Abstract
The aims of this study were to analyze proton pump inhibitor (PPI) users in Germany, defining and classifying them in terms of treatment appropriateness, and to analyze the PPI prescription practices of healthcare providers. The updated DGVS (Deutsche Gesellschaft für Gastroenterologie, Verdauungs-und Stoffwechselkrankheiten) gastroesophageal reflux disease (GERD) treatment guideline (published March 2023) for mild heartburn symptoms recommends carrying out a probatory treatment of mild symptoms via other medication such as antacids, alginates, and H2 blockers before escalating to PPI treatments, if the patient profile allows. This retrospective cross-sectional study was based on data from the IQVIA™ Disease Analyzer database (DA) and included adult patients (18 years or older) in 1006 general and 39 gastroenterological practices in Germany who received at least 1 PPI prescription or alginate between September 2019 and September 2021 (hereinafter referred to as the index period). Analyses included indications associated with PPI prescription, co-diagnoses, co-therapies of PPI patients, duration of PPI therapy, dosages of PPI prescriptions, and proportions of practices prescribing PPIs and alginates. A total of 472 146 patients taking PPIs and 9101 patients taking alginates were available for analysis. Very few patients (4.5%) of the total cohort were treated in complete adherence to treatment guidelines. Conditions such as gastritis and duodenitis (47.2%) and reflux diseases (38.4%) were more frequently associated with PPI prescriptions. The average PPI treatment period lasted 141 days, and 36.6% of patients were treated for >6 months. High doses were prescribed relatively often (ie, 42.8% of esomeprazole prescriptions were 40 mg, 59.1% of lansoprazole prescriptions 30 mg, 28.6% of omeprazole prescriptions 40 mg). With each practice prescribing PPIs to at least 10% of their patients; 72% of general practitioners (GPs) and 8% of GENTS (Gastroenterologists) prescribed alginates. This study highlights that discrepancies exist between clinical guidelines and real-life prescribing practices of PPIs in Germany. Particular attention should be given to the incidence of patients being prescribed high-dose or long-duration PPI with mild indications. These findings are particularly apt considering the publication (March 2023) of new guidelines on the “management of gastroesophageal reflux disease and eosinophilic esophagitis,” by the DGVS.
Keywords: proton pump inhibitor, alginate, primary care, deprescribing
Introduction
Proton pump inhibitors (PPIs) are a class of medications that were introduced clinically in the 1980s and are used to induce a strong and lasting reduction in the production of stomach acid. 1 PPIs target the last step of acid production in the gastric parietal cells, irreversibly inhibiting the hydrogen-potassium ATPase (proton pump) that is responsible for the secretion of H+ ions into the stomach lining, inducing a very significant reduction in gastric acid production. 2 PPIs can be used to treat acid-related gastro-intestinal indications, including conditions such as peptic ulcers and GERD, as well as to prevent damage to the upper GI tract by aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs). 3
As such, PPIs are popular drugs—in Bavaria alone a recent drug-utilization study from 2010 to 2018 found that 64.8 defined daily doses were prescribed per 1000 insured people, per day. 4
While the short-term benefits of PPI usage largely outweigh the risks, it is long-term usage—particularly at high doses—that have brought up intensive discussions on the risk-benefit analysis.5-17 The excessive length of treatment and dosage is also affected by the fact that some patients take PPIs without any indication, and by the extension of the treatment duration in some patients without indication for a PPI, due to rebound acid hypersecretion (RAHS) symptoms after attempted withdrawal.3,18,19 While the observational results of these studies should be interpreted with caution (causation cannot be implied), the evidence suggests that long-term PPI usage should be limited to medically justified situations according to not only the updated national, but also international guidelines, while a reduction in PPI dosage or a switch to other alternative therapies such as antacids, alginates, or H2 blockers might be a better option for the remainder of cases.5,20,21 Heartburn symptoms may occur during a PPI deprescribing phase, and alginates can be prescribed to reduce these symptoms.22-25 Indeed, several studies have already been conducted which focus on PPI dosage reduction or replacement, with encouraging results in certain cases.23,24 Coyle et al showed that 75.1% of 6249 patients were able to step off or down from PPI within 1 year, after receiving educational information and agreeing on a management plan on reducing or stopping PPI therapy. 23
Given the extensive use of PPIs in Germany and the potential negative effects of their long-term and/or high-dosage use, there is a recognized need for a large-scale evaluation of treatment and dosage patterns, along with an evaluation of whether such treatments are adequate or whether patients would benefit from either pursuing alternative symptom management options, or from a reduction in PPI dosage.
As such, this study aimed to analyze PPI users in Germany in terms of appropriateness of treatment, and to analyze the PPI prescription practices of healthcare providers. This study was carried out prior to the publication of the new DGVS guidelines in Germany, however, the findings support its publication and clarify its importance amidst the current culture of PPI prescription.
