Abstract
Background
Neutrophil percentage-to-albumin ratio (NPAR), a new inflammatory marker, has been shown to be associated with poor prognosis in patients with cardiovascular disease. However, limited evidence is available for its role in peritoneal dialysis (PD) patients. Our study aimed at investigating the prognostic value of NPAR for mortality in PD patients.
Patients and Methods
This was a single center retrospective cohort study. A total of 1966 PD patients were enrolled in our study from January 2006 to December 2016 and were followed up until December 2021. Patients were stratified into tertiles according to baseline NPAR levels. The associations between NPAR levels with all-cause and cardiovascular mortality were estimated using Cox proportional hazards models. Receiver operating characteristic (ROC) analysis was performed to compare the mortality predictive values of NPAR and other known biomarkers, such as NLR (neutrophil-to-lymphocyte ratio), PLR (platelet-to-lymphocyte ratio), LHR (low-density lipoprotein cholesterol-to-high-density lipoprotein cholesterol ratio) and MLR (monocyte-to-lymphocyte ratio).
Results
During a median follow-up of 48.1 months, 503 (25.6%) patients died, in which cardiovascular disease (CVD) death dominated 50.3%. Multivariate Cox regression analysis revealed that the highest NPAR tertile was significantly associated with a higher risk of all-cause and cardiovascular mortality (HR 1.51, 95% CI 1.14–1.98; HR 1.57, 95% CI 1.07–2.31; respectively) compared with tertile 1. The AUC values of NPAR were 0.62 (95% CI 0.60–0.65, P < 0.001) for all-cause mortality and 0.61 (95% CI 0.57–0.65, P < 0.001) for cardiovascular mortality.
Conclusion
Our study showed that higher NPAR levels were independently associated with increased risk of all-cause and cardiovascular mortality in PD patients. Notably, our results demonstrated that NPAR exhibited superior predictive value for mortality compared to NLR, PLR, MLR, and LHR.
Keywords: peritoneal dialysis, neutrophil percentage-to-albumin ratio, mortality, inflammation
Introduction
Peritoneal dialysis (PD), as a high-quality and cost-effective therapy, is one of the primary renal replacement therapy (RRT) modalities offered to patients with end stage renal disease (ESRD).1 It is estimated that more than 272,000 patients with ESRD received PD treatment worldwide in 2017, representing approximately 11% of the global dialysis population.1,2 However, PD patients are prone to a condition known as malnutrition-inflammation-atherosclerosis (MIA) syndrome, in which malnutrition, pro-inflammatory cytokines, and atherosclerosis coexist and interact with each other.3 Factors related to dialysis such as PD catheter implantation, bioincompatible PD solution, peritonitis, as well as factors unrelated to dialysis, such as uremic toxins, oxidative stress, volume overload, and endothelial dysfunction, are prevalent in patients with PD. These factors collectively contribute to persistent systemic inflammation and malnutrition in PD patients and ultimately leading to a poor prognosis.4,5 Chronic inflammation has been considered vital for the pathogenesis of MIA syndrome, contributing to the progression of atherosclerosis and increased the risk of cardiovascular disease.6 Previous studies have reported that MIA syndrome is associated with higher all-cause and cardiovascular mortality in PD patients.3,7 Early assessment of the inflammatory state in PD patients is crucial for identifying individuals at a high risk of mortality.
