to Shengli Oilfield Central Hospital from 2018 to 2019 were collected in this study, and the serum was obtained from blood by centrifugation 1500 × g at 4°C and stored at −80°C for further use. Among these sepsis patients, 28 patients had cardiac dysfunction (CD) and the patients included 28 males and 20 females with an average age of 56.75 ± 11.23 years. The patients were diagnosed with sepsis according to the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis‐3). 21 Patients' inclusion criteria were (a) age ≥ 18 years and (b) no history of cancer or hematological malignancy. The patients were excluded if they (a) died within 24 h of admission; (b) were treated with immunosuppression within 6 months; (c) were infected with human immunodeficiency virus and (d) were pregnant or in lactation. In addition, the serum samples of 48 healthy volunteers, who underwent physical examination in our hospital and matched with sepsis patients in both age and sex, were collected as healthy control (including 27 males and 21 females, aged 57.26 ± 11.81 years old). The healthy volunteers had no history of sepsis or malignancy and was confirmed by physical examination to have no significant abnormalities. The study was approved by the Ethics Committee of Shengli Oilfield Central Hospital and each patient provided written informed consent.
2.2. Sepsis animal model constructed using cecal ligation and puncture (CLP)
The C57BL/6 mice (n = 100, aged 8–10 weeks) were purchased from the Shanghai Animal Center (Shanghai, China). The animal experiments were performed following the National Institutes of Health Guidelines for Animal Care, and were approved by the Shengli Oilfield Central Hospital. The sepsis mice model was constructed by CLP surgery as described previously. 22 Briefly, mice were first anesthetized by inhalation of 5% isoflurane, and then a small incision was made in their anterior abdomen. The cecum was exposed and ligated 1 cm proximal to the end of the cecum. Then, the cecum was punctured twice with a sterile 23‐gauge needle. Afterwards, the cecum was placed back into the abdominal cavity and the incision was closed. Sham‐operated mice did not receive cecal ligation or puncture, and the rest of the treatment procedures were the same as those in the sepsis mice model. After surgery, mice were injected with 1 mL normal saline for fluid resuscitation.
2.3. H9C2 cell culture and LPS induction
A myocardial cell line H9C2 was purchased from the Cell Bank of the Chinese Academy of Science (Shanghai, China). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Gibco, CA, USA) at 37°C under 5% CO2. Before further experiments, H9C2 cells were routinely subcultured to 80% fusion. H9C2 cells were stimulated for 6 h using 1 μg/mL LPS to construct in vitro model to mimic the myocardial damage during the development of sepsis.
2.4. Dual‐luciferase reporter assay
The StarBase platform (http://starbase.sysu.edu.cn/index.php) was used to predict the binding sites of miR‐21 and SNHG1, and then dual‐luciferase reporter assay was used to verify their direct binding. The wild‐type (WT) sequences of SNHG1 containing the biding site of miR‐21 and the mutant‐type (MUT) sequences of SNHG1 were cloned into the luciferase reporter vector pmirGLO vector (Promega, Madison, WI, USA). Then, SNHG1‐WT or SNHG1‐MUT vectors were co transfected with miR‐21 mimic or mimic NC into H9C2 cells using Lipofectamine 3000 reagent (Invitrogen, Carlsbad, USA). Forty‐eight hours after transfection, relative luciferase activity was confirmed utilizing the dual‐luciferase reporter assay system (Promega). The firefly luciferase activity was normalized to Renilla luciferase activity. All procedures were performed based on the instructions of manufacturers.
2.5. RNA extraction and real‐time quantitative PCR (RT‐qPCR)
Total RNA was extracted from the serum of participants, heart tissues of mice and H9C2 cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). A NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA) was utilized to verify RNA purity and concentration. Subsequently, a PrimeScript RT reagent Kit (TaKaRa, Otsu, Shiga, Japan) was used for reverse transcription of RNA to synthesize cDNA. The RT‐qPCR was performed using a 7500 Real‐Time PCR System (Applied Biosystems, USA) with SYBR green I Master Mix kit (Invitrogen, Carlsbad, CA, USA) to detect the expression of SNHG1 and miR‐21. The thermocycling conditions were as follows: 95°C for 10 min and followed by 40 cycles of 95°C for 30 s, 60°C for 20 s and 72°C for 30 s. GAPDH and U6 were used as internal controls for SNHG1 and miR‐21. Their expression levels were calculated using the 2−ΔΔCt method. 23 Following were the primer sequences: SNHG1 forward 5’‐CCTAAAGCCACGCTTCTTG‐3′, reverse 5’‐TGCAGGCTGGAGATCCTACT‐3′; miR‐21 forward 5′–3′, reverse 5′–3′; GAPHD forward 5’‐CAAGGTCATCCATGACAACTTTG‐3′, reverse 5’‐GTCCACCACCCTGTTGCTGTAG‐3′; U6 forward 5’‐CTCGCTTCGGCAGCACA‐3′, reverse 5’‐AACGCTTCACGAATTTGCGT‐3′.