Methods
Data Sources
This retrospective cross-sectional study was based on data from IQVIA DA, which contains case-based information provided by office-based physicians (both GPs and specialists) including demographic data, prescriptions, and diagnoses in Germany. This database represents approximately 3% of all German private practices. The database is considered representative of German outpatients and appears to be suitable for epidemiological studies. IQVIA ensures the accuracy, consistency, and completeness of the data. 26
Study Population
This study was designed to include in its main cohort: adult patients (18 years or older) in general and gastroenterological practices in Germany receiving at least 1 PPI (ATC: A02B2) or alginate (defined as having alginic acid listed among its active substances, or as the brand) prescription during the index period. Exclusion criteria included missing documentation of age, sex, and insurance status as well as less than 12 months of observability prior to the first prescription.
Analyses and Definitions
PPI prescription cohorts
All PPI and alginate patients included in the study were classified into 1 (or several, as a patient could belong to more than 1 group) of the 6 distinctive cohorts depending on the details of their PPI prescriptions. Table 1 shows the patterns of PPI use which deviate from guidelines, defined in this study. Additionally, we calculated the proportion of patients who received no co-medication while reducing or coming off PPI. These patients had at least 1 PPI dosage reduction during their treatment period or received no PPI prescription in the 3 months afterwards.
Table 1.
Patterns of PPI Use Which May be Inappropriate.
| Cohort | Definition |
|---|---|
| Patients for whom PPIs are no longer necessary | - Patients with mild conditions whose treatment lasts longer than 8 weeks. - Patients who have not had their prescription reviewed after 8 weeks, that is, patients receiving treatment for longer than 8 weeks who logged no physician visits in the 8 to 12 weeks after their first PPI prescription for each treatment episode. - Patients using PPI for gastroprotection/prophylaxis who no longer take the comedication—defined as patients receiving overlapping NSAID and PPI treatment for whom the PPI treatment episode ends more than 1 week after the NSAID treatment. |
| Patients who have been prescribed PPIs inappropriately | - Patients receiving PPIs without an appropriate indication, defined as being one of the gastroesophageal focus diseases. - Patients who received PPI on their first visit for mild disease—meaning patients that for whom a PPI prescription, a diagnosis for one of the mild diseases listed in Table 2, and no severe disease diagnoses are documented upon the first visit. - Patients receiving an interfering co-medication during a PPI treatment episode. |
| Patients whose PPI dosage can be reduced | - Patients continuously using PPI for longer than 8 weeks. - Patients on high doses PPIs whose dosage remains unchanged in the 8 weeks after the initial prescription. - Patients on polypharmacy (using 5+ concurrent drugs), older patients (age group > 70 years), or patients with impaired liver or kidney function. |
| Patients with an appropriate PPI prescription | - Patients with at least 1 PPI prescription who were excluded in the 3 previous groups. |
| Patients who may benefit from an alginate add-on | - Patients who visit the doctor repeatedly during a long PPI episode (defined as patients with an average of 2 or more visits per month after 8 weeks have elapsed since the start of the PPI episode). - Patients who remain on continuous, high-dose PPI for 3 months or longer. - Patients whose daily PPI dose has gone up, suggesting breakthrough symptoms. - Patients with more severe erosive conditions, that is, Barret’s esophagus or Zollinger-Ellison syndrome. - Patients receiving long-term gastroprotection, that is, under NSAID treatments lasting longer than 6 months. |
| Patients receiving no comedication while reducing or coming off PPI | - Patients who had at least 1 PPI treatment episode whose PPI dosage was reduced during the episode or who received no PPI prescriptions in the 3 months after the end of the treatment episode. Of these, those patients who received no comedication at all during the aforementioned dose reduction or discontinuation episodes were selected for inclusion in this group. |
Diagnoses associated with PPI prescription, comorbidity, and polypharmacy
Diagnoses which were directly linked to PPI prescriptions were analyzed. Diagnoses and symptoms with a proportion of at least 1% included gastritis and duodenitis, functional dyspepsia (ICD-10: K30, K29), reflux disease (ICD-10: K21), heartburn (ICD-10: R12), and ulcers (ICD-10: K25-K28).
The most common comorbidities documented between January 2018 and September 2021 independently of PPI treatment episodes, as well as the most common co-therapies prescribed during a PPI treatment episode were also calculated.
Duration of PPI therapy
The therapy duration was calculated for every PPI prescription based on daily dosage, number of packages, package size, and strength. A continuous PPI treatment episode was calculated as time to therapy discontinuation defined as at least 2 weeks without PPI therapy and presented as a persistence analysis (Figure 4). Along with mean and median duration of continuous PPI treatment episodes, treatment episodes were grouped by duration into 6 categories: under 4 weeks, 4 to 8 weeks, 8 weeks to 3 months, 3 to 6 months, 6 to 12 months, and treatments lasting longer than a year.