Neutrophil is one of the prototypical markers of inflammation. Over the past decade, it has been recognized to play a crucial role not only as a key mediator of acute inflammation, but also in chronic inflammation, such as atherosclerosis.8 Elevated neutrophil counts have been found to be independently associated with an increased risk of death in both hemodialysis (HD) and PD patients.0.9,10 Albumin has numerous functions, such as maintaining plasma osmotic pressure, transporting substances, and acting as antioxidants. PD patients often experience hypoalbuminemia due to dialysis exchange, chronic inflammation, infection, and other related factors.11,12 It has been suggested that serum albumin can evaluate nutritional and inflammatory status.3 Hypoalbuminemia has been identified as a substantial risk factor for the decline of residual renal function (RRF) and all-cause mortality in PD patients.11,13
It is possible that neutrophil percentage-to-albumin ratio (NPAR), comprising of neutrophil percentage and serum albumin, magnifies changes in both of these parameters and has a stronger association with mortality in PD patients, resulting in an enhanced prediction of adverse outcomes for PD patients. At present, there is limited information available on the prognostic value of NPAR in PD patients. This cohort study was conducted to investigate the association of NPAR with mortality in PD patients, as well as to compare the prognostic value of NPAR with several other known inflammatory biomarkers, including neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR) and LDL-C-to-HDL-C ratio (LHR).
Materials and Methods
Study Participants
This was a single-center retrospective observational cohort study. Patients who underwent catheter insertion and peritoneal dialysis at the PD center of The First Affiliated Hospital, Sun Yat-sen University from January 1, 2006 to December 31, 2016 were enrolled in the study. Exclusion criteria were as follows: (1) patients were < 18 years old at the start of PD; (2) treated with PD therapy for less than 3 months; (3) transferred from HD; (4) transferred from failed renal transplantation; (5) patients with a history of malignant tumors; (6) patients suffered from acute infections during the baseline period; (7) patients had hematological diseases, hepatic disease or acquired immune deficiency syndrome; (8) missing baseline neutrophil percentage and/or albumin data. This study was conducted in accordance with the ethical principles of the Helsinki Declaration and was approved by the Research Ethics Committee of The First Affiliated Hospital, Sun Yat-sen University. Written informed consent was provided by all participants.
Data Collection
All baseline demographic, clinical and laboratory data for this study were collected within the first 1–3 months following the PD initiation. Demographic data included age, gender and body mass index (BMI). Clinical data included medical history (cardiovascular disease and diabetes) and comorbidity score. Laboratory data included white blood cell (WBC), neutrophil, lymphocyte, monocyte, hemoglobin, serum albumin, serum creatinine, uric acid, serum phosphorus, albumin-corrected calcium, total cholesterol, triglyceride, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), high-sensitivity C-reactive protein (hs-CRP), residual renal function (RRF) and total urea clearance index (Kt/V), all of which were measured in the biochemical laboratory of The First Affiliated Hospital of Sun Yat-sen University. The comorbidity score was assessed by the Charlson comorbidity index (CCI).14 RRF and total Kt/V were calculated using the PD Adequest software (Baxter Healthcare Corporation, Chicago, IL, USA).
Outcomes
The primary outcome of our study was all-cause mortality, and the secondary outcome was cardiovascular mortality, which was defined as death due to myocardial infarction, cardiac arrhythmia, congestive heart failure, cardiac arrest, cerebrovascular disease, and peripheral vascular disease.15 To determine the cause of death, three nephrologists at our PD center thoroughly reviewed medical records and/or directly communicated with the referring physician. All participants were followed up until death, renal transplantation, transferring to HD or other PD centers, loss of follow-up, or the end of the study (December 31, 2021).
Statistical Analyses
NPAR was calculated as neutrophil percentage divided by serum albumin (expressed in g/L). Participants were categorized into three groups according to their baseline NPAR levels: tertile 1 (NPAR < 1.64), tertile 2 (1.64 ≤ NPAR < 1.93), and tertile 3 (NPAR ≥ 1.93). Continuous variables were characterized as mean ± standard deviation for normally distributed data or median (interquartile ranges) for non-normal distribution. Categorical variables were presented as frequencies and percentages. Differences in continuous or categorical variables among groups were compared by chi-squared tests, One-way ANOVA, or Kruskal–Wallis tests, as appropriate. Spearman rank correlation analysis and Pearson correlation analysis were applied to examine the correlations between NPAR and other clinical parameters. Kaplan-Meier curves were performed to estimate the cumulative survival rate, and the log rank test was used to compare the differences among the three groups. The associations between NPAR levels and all-cause and cardiovascular mortality were investigated using multivariate Cox proportional hazards models. To assess the prognostic values of NPAR for mortality, we conducted the receiver operating characteristic (ROC) curve analysis and calculated the area under the curve (AUC). A two-tailed P value of < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software, version 25.0 (IBM SPSS, Chicago, IL, USA) and MedCalc software, version 20.218 (Broekstraat, Mariakerke, Belgium).