2.6. Experimental group design and treatments
The aloin (>97%, dissolved in 0.5% DMSO) was purchased from Sigma‐Aldrich (St. Louis, MO, USA). The mice were randomly divided into ten groups (n = 10/group): (1) sham group (mice received sham operation); (2) CLP group (mice received CLP operation); (3) CLP + vehicle group; (4) CLP + aloin group; (5) CLP + pcDNA3.1 group; (6) CLP + pcDNA3.1‐SNHG1 group; (7) CLP + mimic NC group; (8) CLP + miR‐21 mimic group; (9) CLP + aloin + si‐SNHG1 group; (10) CLP + aloin + miR‐21 mimic group. At 12 and 50 h after CLP surgery, the mice in groups (3) and (4) were administered with aloin (12.4 mg/kg) and vehicle (equal volume) by intravenous injection, respectively. The mice in groups (5), (6), (7) and (8) were treated with the indicated vectors, respectively, including pcDNA3.1, pcDNA3.1‐SNHG1, mimic NC and miR‐21 mimic, by tail vein injection after CLP treatment. In groups (9) and (10), the mice received si‐SNHG1 and miR‐21 mimic, respectively, by tail vein injection after fluid resuscitation following the CLP treatment. All above vectors were synthesized by the GenePharma (Shanghai, China). Three days after CLP surgery, cardiac function was checked and blood samples were retained.
The H9C2 cells were also divided into ten groups: (1) control group (received no treatment); (2) LPS group (received LPS treatment); (3) LPS + vehicle group; (4) LPS + aloin group; (5) LPS + pcDNA3.1 group; (6) LPS + pcDNA3.1‐SNHG1 group; (7) LPS + mimic NC group; (8) LPS + miR‐21 mimic group; (9) LPS + aloin + si‐SNHG1 group; (10) LPS + aloin + miR‐21 mimic. Two hours before LPS (1 g/mL, 6 h) treatment, cells in (3) group were treated with 400 μM aloin and cells in (4) group were treated with equal volume of vehicle. In the groups (5), (6), (7) and (8), cells were transfected with the indicated vectors, respectively, using Lipofectamine 3000 (Thermo Fisher Scientific, Inc.) before LPS. The cells in the (9) and (10) groups, except receiving aloin treatment, were also transfected with the indicated vectors using Lipofectamine 3000 (Thermo Fisher Scientific, Inc.). The cell transfection vectors were obtained from GenePharma (Shanghai, China).
2.7. Evaluation of myocardial damage
To reflect the situation of myocardial damage in mice, the ultrasound examination, hemodynamic analysis as well as detection of myocardial damage markers were performed on mice. The left ventricular ejection fraction (LVEF) was calculated using Doppler echocardiography (GEL‐400). The MedLab6.0 software in HRV&BRS (FDP‐1) analysis system was used to examine and calculate the maximum rate of rise/fall of left ventricle pressure (±dp/dtmax). An enzyme‐linked immunosorbent assay (ELISA) kit (Bioscience, San Diego, CA, USA) was used to measure the levels of serum cardiac troponin I (cTnI) and brain natriuretic peptide (BNP).
2.8. Enzyme‐linked immunosorbent assay (ELISA)
In addition to detecting the levels of cTnI and BNP, the ELISA kit (Beyotime, Shanghai, China) was also used to analyze the levels of proinflammatory factor IL‐1β and TNF‐α in the blood of mice and the supernatant of H9C2 cells following the protocols of manufacture. In brief, specific antibodies were coated onto 96‐well microtiter plates. The samples to be tested were added into plates and incubated at room temperature for 2 h. After washing with wash buffer for 5 times, the plates were added with biotinylated antibodies at room temperature for 1 h. Then, HRP‐conjugated streptavidin was added in the plates for 20 min at room temperature. Color development reagent TMB was added and incubated for 20 min away from light after 5 times of washing. After that, the absorbance at 450 nm was measured.
2.9. Cell counting kit (CCK)‐8 assay
Cell Counting Kit‐8 (CCK‐8) assay was utilized to assess the viability of H9C2 cells. H9C2 cells were seeded into 96‐well plates (5000 cells/well) and incubated at 37°C. After 24 h of incubation, 10 μL CCK‐8 reagent was added into the cells and all the cells were incubated for an additional 2 h. At last, the optical density (OD) values of all plates were detected at 450 nm using a microplate analyzer (Bio‐Rad Laboratories, Inc.) to reflect H9C2 cell viability.
2.10. Statistical analysis
The results of data analysis were expressed as mean ± SD. SPSS 22.0 (SPSS Inc., Chicago, IL) and GraphPad Prism 7.0 software (GraphPad Software, Inc., USA) were used for the statistical analyses. The distribution of data was checked using Kolmogorov–Smirnov test. Differences between two groups and among multiple groups were compared by unpaired Student's t test and one‐way ANOVA followed by Tukey's post hoc test, respectively. Pearson correlation coefficient was used to evaluate the correlation between SNHG1 and miR‐21 in patients with sepsis. p < 0.05 was considered statistically significant.
3. RESULTS
3.1. Deregulated expression of the SNHG1/miR‐21 axis in sepsis patients
Through StarBase platform prediction, a putative binding site of miR‐21 was identified in the 3’‐UTR of SNHG1 (Figure 1A). Subsequently, the interaction between miR‐21 and SNHG1 was confirmed by a dual‐luciferase reporter assay. Relative luciferase activity of SNHG1‐WT was significantly suppressed following the overexpression of miR‐21 in H9C2 cells (p < 0.05), whereas there were no significant differences in the relative luciferase activity of SNHG1‐MUT in H9C2 cells (Figure 1B). In addition, SNHG1 expression was decreased (p < 0.001, Figure 1C) and miR‐21 expression was increased (p < 0.001, Figure 1D) in sepsis patients compared with that in healthy volunteers, and SNHG1 was found to be negatively correlated with miR‐21 in sepsis patients (r = −0.515, p < 0.001; Figure 1E). Moreover, SNHG1 expression was also decreased (p < 0.001, Figure 1F) and miR‐21 expression was also increased (p < 0.001, Figure 1G) in sepsis patients with CD compared with that sepsis patients without CD.