Figure 4.
Persistence analyses for PPI therapy depending on the user cohort.
Dosages of PPI prescriptions
A 2-tier system was used to determine the daily dosage: If the prescriber had entered a daily dosage directly in the database, that figure was used, while for prescriptions in which this information was missing, the daily dosage was estimated based on the median time between equivalent prescriptions of the same substance, package size, and strength.
PPI prescriber analyses
The proportions of practices prescribing PPIs and alginates as well as practices prescribing PPIs upon first patient visit were calculated based on GP and Gastroenterology practices in the database. In addition, proportions of practices prescribing PPIs and alginates to at least 10% of their patients were shown.
Results
Study Population and Baseline Characteristics
The database contains 1006 and 39 GP and gastroenterologist practices continuously delivering data between January 2018 and September 2019 with 9 310 670 and 1 041 189 respective patients (including those with visit dates preceding the index period). Of these, 567 726 and 12 113 patients respectively received at least 1 PPI or alginate prescription during the index period, of which in turn 472 146 and 9101 respectively had at least 1 year of observability before their first PPI prescription.
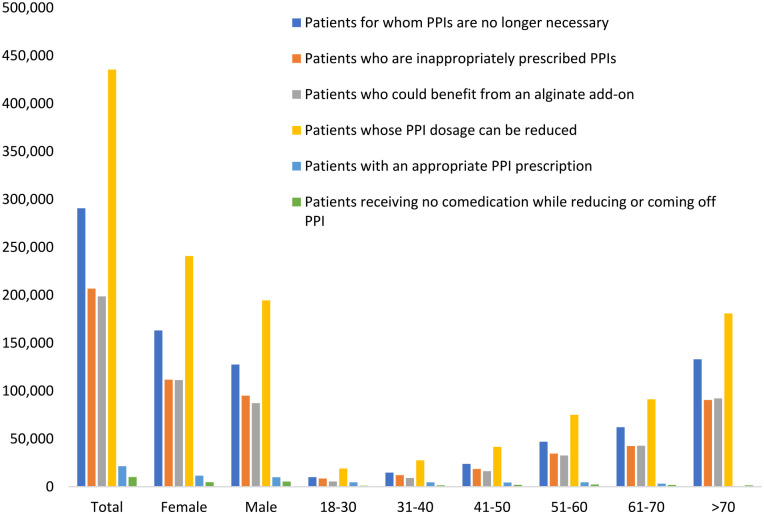
Overall, most PPI patients tend to be in older age cohorts, with 38% belonging to the >70 cohort, 21% to the 61 to 70 cohort, and 18% to the 51 to 60 cohort (Figure 1). The sex distribution is (55% female, 45% male; Figure 1), and most patients on PPI treatment are covered by statutory health insurance (92% SHI, 8% PHI). The regional distribution is roughly aligned with the overall population, with 83% of patients in the West and 17% in the East, and most patients are treated by GPs (96%) rather than by gastroenterologists (4%).
Figure 1.
User cohorts striated by age and sex.
Defining PPI Prescription Cohorts
In this study we looked at PPI users, which fell into a number of cohorts. These cohorts are defined as 1 of 6 groups (Table 1), addressing presence or absence of an appropriate indication for PPI use, duration of use, dose, and potential for dose or use reduction or alternative treatment. Most patients (96.5%) of the total study population were classified to at least one of the defined groups, since >70% were counted in 2 or 3 groups at the same time. The significant majority (92.2%) could benefit from a reduction in their PPI dosage, while 62% no longer need PPIs, 44% were inappropriately prescribed PPIs, and 42% would benefit from an alginate add-on (Table 2). Furthermore, very few patients (1.4% of the total) are receiving alginates with GERD or nonerosive reflux disease (NERD), and out of the total number of patients, only 2% received no comedication while coming off PPI and virtually none (0.2%) received alginates while coming off PPI.
Table 2.
Classification of PPI Patients in Terms of Appropriateness of the PPI Use.
| Group | Patients (472 146 = 100%) (N%) |
|---|---|
| Patients for whom PPIs are no longer necessary | 290 742 (62) |
| Patients who have been prescribed PPIs inappropriately | 206 840 (44) |
| Patients whose PPI dosage can be reduced | 435 427 (92) |
| Patients with an appropriate PPI prescription | 21 458 (5) |
| Patients who may benefit from an alginate add-on | 198 696 (42) |
| Patients receiving no comedication while reducing or coming off PPI | 10 108 (2) |
Patients may belong to more than 1 cohort.