Results
Baseline Demographic and Clinical Characteristics
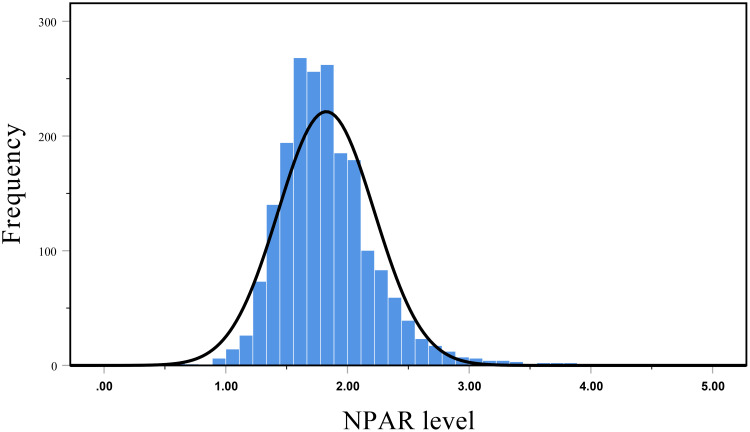
The flow chart illustrating the inclusion of participants was shown in Figure 1. A total of 1966 participants were enrolled in the cohort study. The mean age was 46.8 ± 15.1 years, 60.3% of patients were male, 25.0% were diabetes, and 33.7% had a history of CVD. Figure 2 revealed an approximately normal distribution of baseline NPAR levels in the overall study population. The average value of NPAR was 1.82 ± 0.40. The baseline demographic and clinical characteristics of the patients stratified by NPAR tertiles are shown in Table 1. Patients with higher NPAR were older, higher proportion of female, more likely to have a history of diabetes and CVD. In addition, patients in tertile 3 had higher CCI, corrected-calcium, neutrophil count, monocyte count, and hs-CRP levels, but lower hemoglobin, albumin, uric acid, lymphocyte count, RRF and serum creatinine levels (P < 0.05). Nevertheless, there were no significant differences in serum phosphorus, triglyceride, total cholesterol, HDL-C, LDL-C and total Kt/V among the three groups (P > 0.05).
Figure 1.
Flow chart of the participants in the study cohort.
Abbreviations: PD, peritoneal dialysis; HD, hemodialysis; NPAR, neutrophil percentage-to-albumin ratio; CVD, cardiovascular disease.
Figure 2.
Distribution of baseline NPAR levels in the cohort.
Table 1.