FIGURE 1.
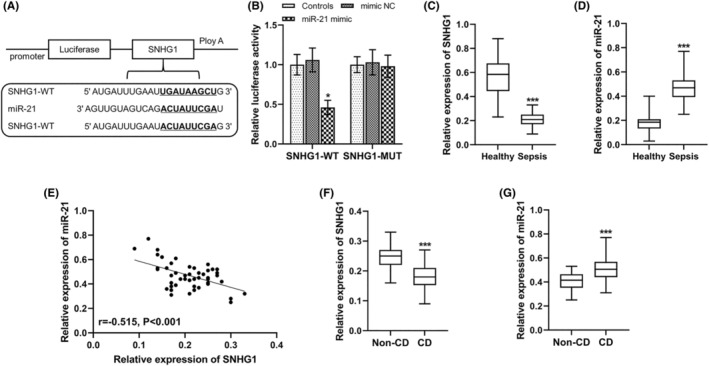
Aberrant expression of the SNHG1/miR‐21 axis in sepsis patients. (A) The putative binding site between miR‐21 and SNHG1 were predicted by StarBase platform. (B) miR‐21 upregulation significantly inhibited the relative luciferase activity in SNHG1‐WT group in H9C2 cells. (C,D) SNHG1 and miR‐21 expression levels in healthy controls (n = 48) and sepsis patients (n = 48). (E) Negative correlation between SNHG1 and miR‐21 was found. (F,G) SNHG1 and miR‐21 expression levels in sepsis patients with (n = 28) and without CD (n = 20). *p < 0.05, ***p < 0.001 versus Controls or healthy controls or non‐CD patients. CD, cardiac dysfunction; MUT, mutant‐type; WT, wide‐type.
3.2. Aloin alleviates myocardial damage and inflammation in sepsis mice induced by CLP
By CLP treatment, we observed the increased levels of cTnI and BNP, decreased LVEF and +dp/dtmax and increased –dp/dtmax (all p < 0.01, Figure 2A–D), demonstrating the myocardial damage of mice. Additionally, the concentrations of IL‐1β and TNF‐α were elevated in mice treated by CLP, which demonstrated that the inflammatory responses of mice were activated (both p < 0.001, Figure 2E,F). The above findings indicated that the sepsis mice model with myocardial damage was successfully constructed. What is more important is that aloin treatment decreased the cTnI, BNP and –dp/dtmax, increased the LVEF and +dp/dtmax, and decreased IL‐1β and TNF‐α in sepsis mice model (all p < 0.01, Figure 2). Thus, aloin improved the myocardial damage and inflammatory responses of sepsis mice model.
FIGURE 2.
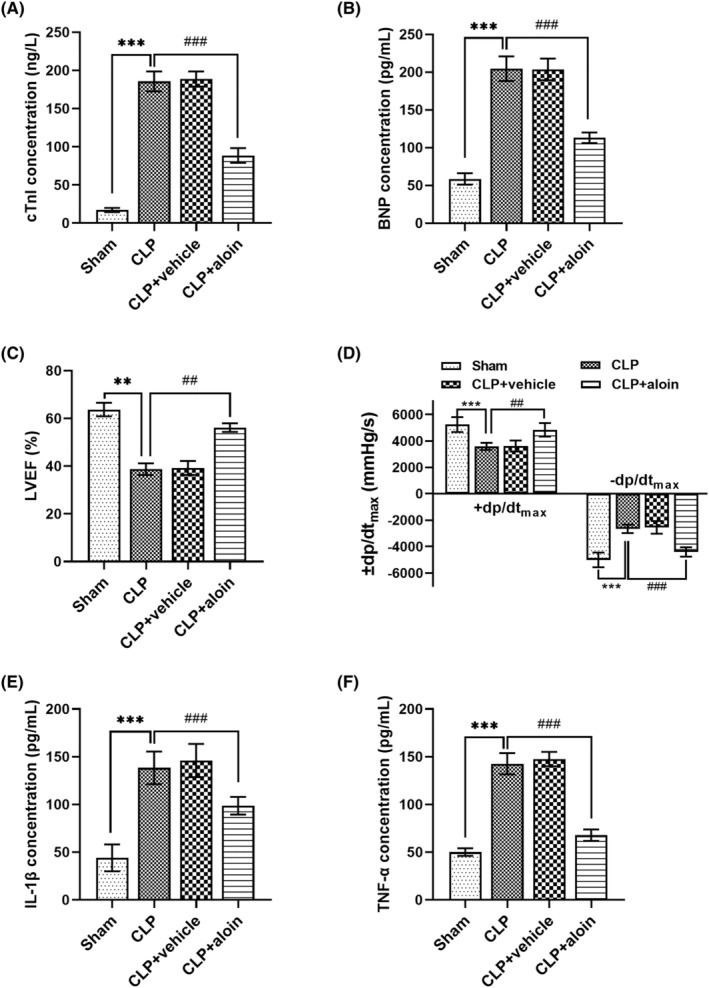
(A,B) The myocardial damage markers cTnI and BNP were increased by CLP in mice, but was reduced by aloin treatment in CLP‐treated mice model. (C,D) CLP treatment had inhibitory effects on LVEF and +dp/dtmax, and had promotion effects on –dp/dtmax, but aloin alleviated these effects. (E,F) CLP treatment increased the levels of IL‐1β and TNF‐α in mice, and the effects of CLP on mice were alleviated by aloin. **p < 0.01, ***p < 0.001 versus Sham group; ## p < 0.01, ### p < 0.001 versus CLP group. BNP, brain natriuretic peptide; CLP, cecal ligation and puncture; cTnI, cardiac troponin I; IL‐1β, interleukin 1β; LVEF, left ventricular ejection fraction; TNF‐α, tumor necrosis factor alpha.
