Abstract
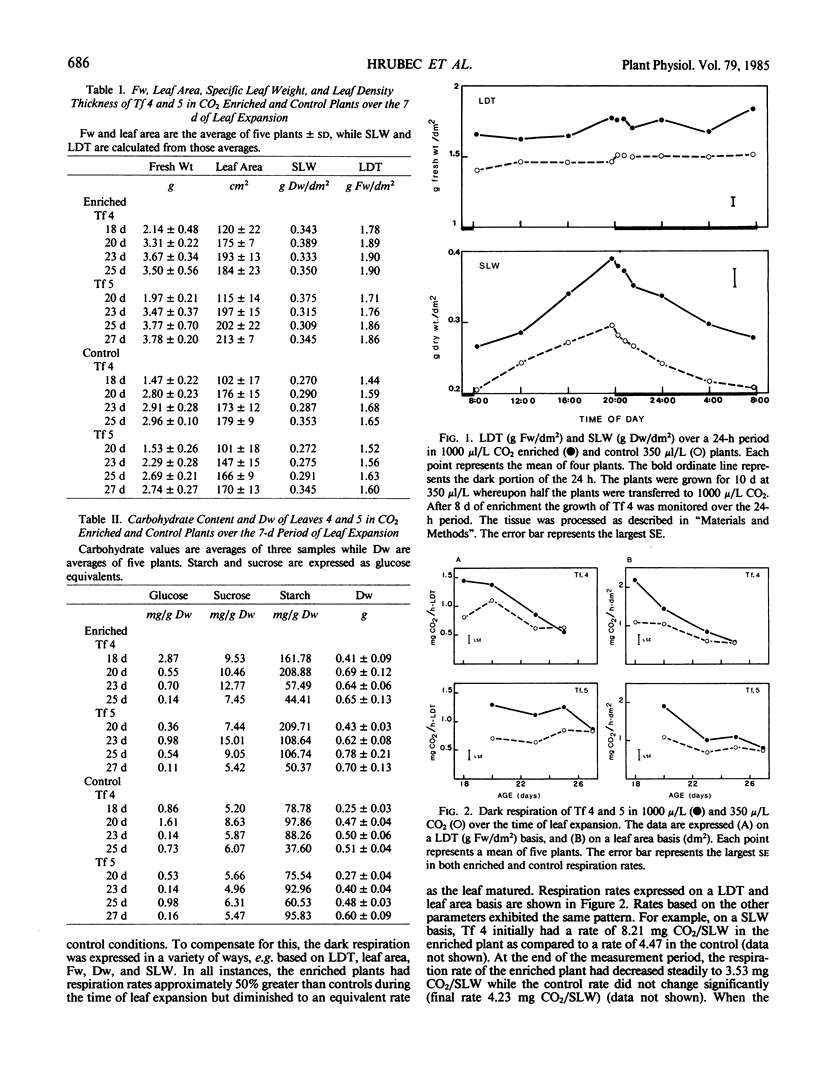
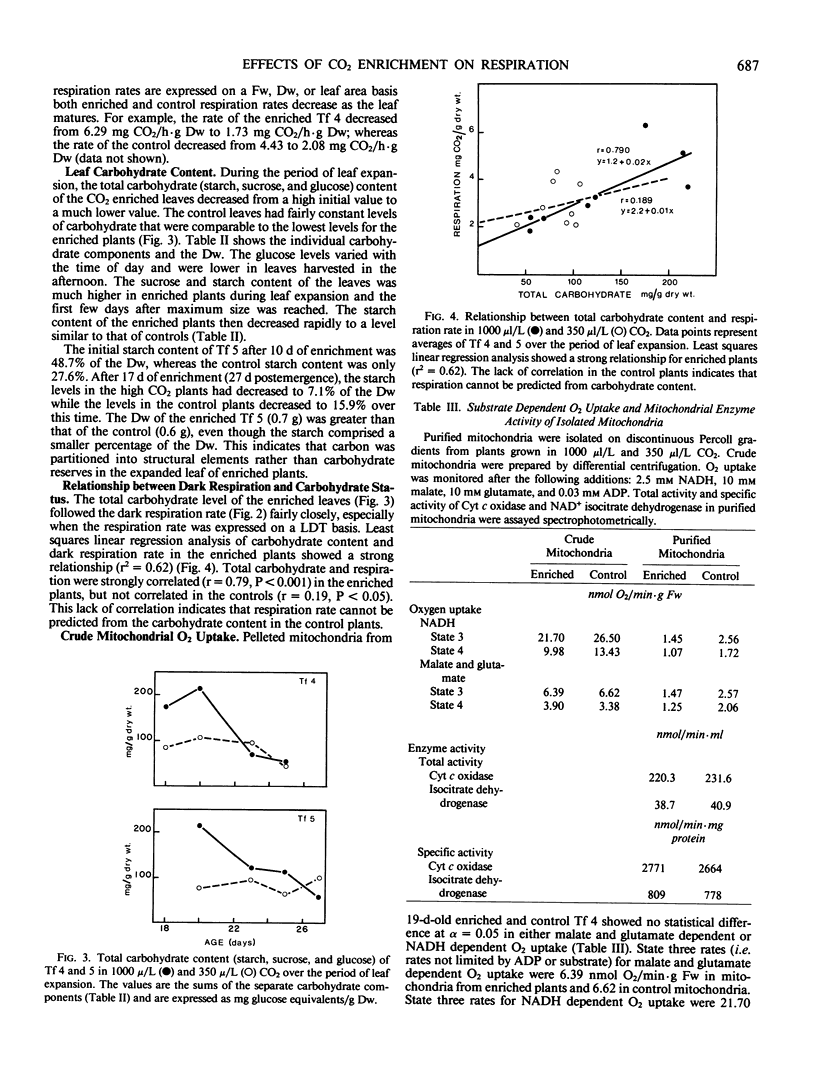
During the period of most active leaf expansion, the foliar dark respiration rate of soybeans (Glycine max cv Williams), grown for 2 weeks in 1000 microliters CO2 per liter air, was 1.45 milligrams CO2 evolved per hour leaf density thickness, and this was twice the rate displayed by leaves of control plants (350 microliters CO2 per liter air). There was a higher foliar nonstructural carbohydrate level (e.g. sucrose and starch) in the CO2 enriched compared with CO2 normal plants. For example, leaves of enriched plants displayed levels of nonstructural carbohydrate equivalent to 174 milligrams glucose per gram dry weight compared to the 84 milligrams glucose per gram dry weight found in control plant leaves. As the leaves of CO2 enriched plants approached full expansion, both the foliar respiration rate and carbohydrate content of the CO2 enriched leaves decreased until they were equivalent with those same parameters in the leaves of control plants. A strong positive correlation between respiration rate and carbohydrate content was seen in high CO2 adapted plants, but not in the control plants.
Mitochondria, isolated simultaneously from the leaves of CO2 enriched and control plants, showed no difference in NADH or malate-glutamate dependent O2 uptake, and there were no observed differences in the specific activities of NAD+ linked isocitrate dehydrogenase and cytochrome c oxidase. Since the mitochondrial O2 uptake and total enzyme activities were not greater in young enriched leaves, the increase in leaf respiration rate was not caused by metabolic adaptations in the leaf mitochondria as a response to long term CO2 enrichment. It was concluded, that the higher respiration rate in the enriched plant's foliage was attributable, in part, to a higher carbohydrate status.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azcón-Bieto J. Inhibition of photosynthesis by carbohydrates in wheat leaves. Plant Physiol. 1983 Nov;73(3):681–686. doi: 10.1104/pp.73.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcón-Bieto J., Lambers H., Day D. A. Effect of photosynthesis and carbohydrate status on respiratory rates and the involvement of the alternative pathway in leaf respiration. Plant Physiol. 1983 Jul;72(3):598–603. doi: 10.1104/pp.72.