Diagnoses Associated With PPI Prescription, Comorbidity, and Polypharmacy
Figure 2 shows diagnoses associated with PPI prescriptions. Conditions such as gastritis, duodenitis and dyspepsia (48.3%), and reflux diseases (38.4%) were more frequent than ulcers (5.1%), for example. For example, 52.0% were diagnosed with dorsalgia, 49.0% with hypertension, 28.7% with lipid metabolism disorders, 24.5% with abnormal pain or pelvic pain, 20.4% with depression, and 20.0% with urinary system diseases. Finally, comorbidities are often linked to polypharmacy, where 32.8% of subjects were prescribed beta blockers, 31.9% non-steroid antirheumatics, 26.0% statins, 19.5% ACE inhibitors, 19.5% thyroid preparations, 18.5% calcium antagonists, and 17.8% diuretics.
Figure 2.
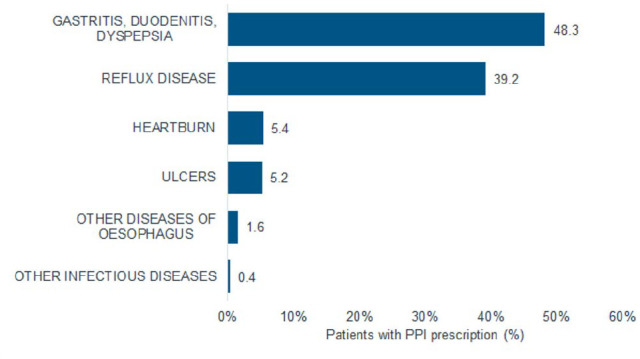
Diagnoses associated with PPI prescription.
Duration of PPI Therapy and Persistence Analyses
The average PPI treatment episode was 141 days, with a median treatment duration of 95 days. A short therapy duration of up to 8 weeks was observed in just 16.1% of patients, with 26.3% treated for 3 to 6 months, 13.2% for 6 to 12 months, and 23.2% for more than 12 months (Figure 3). Figure 4 additionally displays persistence analysis depending on the user cohort which shows that the cohort of patients with proper PPI treatment has 2 sharp drop-offs in persistence after the first and second months, whereas for the other cohorts treated patients tend to persist for longer, though a noticeable drop is evident in between months 2 and 4 in most cohorts with all cohorts dropping more than 40% of their patients in those months (except for the cohort of patients who can reduce their PPI dosage, which shows a much smoother and continuous persistence court).
Figure 3.
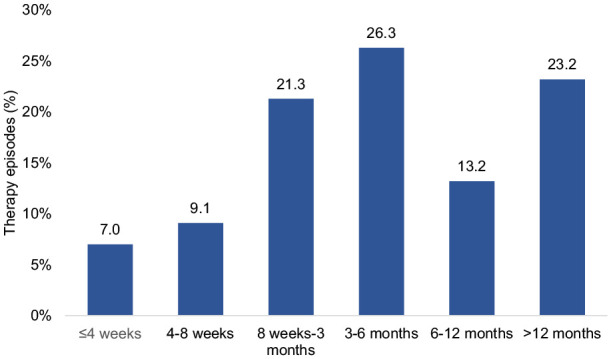
Duration of PPI therapy.
After 12 months, the cohorts of patients on adequate PPI treatment and who receive no comedication while reducing or coming off PPI see most of their patients drop off, whereas for all other cohorts there is a remanent of 10% to 20% of the initial patients that are still treated after a year (Figure 4).
Dosages of PPI Prescriptions
Table 3 shows the prescribed doses of PPI drugs. Doses did vary depending on the PPI drug, for example: 49.5% of esomeprazole prescriptions had a dose of 40 or 80 mg compared to 47.2% of pantoprazole patients and 31.2% of omeprazole patients. In total, high doses were prescribed more often than low doses (Table 4).
Table 3.
PPI Doses Prescribed.
| Daily dosage (mg) | Esomeprazole (n, %) | Lansoprazole (n, %) | Omeprazole (n, %) | Pantoprazole (n, %) | Rabeprazole (n, %) |
|---|---|---|---|---|---|
| 5 | 1746 (4.6) | 39 (1.7) | 8370 (9.1) | 27 956 (6.4) | 199 (10.9) |
| 10 | 2317 (6.1) | 736 (31.9) | 11 891 (12.9) | 47 890 (11.0) | 465 (25.4) |
| 20 | 12 298 (32.6) | 170 (7.4) | 40 255 (43.8) | 124 678 (28.7) | 1041 (56.8) |
| 30 | 2703 (7.2) | 1364 (59.1) | 2729 (3.0) | 29 007 (6.7) | — |
| 40 | 16 127 (42.8) | — | 26 268 (28.6) | 180 213 (41.4) | 127 (6.9) |
| 80 | 2527 (6.7) | — | 2424 (2.6) | 25 370 (5.8) | — |
The doses shown here are those available in Germany, any doses not shown are unavailable in this region, therefore not included.
Table 4.