3.3. Aloin protects against LPS‐induced cell injury in H9C2 cells
For H9C2 cells, the cell viability was markedly inhibited and the secretion of IL‐1β and TNF‐α was markedly promoted by LPS stimulation (all p < 0.001, Figure 3), suggesting that impaired myocardial cell model was constructed by LPS stimulation. More importantly, for H9C2 cells receiving LPS stimulation, the cell viability was increased (p < 0.001, Figure 3A), and the inflammatory cytokine levels were decreased by aloin treatment (all p < 0.001, Figure 3B,C).
FIGURE 3.
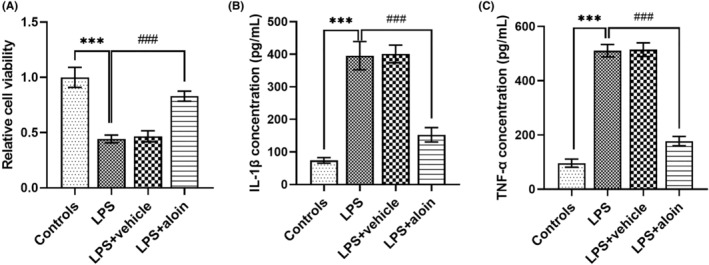
Aloin protected H9C2 cells against LPS‐induced cell injury. (A) LPS suppressed the viability of H9C2 cells, but aloin promoted the cell viability of LPS‐induced H9C2 cells. (B,C) The IL‐1β and TNF‐α levels of H9C2 cells were increased by LPS, and these effects of LPS were alleviated by aloin. ***p < 0.001 versus Controls; ### p < 0.001 versus LPS group. IL‐1β, interleukin 1β; LPS, lipopolysaccharide; TNF‐α, tumor necrosis factor alpha.
3.4. Regulatory effects of aloin on the expression of the SNHG1/miR‐21 axis
To further investigate the potential molecular mechanism of aloin's drug action, the relationship of the SNHG1/miR‐21 axis with aloin was studied. We observed that SNHG1 expression was promoted and miR‐21 expression was inhibited by aloin in the mice model (all p < 0.001, Figure 4A,B) and H9C2 cell model (all p < 0.001, Figure 4C,D).
FIGURE 4.
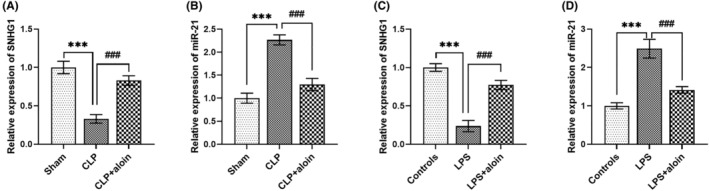
Aloin regulated the expression of SNHG1 and miR‐21. (A–D) Aloin could upregulated SNHG1 expression but downregulated miR‐21 expression in the mice model and cell model. ***p < 0.001 versus Sham group or controls; ### p < 0.001 versus CLP group or LPS group. CLP, cecal ligation and puncture; LPS, lipopolysaccharide.
3.5. Influences of the SNHG1/miR‐21 axis on sepsis‐related myocardial damage in vivo and in vitro
The expression of SNHG1 was increased by pcDNA3.1‐SNHG1, and miR‐21 expression was increased by miR‐21 mimic in sepsis mice model (all p < 0.001, Figure 5A,B). SNHG1 upregulation significantly improved myocardial damage and inflammation in mice model that evidenced by the decreased cTnI, BNP, −dp/dtmax, increased LVEF and +dp/dtmax, and reduced IL‐1β and TNF‐α levels (all p < 0.001, Figure 5C–H). By contrary, miR‐21 upregulation in mice model significantly aggravated myocardial damage and inflammation that evidenced by increased cTnI, BNP, −dp/dtmax, decreased LVEF and +dp/dtmax, and increased IL‐1β and TNF‐α levels (all p < 0.01, Figure 5C–H). As shown in Figure 5I,J, SNHG1 was upregulated by pcDNA3.1‐SNHG1, and miR‐21 was upregulated by miR‐21 mimic in H9C2 cell model (all p < 0.001). SNHG1 upregulation promoted, and miR‐21 upregulation inhibited the viability of LPS‐induced H9C2 cells (all p < 0.001, Figure 5K). The inflammation of LPS‐induced H9C2 cells was inhibited by SNHG1 upregulation, and was promoted by miR‐21 upregulation (all p < 0.001, Figure 5L,M).
FIGURE 5.