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcón-Bieto J., Osmond C. B. Relationship between Photosynthesis and Respiration: The Effect of Carbohydrate Status on the Rate of CO(2) Production by Respiration in Darkened and Illuminated Wheat Leaves. Plant Physiol. 1983 Mar;71(3):574–581. doi: 10.1104/pp.71.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Hrubec T. C., Robinson J. M., Donaldson R. P. Isolation of mitochondria from soybean leaves on discontinuous percoll gradients. Plant Physiol. 1985 Apr;77(4):1010–1012. doi: 10.1104/pp.77.4.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latzko E., Gibbs M. Measurement of the intermediates of the photosynthetic carbon reduction cycle, using enzymatic methods. Methods Enzymol. 1972;24:261–268. doi: 10.1016/0076-6879(72)24073-3. [DOI] [PubMed] [Google Scholar]
- Liang Z., Yu C., Huang A. H. Isolation of spinach leaf peroxisomes in 0.25 molar sucrose solution by percoll density gradient centrifugation. Plant Physiol. 1982 Oct;70(4):1210–1212. doi: 10.1104/pp.70.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen G. G., McClendon J. H. Dependence of photosynthetic rates on leaf density thickness in deciduous woody plants grown in sun and shade. Plant Physiol. 1983 Jul;72(3):674–678. doi: 10.1104/pp.72.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M. Photosynthetic Carbon Metabolism in Leaves and Isolated Chloroplasts from Spinach Plants Grown under Short and Intermediate Photosynthetic Periods. Plant Physiol. 1984 Jun;75(2):397–409. doi: 10.1104/pp.75.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMON E. W. The effect of digitonin on the cytochrome c oxidase activity of plant mitochondria. Biochem J. 1958 May;69(1):67–74. doi: 10.1042/bj0690067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvius J. E., Kremer D. F., Lee D. R. Carbon assimilation and translocation in soybean leaves at different stages of development. Plant Physiol. 1978 Jul;62(1):54–58. doi: 10.1104/pp.62.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne J. H., Koller H. R. Influence of assimilate demand on photosynthesis, diffusive resistances, translocation, and carbohydrate levels of soybean leaves. Plant Physiol. 1974 Aug;54(2):201–207. doi: 10.1104/pp.54.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volenec J. J., Nelson C. J. Carbohydrate metabolism in leaf meristems of tall fescue : I. Relationship to genetically altered leaf elongation rates. Plant Physiol. 1984 Mar;74(3):590–594. doi: 10.1104/pp.74.3.590. [DOI] [PMC free article] [PubMed] [Google Scholar]