Diseases and ICD-10 Coding Associated With PPI Prescriptions.
| Category | Severity | ICD-10 code | Description |
|---|---|---|---|
| Gastro-oesophageal focus diseases | Mild | B980 | H. pylori infection |
| K219 | NERD | ||
| K29 | Gastritis and duodenitis | ||
| K300 | Functional dyspepsia | ||
| R120 | Heartburn | ||
| Severe | E164 | Zollinger-Ellison Syndrome | |
| K25 | Gastric ulcer | ||
| K26 | Duodenal ulcer | ||
| K27 | Peptic ulcer | ||
| K210 | Erosive GORD | ||
| K227 | Barret’s oesophagus | ||
| Other relevant diseases | Does not apply | A047 | Clostridium difficile infection |
| A049 | Small intestinal bacterial Overgrowth | ||
| B012 | Pneumonia | ||
| J12-J16 and J18 | Pneumonia |
PPI Prescriber Analyses
Each of the 1045 practices in the database prescribed PPIs to at least 10% of its patients, with >95% of practices prescribing PPIs to at least 10% of their patients upon the first visit. All practices were also involved in deprescribing for more than 10% of their patients and prescribe co-medication alongside PPI. Interestingly, GPs were far more likely to review a patient and stop PPI prescriptions after 4 weeks than gastroenterologists (97% of GPs do this for more than 10% of their patients vs 21% of gastroenterologists).
Discussion
This retrospective cross-sectional study represents a large sample of 472 146 patients in Germany, has resulted in several findings regarding the current usage and practices in proton pump inhibitor treatment in Germany.
First, nearly all practices used in the sample—both GPs and gastroenterologists—prescribed PPIs to more than 10% of their patients, with PPIs representing 5% of all prescriptions by GPs and 16% of all prescriptions by gastroenterologists during the index period. Furthermore, several of the gastroesophageal diseases such as reflux disease, gastritis and duodenitis, heartburn, or gastric ulcers were found to be among the most common diagnoses linked by physicians to PPI prescriptions. NSAIDs were also found to be one of the co-medications most prescribed alongside PPIs, suggesting their common use as gastroprotection.
PPIs are known to be widely used in Germany in the treatment of many conditions.27,28 However, our study has shown that only a very small number of patients receiving PPIs were treated in line with guideline recommendations. Overall, 92% of the total cohort of PPI patients were shown to be likely to benefit from a reduction in dosage, 62% should be able to stop treatment altogether, and 44% were found to be receiving a course of PPI treatment not in line with guidance and appropriate treatment. In addition, PPI treatments tended to be much longer than the 4 to 8 weeks that are recommended for most cases and were also prescribed at higher doses than the recommended dose.29-31 In addition, most PPI patients had several poly-pharmacies, these could be unnecessary and should be reduced where applicable to reduce risk of drug interactions.
It should be noted that having a high number of inappropriate PPI prescriptions and excessively long-term administration of the medication is not solely a German problem. In 2017, a Canadian guideline was published with the aim of facilitating PPI deprescribing. 32 Deprescribing is a process involving structured dose reduction and discontinuation of a medication for which there is no longer an indication, or where the potential harm outweighs the benefit. This Both Canadian and American guideline recommended reducing doses, stopping, or using low doses in patients who have completed 4 weeks of PPI treatment for heartburn or mild to moderate gastroesophageal reflux disease or esophagitis, and whose symptoms have been alleviated.32,33 Various deprescribing strategies have been developed and trialed in clinical practice among small patient populations.34 -39 Rudelle and Laroche 40 have determined the level of knowledge and attitude of GPs with respect to the adverse effects caused by PPIs in France. Some 80.4% of GPs were willing to deprescribe PPIs where such side effects arose. 40
Heartburn symptoms may occur during a PPI deprescribing phase, and alginates can be prescribed to reduce these symptoms.23 -25
In the present study, alginate prescription was found to be comparatively rare in patient with GERD indication, in GP and gastroenterology practices prescribing them at all, and 50% and 77% respectively prescribing them in combination with PPIs. We also found that 42% of PPI patients could benefit from an alginate add-on, while just 1.4% of patients with GERD diagnoses have received such an add-on, and barely 0.2% of patients are receiving alginates while coming off PPI. In Germany alginates, if an OTC medicinal product, can be prescribed via green prescription by the GP or GENT and patients do have the opportunity to asked for reimbursement with their general health insurance on a case-by-case situation. There is a published list of general health insurances and their reimbursement limit for non Rx products (OTC-Satzungsleistungen der Krankenkassen—DeutschesApothekenPortal). 41 In a randomized, double-blind, double-dummy study in patients with heartburn without alarm symptoms on symptoms on 2 to 6 days in the previous week, a 14-day therapy with an alginate (4 times a day) was not inferior to a therapy with alginate (4 times a day) was not inferior to treatment with 20 mg omeprazole. 42 Alginates may represent a comparably effective alternative to PPI. 43
Retrospective primary care database analyses are generally limited by the validity and completeness of the data analyzed. The following limitations that are relevant to the present study should be mentioned: first, practices only report on diagnoses based on international classification of diseases (ICD) codes; no detailed information on symptoms or severity is available in the database. Second, prescriptions are not always reported with an associated diagnosis, nor is there an indicated date for the end of the condition if or when it resolves. Third, some low-dose PPIs are also available in pharmacies without prescription, and the database does not include data on the use of PPIs purchased over the counter by patients. Fourth, no data are available on socioeconomic status and lifestyle-related risk factors (smoking, alcohol, and physical activity). Fifth, patients can only be observed in a single practice; if they receive a diagnosis or prescription from another physician or in hospital, such prescriptions are not documented. However, this study also has several strengths including a very large sample size, use of data from clinical practice, and access to data from both GP and GENT practices.