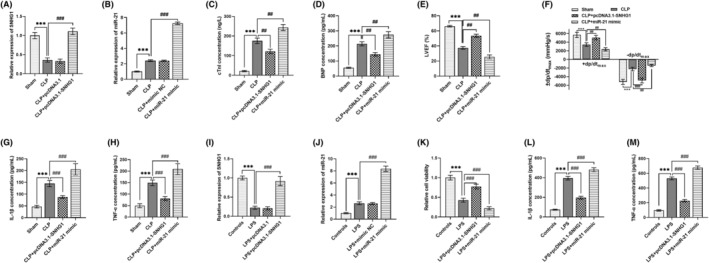
Effects of SNHG1/miR‐21 axis on sepsis‐related myocardial damage in mice model and cell model. (A,B) In CLP treated mice model, SNHG1 expression was upregulated by pcDNA3.1‐SNHG1, and miR‐21 was upregulated by miR‐21 mimic. (C–F) SNHG1 upregulation reduced but miR‐21 upregulation increased the levels of cTnI, BNP and –dp/dtmax in mice model. Besides, SNHG1 upregulation increased but miR‐21 upregulation reduced LVEF and +dp/dtmax in mice model. (G,H) The levels of IL‐1β and TNF‐α in mice model were reduced by SNHG1 upregulation and were increased by miR‐21 upregulation. (I,J) In H9C2 cell model, the pcDNA3.1‐SNHG1 transfection and miR‐21 mimic transfection upregulated SNHG1 and miR‐21 expression, respectively. (K) The viability of LPS‐induced H9C2 cells was promoted by SNHG1 upregulation and was inhibited by miR‐21 upregulation. (L,M) SNHG1 upregulation suppressed and miR‐21 upregulation promoted the IL‐1β and TNF‐α levels of LPS‐induced H9C2 cells. ***p < 0.001 versus Shame group or controls; ## p < 0.01, ### p < 0.001 versus CLP group or LPS group. BNP, brain natriuretic peptide; CLP, cecal ligation and puncture; cTnI, cardiac troponin I; IL‐1β, interleukin 1β; LPS, lipopolysaccharide; LVEF, left ventricular ejection fraction; TNF‐α, tumor necrosis factor alpha.
3.6. SNHG1/miR‐21 axis mediates the cardioprotective role of aloin in sepsis
In the mice model receiving aloin, SNHG1 expression was reduced by si‐SNHG1, and miR‐21 expression was increased by miR‐21 mimic as well as SNHG1 downregulation (all p < 0.001, Figure 6A). The cTnI, BNP, −dp/dtmax, IL‐1β and TNF‐α were upregulated, and the LVEF and +dp/dtmax were downregulated by both SNHG1 downregulation and miR‐21 upregulation in aloin‐treated mice model (all p < 0.01, Figure 6B–E), suggesting the mediating role of SNHG1/miR‐21 axis in myocardial damage and inflammation of mice model. As presented in Figure 6F, in H9C2 cell model receiving aloin, the si‐SNHG1 transfection inhibited the expression of SNHG1, and the miR‐21 mimic and SNHG1 downregulation promoted the expression of miR‐21 (all p < 0.001). SNHG1 downregulation and miR‐21 upregulation both reduced the cell viability and increased the levels of inflammatory factors of aloin‐treated cell model (all p < 0.001, Figure 6G,H), suggesting that SNHG1 and miR‐21 mediated the role of aloin in sepsis cell model.
FIGURE 6.
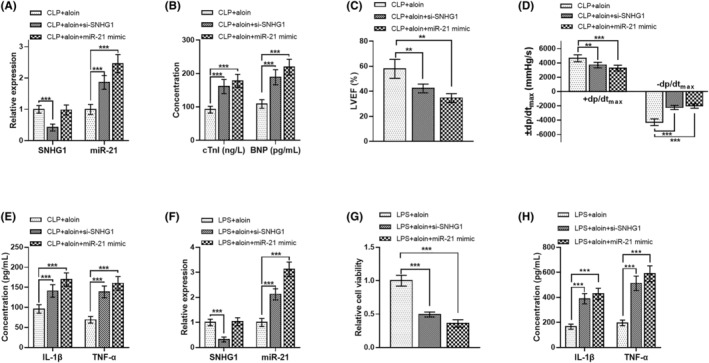
The cardioprotective role of aloin in sepsis was mediated by the SNHG1/miR‐21 axis. (A) The si‐SNHG1 decreased SNHG1 expression and miR‐21 mimic and SNHG1 increased miR‐21 expression in the aloin‐treated mice model. (B–E) The cTnI, BNP, −dp/dtmax, IL‐1β and TNF‐α of aloin‐treated mice model were increased, and the LVEF and +dp/dtmax of aloin‐treated mice model were decreased by SNHG1 downregulation and miR‐21 upregulation. (F) The si‐SNHG1 decreased SNHG1 expression, and miR‐21 mimic and SNHG1 downregulation increased miR‐21 expression in aloin‐treated cell model. (G,H) In aloin‐treated cell model, the cell viability was decreased and the inflammatory cytokines (IL‐1β and TNF‐α) levels were increased by SNHG1 downregulation and miR‐21 upregulation. **p < 0.01, ***p < 0.001 versus CLP + aloin group or LPS + aloin group. BNP, brain natriuretic peptide; CLP, cecal ligation and puncture; cTnI, cardiac troponin I; IL‐1β, interleukin 1β; LPS, lipopolysaccharide; LVEF, left ventricular ejection fraction; TNF‐α, tumor necrosis factor alpha.
4. DISCUSSION
CLP surgery has been widely used to induce sepsis animal models. 24 Many previous studies have analyzed sepsis associated myocardial damage by using CLP induced animal models. For example, a study by Sang et al. has investigated the potential of miR‐214‐3p in sepsis‐induced myocardial damage using mice that underwent CLP surgery. 25 The protective effects of dexmedetomidine on sepsis‐induced myocardial injury were studied in a male mice model of CLP. 26 In the current study, we also used CLP surgery to successfully construct a sepsis mice model, which could be demonstrated by aggravated myocardial damage and inflammatory response. In addition, LPS‐induced H9C2 cells were routinely used as a cell model of sepsis myocardial damage. 27 , 28 In this study, a myocardial cell model of sepsis was successfully constructed, as evidenced by reduced cell viability and increased inflammation in H9C2 cells.