Conclusion
This study highlights that discrepancies exist between clinical guidelines for GERD and real-life prescribing practices of PPIs in Germany. Particular attention should be given to the incidence of patients being prescribed high-dose or long-duration PPI with mild indications. These findings are particularly apt considering the publication of new guidelines on the management of “Gastroesophageal reflux disease and eosinophilic esophagitis,” by the DGVS. 42 Deprescribing of PPI is required in many patients, while alginate use may be an appropriate supporting therapy in many cases. There is a clear need to bring more awareness to doctors in reevaluating patients’ diagnosis, who are on long term PPI therapy, which would be beneficial for patients’ safety (concerning long term side effects) and in reducing the burden on the health care system. A clear method with which to eliminate or reduce PPI doses from long term PPI treatment cases could be helpful and encourage doctors to follow the treatment guidelines more closely.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MH, JW, CC, and KP are employees of Reckitt Benckiser, while EI and KK are employees of IQVIA.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: All funding for the research and writing of this manuscript was provided by Reckitt Benckiser.
ORCID iD: Karel Kostev  https://orcid.org/0000-0002-2124-7227
https://orcid.org/0000-0002-2124-7227
References
- 1. Chubineh S, Birk J. Proton pump inhibitors: the good, the bad, and the unwanted. South Med J. 2012;105:613-618. doi: 10.1097/smj.0b013e31826efbea [DOI] [PubMed] [Google Scholar]
- 2. Sachs G, Shin JM, Howden CW. Review article: the clinical pharmacology of proton pump inhibitors. Aliment Pharmacol Ther. 2006;23 Suppl 2:2-8. doi: 10.1111/j.1365-2036.2006.02943.x [DOI] [PubMed] [Google Scholar]
- 3. Savarino E, Anastasiou F, Labenz J, Hungin APS, Mendive J. Holistic management of symptomatic reflux: rising to the challenge of proton pump inhibitor overuse. BJGP. 2022;72(724):541-544. doi:10.3399%2Fbjgp22X721157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ruckert-Eheberg IM, Nolde M, Ahn N, et al. Who gets prescriptions for proton pump inhibitors and why? A drug-utilization study with claims data in Bavaria, Germany, 2010–2018. Eur J Clin Pharmacol. 2022;78(4):657-667. doi: 10.1007/s00228-021-03257-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Farrell B, Lass E, Moayyedi P, Ward D, Thompson W. Reduce unnecessary use of proton pump inhibitors. BMJ. 2022;379:e069211. doi: 10.1136/bmj-2021-069211 [DOI] [PubMed] [Google Scholar]
- 6. Antoniou T, Macdonald EM, Hollands S, et al. Proton pump inhibitors and the risk of acute kidney injury in older patients. CMAJ Open. 2015;3:166-171. doi: 10.9778/cmajo.20140074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Castellana C, Pecere S, Furnari M, et al. Side effects of long-term use of proton pump inhibitors: practical considerations. Pol Arch Intern Med. 2021;131(6):541-549. doi: 10.20452/pamw.15997 [DOI] [PubMed] [Google Scholar]
- 8. Charlot M, Grove EL, Hansen PR, et al. Proton pump inhibitor use and risk of adverse cardiovascular events in aspirin treated patients with first time myocardial infarction. BMJ. 2011;342:d2690. doi: 10.1136/bmj.d2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Giuliano C, Wilhelm SM, Kale-Pradhan PB. Are proton pump inhibitors associated with the development of community-acquired pneumonia? A meta-analysis. Expert Rev Clin Pharmacol. 2012;5:337-344. doi: 10.1586/ecp.12.20 [DOI] [PubMed] [Google Scholar]
- 10. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265:419-428. doi: 10.1007/s00406-014-0554-0 [DOI] [PubMed] [Google Scholar]
- 11. Hart E, Dunn TE, Feuerstein S, Jacobs DM. Proton pump inhibitors and risk of acute and chronic kidney disease: a retrospective cohort study. Pharmacotherapy. 2019;39(4): 443-453. doi: 10.1002/phar.2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics. Am J Gastroenterol. 2012;107:1011-1019. doi: 10.1038/ajg.2012.108 [DOI] [PubMed] [Google Scholar]
- 13. Kwok CS, Yeong JK-Y, Loke YK. Risk of fractures with acid-suppressing medication. Bone. 2011;48:768-776. doi: 10.1016/j.bone.2010.12.015 [DOI] [PubMed] [Google Scholar]
- 14. Loosen SH, Kostev K, Luedde M, et al. Long-term use of proton pump inhibitors (PPIs) is associated with an increased risk of type 2 diabetes. Gut. 2022;71:1687-1688. doi: 10.1136/gutjnl-2021-326297 [DOI] [PubMed] [Google Scholar]
- 15. Muheim L, Signorell A, Markun S, et al. Potentially inappropriate proton-pump inhibitor prescription in the general population: a claims-based retrospective time trend analysis. Therap Adv Gastroenterol. 2021;14:1756284821998928. doi: 10.1177/1756284821998928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rababa M, Rababa’h A. The inappropriate use of proton pump inhibitors and its associated factors among community-dwelling older adults. Heliyon. 2021;7(7):e07595. doi: 10.1016/j.heliyon.2021.e07595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Savarino V, Marabotto E, Zentilin P, et al. The appropriate use of proton-pump inhibitors. Minerva Med. 2018;109(5): 386-399. doi: 10.23736/s0026-4806.18.05705-1 [DOI] [PubMed] [Google Scholar]
- 18. Haastrup P, Paulsen MS, Begtrup LM, Hansen JM, Jarbøl DE. Strategies for discontinuation of proton pump inhibitors. Fam Pract. 2014;31:625-630. doi: 10.1093/fampra/cmu050 [DOI] [PubMed] [Google Scholar]
- 19. Koop H. Verordnungspraxis und Risiken von Protonenpumpenblockern – Fiktion und Fakten [Prescription and risks of proton pump inhibitor: fiction and facts]. Z Gastroenterol. 2018;56(3):264-274. doi: 10.1055/s-0043-125340 [DOI] [PubMed] [Google Scholar]
- 20. Freedberg DE, Kim LS, Yang Y-X. The Risks benefits of long-term use of proton pump inhibitors. Gastroenterology. 2017;152:706-715. doi: 10.1053/j.gastro.2017.01.031 [DOI] [PubMed] [Google Scholar]
- 21. Heidelbaugh JJ, Goldberg KL, Inadomi JM. Overutilization of proton pump inhibitors. Am J Gastroenterol. 2009;104: 27-32. doi: 10.1038/ajg.2009.49 [DOI] [PubMed] [Google Scholar]
- 22. Barberio B, Visaggi P, Savarino E, Bortoli N, Black CJ, Ford A. Comparison of acid-lowering drugs for endoscopy negative reflux disease: systematic review and network meta-analysis. Neurogastroenterol. Motil. 2022;35:e14469. doi: 10.1111/nmo.14469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coyle C, Symonds R, Allan J, et al. Sustained proton pump inhibitor deprescribing among dyspeptic patients in general practice: a return to self-management through a programme of education and alginate rescue therapy. A prospective interventional study. BJGP Open. 2019;3(3):bjgpopen19X101651. doi: 10.3399/bjgpopen19x101651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee TJ, Fennerty MB, Howden CW. Systematic review. Aliment Pharmacol Ther. 2004;20:1241-1251. doi: 10.1111/j.1365-2036.2004.02289.x [DOI] [PubMed] [Google Scholar]
- 25. Vales A, Coyle C, Plehhova K, Hobson A, Woodland P. Randomised clinical trial: the use of alginates during pre investigation proton pump inhibitor wash-out and their impact on compliance and symptom burden. BMJ Open Gastroenterol. 2023;10(1):e001026. doi: 10.1136/bmjgast-2022-001026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rathmann W, Bongaerts B, Carius HJ, Kruppert S, Kostev K. Basic characteristics and representativeness of the German Disease Analyzer database. Int J Clin Pharmacol Ther. 2018;56(10):459-466. doi: [DOI] [PubMed] [Google Scholar]
- 27. Lanas A. We are using too many PPIs, and we need to stop: a European perspective. Am J Gastroenterol. 2016;111(8): 1085-1086. doi: 10.1038/ajg.2016.166 [DOI] [PubMed] [Google Scholar]
- 28. Mössner J. Magen-Darm-Mittel und Lebertherapeutika. In: Schwabe U, Paffrath D. (eds) Arzneiverordnungs-Report. Springer, 2015:757-788. [Google Scholar]