Accumulating evidence has indicated that many bioactive products of Chinese herbal medicine have attracted attention due to their great therapeutic potential in various human diseases, including sepsis and its associated complications. For example, naringin can attenuate the inflammatory responses and myocardial injury induced by sepsis by regulating the PI3K/AKT/NF‐κB pathway. 29 Xu et al. have provided evidence that shenfu injection, a Chinese herbal medicine, can prevent sepsis induced myocardial injury via the inhibition of myocardial apoptosis. 30 Matrine, as a bio‐active agent of several Chinese medical herbs, can improve sepsis‐associated cardiac dysfunction by increasing cardiac myoblast viability and inhibiting inflammatory responses. 31 In this study, we demonstrated that aloin could attenuate myocardial damage and inflammatory responses in a mice model of sepsis. In addition, in the LPS‐stimulated H9C2 cells, aloin increased myocardial cell viability and decreased the levels of inflammatory cytokines, further indicating the cardioprotective effects of aloin in sepsis. Notably, the cardioprotective effects of aloin have been found in arsenic trioxide‐induced myocardial membrane damage 8 and doxorubicin‐induced cardiotoxicity. 9 In addition, aloin has been reported to mitigate sepsis‐related inflammatory responses and organ injury. 10 Thus, aloin may function as a potential natural drug for sepsis‐related myocardial damage through increasing myocardial cell viability and decreasing inflammatory responses.
More and more studies have highlighted the role of non‐coding RNAs in organ injury, including myocardial injury, in sepsis. For instance, lncRNA taurine up‐regulated gene 1 (TUG1) has been shown to attenuate the acute lung injury induced by sepsis through miR‐34b‐5p and GRB2 associated binding protein 1 (GAB1). 32 miR‐193‐3p alleviates myocardial damage and inflammation of mice with sepsis by targeting signal transducers and activators of transcription 3 (STAT3). 33 A study by Sun et al. has shown that lncRNA KCNQ1OT1 regulates myocardial damage and inflammatory responses during sepsis via the miR‐192‐5p/XIAP axis. 27 LncRNA nuclear enriched abundant transcript 1 (NEAT1) can regulate sepsis‐induced myocardial cell injury and inflammation via targeting miR‐144‐3p. 34 Of note, SNHG1 and miR‐21 have been found to be related to sepsis‐related cardiac diseases. 14 , 18 In this study, SNHG1 could directly bind to miR‐21. Notably, SNHG1 has been found to act as a ceRNA for miR‐21 and bind to miR‐21 in the previous studies. 16 , 17 In addition, downregulated SNHG1 and upregulated miR‐21 were observed in sepsis patients and sepsis patients with CD, which was consistent with previous studies. 14 , 18 Moreover, the expression of SNHG1 and miR‐21 in mice and cell models could be regulated by aloin, which has a protective effect on myocardial damage caused by sepsis. Furthermore, SNHG1 upregulation could improve the myocardial damage and inflammation in mice model, and enhanced the viability and inflammation of cell model. Nevertheless, the effect of miR‐21 upregulation on myocardium was opposite to that of SNHG1. Therefore, the SNHG1/miR‐21 axis may be correlated with the progression of sepsis‐related myocardial damage.
Increasing studies have emphasized the role of non‐coding RNAs in the therapeutic mechanisms of different drugs, including active components of traditional Chinese medicines. For instance, through decreasing miR‐132 expression, luteolin can alleviate LPS‐induced bronchopneumonia injury in vitro and in vivo. 35 Song et al. have found that gracillin can improve LPS‐induced acute cardiac injury in mice through inhibiting apoptosis and inflammation by regulating miR‐29a. 36 Sevoflurane has been reported to protect cardiomyocytes from hypoxia/reperfusion injury through the LINC01133/miR‐30a‐5p axis. 37 The study by Liu et al. has revealed that matrine exerts protective effects in sepsis‐associated cardiac dysfunction by modulating the PTENP1/miR‐106b‐5p axis. 31 The use of aloin for cardiac disease treatment is currently increasing, however, its function through regulation of non‐coding RNAs is poorly studied. Notably, there has a study reporting that the ameliorative effects of aloin on osteopenic disorders were mediated by miR‐21. 12 In the present study, considering the relationship of SNHG1/miR‐21 axis with aloin and the important role of the SNHG1/miR‐21 axis on sepsis‐related myocardial damage in in vivo and in vitro models, we further explored whether the SNHG1/miR‐21 axis could modulate the effects of aloin. This study found that overexpression of miR‐21 significantly reversed the cardioprotective effects of aloin on sepsis. Importantly, a study by Zhang et al. has shown that miR‐21 mediated the effects of natural drug hyperoside on sepsis‐associated cardiac dysfunction. 15 Therefore, we concluded that the cardioprotective effect of aloin in sepsis might be mediated by miR‐21. For SNHG1, its downregulation markedly reversed the protective effects of aloin against sepsis‐induced myocardial damage. Taken together, the SNHG1/miR‐21 axis might be involved in the cardioprotective effects of aloin against sepsis‐induced myocardial damage.
This study is the first to explore the role of SNHG1/miR‐21 axis in the effect of aloin in sepsis‐induced myocardial damage. However, this study had some limitations. At first, the study sample was small; thus, a large cohort is needed in further studies. Second, the target genes of miR‐21 that may be involved in sepsis‐induced myocardial damage were not analyzed in this study, and further studies to comprehensively understand the action mechanism of aloin in sepsis‐induced myocardial damage are warranted. It has been found that miR‐21 controls sepsis‐associated cardiac dysfunction via regulating SH3 domain‐containing protein 2 (SORBS2). 14 In addition, miR‐21 can target A20 to mediate septic shock. 38 Thus, we speculated that SORBS2 and A20 may play a role in sepsis‐induced myocardial damage as targets of miR‐21. Further studies are necessary to explore that whether SNHG1/miR‐21 mediated the function of aloin through targeting SORBS2 and A20.