- 29. Fischbach W, Malfertheiner P, Lynen Jansen P, et al. S2k-Guideline Helicobacter pylori and gastroduodenal ulcer disease. Z Gastroenterol. 2017;55(2):167-206. English. doi: 10.1055/s-0042-102967 [DOI] [PubMed] [Google Scholar]
- 30. Koop H, Fuchs KH, Labenz J, et al. Guideline: gastroesophageal reflux disease guided by the German Society of Gastroenterology. Z Gastroenterol. 2014;52(11):1299-1346. doi: 10.1055/s-0034-1385202 [DOI] [PubMed] [Google Scholar]
- 31. Madisch A, Andresen V, Enck P, Labenz J, Frieling T, Schemann M. The diagnosis and treatment of functional dyspepsia. Dtsch Arztebl Int. 2018;115(13):222-232. doi:10.3238%2Farztebl.2018.0222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors: evidence-based clinical practice guideline. Can Fam Physician. 2017;63(5):354-364. [PMC free article] [PubMed] [Google Scholar]
- 33. Targownik LE, Fisher DA, Saini SD. AGA clinical practice update on de-prescribing of proton pump inhibitors: expert review. Gastroenterology. 2022;162(4):1334-1342. doi: 10.1053/j.gastro.2021.12.247 [DOI] [PubMed] [Google Scholar]
- 34. Coffey CP, Barnette DJ, Wenzke JT, Lawrence J. Implementing a systematic approach to deprescribing proton pump inhibitor therapy in older adults. Sr Care Pharm. 2019;34(1):47-55. doi: 10.4140/tcp.n.2019.47 [DOI] [PubMed] [Google Scholar]
- 35. Ikeji C, Williams A, Hennawi G, Brandt NJ. Patient and provider perspectives on deprescribing proton pump inhibitors. J Gerontol Nurs. 2019;45(10):9-17. doi: 10.3928/00989134-20190912-03 [DOI] [PubMed] [Google Scholar]
- 36. Odenthal DR, Philbrick AM, Harris IM. Successful deprescribing of unnecessary proton pump inhibitors in a primary care clinic. J Am Pharm Assoc. 2020;60(1):100-104. doi: 10.1016/j.japh.2019.08.012 [DOI] [PubMed] [Google Scholar]
- 37. Rieckert A, Becker A, Donner-Banzhof N, et al. Reduction of the long-term use of proton pump inhibitors by a patient-oriented electronic decision support tool (arriba-PPI): study protocol for a randomized controlled trial. Trials. 2019;20(1):636. doi:10.1186%2Fs13063-019-3728-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thompson W, Farrell B, Welch V, et al. Continuation or deprescribing of proton pump inhibitors: a consult patient decision aid. Can Pharm J. 2018;152(1):18-22. doi:10.1177%2F1715163518816719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fuentes-Valenzuela E, Díez Redondo P, Tejedor-Tejada J, Nájera-Muñoz R, Sánchez-Delgado L, Maroto-Martín C. Tratamiento con inhibidores de la bomba de protones. ¿Realmente lo necesita el paciente? [Proton-pump inhibitors treatment. Does your patient really need it?]. Semergen. 2022; 48(2):82-87. doi: 10.1016/j.semerg.2021.08.002 [DOI] [PubMed] [Google Scholar]
- 40. Rudelle K, Laroche ML. Connaissances et attitudes des médecins généralistes à l’égard des effets indésirables des inhibiteurs de la pompe à protons [The general practitioner’s knowledge and attitude towards proton pump inhibitors adverse effects]. Therapie. 2020;75(3):253-260. doi: 10.1016/j.therap.2019.07.008 [DOI] [PubMed] [Google Scholar]
- 41. GmbH, DAP Networks. OTC-Satzungsleistungen Der Krankenkassen. DeutschesApothekenPortal, July 2023. https://www.deutschesapothekenportal.de/rezept-retax/retax-arbeitshilfen/otc-arzneimittel/otc-satzungsleistungen-der-krankenkassen/ (accessed 15 November 2023). [Google Scholar]
- 42. Madisch A, Koop H, Miehlke S, et al. S2k-Leitlinie Gastroösophageale Refluxkrankheit und eosinophile Ösophagitis der Deutschen Gesellschaft für Gastroenterologie, Verdauungs-und Stoffwechselkrankheiten (DGVS). DGVS. 2023. AWMF-Regsiternummer: 021-013. 021-013l_S2k_Gastrooesophageale-Refluxkrankheit-eosinophile_Oesophagitis_2023-03.pdf (awmf.org) [Google Scholar]
- 43. Pouchain D, Bigard M-A, Liard F, et al. Gaviscon® vs. omeprazole in symptomatic treatment of moderate gastroesophageal reflux. a direct comparative randomised trial. BMC Gastroenterol. 2012;12:18. doi: 10.1186/1471-230x-12-18 [DOI] [PMC free article] [PubMed] [Google Scholar]