In conclusion, the findings of this study demonstrate that the SNHG1/miR‐21 axis is aberrantly expressed in sepsis patients with CD. In addition, aloin may alleviate the sepsis‐related myocardial damage through facilitating cardiac cell viability and inhibiting inflammatory responses by regulation of the SNHG1/miR‐21 axis. Therefore, aloin may be a promising agent for the therapy of sepsis‐related myocardial damage. This study may contribute to the development of novel strategies for the therapy of sepsis‐related myocardial damage.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
CONSENT TO PARTICIPATE
A signed written informed consent was obtained from each patient.
CONSENT FOR PUBLICATION
Written informed consent for publication was obtained from each participant.
ACKNOWLEDGEMENTS
Not applicable.
Peng J, Li S, Han M, Gao F, Qiao L, Tian Y. SNHG1/miR‐21 axis mediates the cardioprotective role of aloin in sepsis through modulating cardiac cell viability and inflammatory responses. J Clin Lab Anal. 2023;37:e24985. doi: 10.1002/jcla.24985
Jin Peng, Shuyuan Li, Lujun Qiao and Yonggang Tian authors contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Salomao R, Ferreira BL, Salomao MC, Santos SS, Azevedo LCP, Brunialti MKC. Sepsis: evolving concepts and challenges. Braz J Med Biol Res. 2019;52(4):e8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Waisbren BA. Bacteremia due to gram‐negative bacilli other than the Salmonella; a clinical and therapeutic study. AMA Arch Intern Med. 1951;88(4):467‐488. [DOI] [PubMed] [Google Scholar]
- 3. Lv X, Wang H. Pathophysiology of sepsis‐induced myocardial dysfunction. Mil Med Res. 2016;3:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee W, Yang S, Lee C, et al. Aloin reduces inflammatory gene iNOS via inhibition activity and p‐STAT‐1 and NF‐kappaB. Food Chem Toxicol. 2019;126:67‐71. [DOI] [PubMed] [Google Scholar]
- 5. Luo X, Zhang H, Wei X, et al. Aloin suppresses lipopolysaccharide‐induced inflammatory response and apoptosis by inhibiting the activation of NF‐kappaB. Molecules. 2018;23(3):517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ma Y, Tang T, Sheng L, et al. Aloin suppresses lipopolysaccharideinduced inflammation by inhibiting JAK1STAT1/3 activation and ROS production in RAW264.7 cells. Int J Mol Med. 2018;42(4):1925‐1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang Z, Rui W, Wang ZC, Liu DX, Du L. Anti‐proliferation and anti‐metastasis effect of barbaloin in non‐small cell lung cancer via inactivating p38MAPK/Cdc25B/Hsp27 pathway. Oncol Rep. 2017;38(2):1172‐1180. [DOI] [PubMed] [Google Scholar]
- 8. Birari LA, Mahajan UB, Patil KR, et al. Aloin protects against arsenic trioxide‐induced myocardial membrane damage and release of inflammatory cytokines. Naunyn Schmiedebergs Arch Pharmacol. 2020;393(8):1365‐1372. [DOI] [PubMed] [Google Scholar]
- 9. Birari L, Wagh S, Patil KR, et al. Aloin alleviates doxorubicin‐induced cardiotoxicity in rats by abrogating oxidative stress and pro‐inflammatory cytokines. Cancer Chemother Pharmacol. 2020;86(3):419‐426. [DOI] [PubMed] [Google Scholar]
- 10. Lee W, Jeong GS, Baek MC, Ku SK, Bae JS. Renal protective effects of aloin in a mouse model of sepsis. Food Chem Toxicol. 2019;132:110651. [DOI] [PubMed] [Google Scholar]
- 11. Matsui M, Corey DR. Non‐coding RNAs as drug targets. Nat Rev Drug Discov. 2017;16(3):167‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Madhyastha R, Madhyastha H, Pengjam Y, Nurrahmah QI, Nakajima Y, Maruyama M. The pivotal role of microRNA‐21 in osteoclastogenesis inhibition by anthracycline glycoside aloin. J Nat Med. 2019;73(1):59‐66. [DOI] [PubMed] [Google Scholar]
- 13. Zhu WD, Xu J, Zhang M, Zhu TM, Zhang YH, Sun K. MicroRNA‐21 inhibits lipopolysaccharide‐induced acute lung injury by targeting nuclear factor‐kappaB. Exp Ther Med. 2018;16(6):4616‐4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang H, Bei Y, Shen S, et al. miR‐21‐3p controls sepsis‐associated cardiac dysfunction via regulating SORBS2. J Mol Cell Cardiol. 2016;94:43‐53. [DOI] [PubMed] [Google Scholar]
- 15. Zhang J, Liu Y, Liu L. Hyperoside prevents sepsis‐associated cardiac dysfunction through regulating cardiomyocyte viability and inflammation via inhibiting miR‐21. Biomed Pharmacother. 2021;138:111524. [DOI] [PubMed] [Google Scholar]
- 16. Li W, Dong X, He C, et al. LncRNA SNHG1 contributes to sorafenib resistance by activating the Akt pathway and is positively regulated by miR‐21 in hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2019;38(1):183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tan J, Wang W, Song B, Song Y, Meng Z. Integrative analysis of three novel competing endogenous RNA biomarkers with a prognostic value in lung adenocarcinoma. Biomed Res Int. 2020;2020:2837906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luo S, Huang X, Liu S, Zhang L, Cai X, Chen B. Long non‐coding RNA small nucleolar RNA host gene 1 alleviates sepsis‐associated myocardial injury by modulating the miR‐181a‐5p/XIAP axis in vitro. Ann Clin Lab Sci. 2021;51(2):231‐240. [PubMed] [Google Scholar]
- 19. Yan SM, Li H, Shu Q, Wu WJ, Luo XM, Lu L. LncRNA SNHG1 exerts a protective role in cardiomyocytes hypertrophy via targeting miR‐15a‐5p/HMGA1 axis. Cell Biol Int. 2020;44(4):1009‐1019. [DOI] [PubMed] [Google Scholar]
- 20. Liang S, Ren K, Li B, et al. LncRNA SNHG1 alleviates hypoxia‐reoxygenation‐induced vascular endothelial cell injury as a competing endogenous RNA through the HIF‐1alpha/VEGF signal pathway. Mol Cell Biochem. 2020;465(1–2):1‐11. [DOI] [PubMed] [Google Scholar]
- 21. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis‐3). Jama. 2016;315(8):801‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou Y, Song Y, Shaikh Z, et al. MicroRNA‐155 attenuates late sepsis‐induced cardiac dysfunction through JNK and beta‐arrestin 2. Oncotarget. 2017;8(29):47317‐47329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25(4):402‐408. [DOI] [PubMed] [Google Scholar]
- 24. Deng Q, Zhao T, Pan B, et al. Protective effect of Tubastatin a in CLP‐induced lethal sepsis. Inflammation. 2018;41(6):2101‐2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sang Z, Zhang P, Wei Y, Dong S. miR‐214‐3p attenuates sepsis‐induced myocardial dysfunction in mice by inhibiting autophagy through PTEN/AKT/mTOR pathway. Biomed Res Int. 2020;2020:1409038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang C, Yuan W, Hu A, et al. Dexmedetomidine alleviated sepsisinduced myocardial ferroptosis and septic heart injury. Mol Med Rep. 2020;22(1):175‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sun F, Yuan W, Wu H, et al. LncRNA KCNQ1OT1 attenuates sepsis‐induced myocardial injury via regulating miR‐192‐5p/XIAP axis. Exp Biol Med (Maywood). 2020;245(7):620‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen T, Zhu C, Ye C. LncRNA CYTOR attenuates sepsis‐induced myocardial injury via regulating miR‐24/XIAP. Cell Biochem Funct. 2020;38(7):976‐985. [DOI] [PubMed] [Google Scholar]
- 29. Sun LJ, Qiao W, Xiao YJ, Cui L, Wang X, Ren WD. Naringin mitigates myocardial strain and the inflammatory response in sepsis‐induced myocardial dysfunction through regulation of PI3K/AKT/NF‐kappaB pathway. Int Immunopharmacol. 2019;75:105782. [DOI] [PubMed] [Google Scholar]
- 30. Xu P, Zhang WQ, Xie J, Wen YS, Zhang GX, Lu SQ. Shenfu injection prevents sepsis‐induced myocardial injury by inhibiting mitochondrial apoptosis. J Ethnopharmacol. 2020;261:113068. [DOI] [PubMed] [Google Scholar]
- 31. Liu Y, Liu L, Zhang J. Protective role of matrine in sepsis‐associated cardiac dysfunction through regulating the lncRNA PTENP1/miR‐106b‐5p axis. Biomed Pharmacother. 2021;134:111112. [DOI] [PubMed] [Google Scholar]
- 32. Qiu N, Xu X, He Y. LncRNA TUG1 alleviates sepsis‐induced acute lung injury by targeting miR‐34b‐5p/GAB1. BMC Pulm Med. 2020;20(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pan J, Alexan B, Dennis D, Bettina C, Christoph LIM, Tang Y. microRNA‐193‐3p attenuates myocardial injury of mice with sepsis via STAT3/HMGB1 axis. J Transl Med. 2021;19(1):386. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34. Wei JL, Wu CJ, Chen JJ, et al. LncRNA NEAT1 promotes the progression of sepsis‐induced myocardial cell injury by sponging miR‐144‐3p. Eur Rev Med Pharmacol Sci. 2020;24(2):851‐861. [DOI] [PubMed] [Google Scholar]
- 35. Liu X, Meng J. Luteolin alleviates LPS‐induced bronchopneumonia injury in vitro and in vivo by down‐regulating microRNA‐132 expression. Biomed Pharmacother. 2018;106:1641‐1649. [DOI] [PubMed] [Google Scholar]
- 36. Song YX, Ou YM, Zhou JY. Gracillin inhibits apoptosis and inflammation induced by lipopolysaccharide (LPS) to alleviate cardiac injury in mice via improving miR‐29a. Biochem Biophys Res Commun. 2020;523(3):580‐587. [DOI] [PubMed] [Google Scholar]
- 37. Yu Z, Ren Q, Yu S, Gao X. Sevoflurane protects cardiomyocytes against hypoxia/reperfusion injury via LINC01133/miR‐30a‐5p axis. Biosci Rep. 2020;40(12):BSR20200713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xue Z, Xi Q, Liu H, et al. miR‐21 promotes NLRP3 inflammasome activation to mediate pyroptosis and endotoxic shock. Cell Death Dis. 2019;10(6):461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