Baseline Characteristics of Individuals Stratified by Tertiles of Baseline NPAR Levels
| Variables | Total | NPAR | P-value | ||
|---|---|---|---|---|---|
| (n = 1966) | < 1.64 (n = 651) | 1.64–1.93 (n = 655) | ≥ 1.93 (n = 660) | ||
| Age (years) | 46.83 ± 15.12 | 43.05 ± 14.15 | 45.88 ± 14.61 | 51.51 ± 15.35 | < 0.001 |
| Men (n, %) | 1186 (60.3%) | 431 (66.2%) | 397 (60.6%) | 358 (54.2%) | < 0.001 |
| Body mass index (kg/m2) | 21.64 ± 3.13 | 21.36 ± 3.06 | 21.81 ± 3.13 | 21.76 ± 3.18 | 0.019 |
| History of diabetes (n, %) | 491 (25.0%) | 84 (12.9%) | 160 (24.4%) | 247 (37.4%) | < 0.001 |
| History of CVD (n, %) | 662 (33.7%) | 193 (29.6%) | 205 (31.3%) | 264 (40.0%) | < 0.001 |
| Charlson comorbidity index | 3.46 ± 1.79 | 3.03 ± 1.56 | 3.32 ± 1.73 | 4.04 ± 1.93 | < 0.001 |
| Hemoglobin (g/L) | 100.65 ± 21.89 | 107.11 ± 20.96 | 101.47 ± 20.97 | 93.43 ± 21.56 | < 0.001 |
| Serum albumin (g/L) | 36.77 ± 5.06 | 40.15 ± 3.90 | 37.56 ± 3.57 | 32.65 ± 4.44 | < 0.001 |
| Uric acid (µmol/L) | 428.67 ± 95.00 | 439.01 ± 94.94 | 429.51 ± 89.70 | 417.61 ± 99.04 | < 0.001 |
| Phosphorus (mmol/L) | 1.41 ± 0.41 | 1.44 ± 0.42 | 1.40 ± 0.38 | 1.39 ± 0.42 | 0.106 |
| Corrected-calcium (mmol/L) | 2.67 ± 1.55 | 2.52 ± 1.27 | 2.65 ± 1.54 | 2.82 ± 1.79 | 0.002 |
| Total cholesterol (mmol/L) | 5.01 ± 1.33 | 5.00 ± 1.26 | 4.97 ± 1.28 | 5.06 ± 1.44 | 0.415 |
| Triglyceride (mmol/L) | 1.40 (1.00–2.01) | 1.42 (1.02–2.04) | 1.36 (1.00–1.90) | 1.42 (0.97–2.07) | 0.319 |
| HDL-C (mmol/L) | 1.23 ± 0.43 | 1.26 ± 0.44 | 1.21 ± 0.38 | 1.22 ± 0.48 | 0.085 |
| LDL-C (mmol/L) | 2.96 ± 1.00 | 2.92 ± 0.96 | 2.94 ± 0.93 | 3.00 ± 1.10 | 0.286 |
| hs-CRP (mg/L) | 1.66 (0.58–5.21) | 1.16 (0.49–3.21) | 1.67 (0.63–5.26) | 2.51 (0.80–8.39) | < 0.001 |
| Neutrophil (109/L) | 4.56 ± 1.72 | 3.70± 1.21 | 4.52 ± 1.38 | 5.43 ± 2.00 | < 0.001 |
| Lymphocyte (109/L) | 1.53 ± 0.58 | 1.75 ± 0.62 | 1.50 ± 0.52 | 1.34 ± 0.52 | < 0.001 |
| Monocyte (109/L) | 0.45 ± 0.20 | 0.44 ± 0.19 | 0.43 ± 0.19 | 0.47 ± 0.23 | 0.012 |
| Total Kt/V | 2.48 ± 0.80 | 2.48 ± 0.76 | 2.52 ± 0.92 | 2.45 ± 0.70 | 0.357 |
| RRF (mL/min/1.73 m2) | 3.31 (1.95–5.25) | 3.48 (2.03–5.50) | 3.47 (2.15–5.28) | 2.91 (1.55–4.83) | < 0.001 |
| Serum creatinine (µmol/L) | 716 (581–920) | 747 (604–948) | 729 (594–938) | 681 (547–858) | < 0.001 |
Note: P<0.05 is considered statistically significant.
Abbreviations: NPAR, neutrophil percentage-to-albumin ratio; CVD, cardiovascular disease; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; hs-CRP, high sensitive C-reactive protein; RRF, residual renal function; Kt/V, urea clearance index.
Clinical Characteristics Associated with NPAR Levels
The results of association analysis between NPAR and the other clinical variables (Table 2) showed that NPAR levels were positively correlated with age, gender, BMI, CCI, corrected-calcium, total cholesterol, LDL-C, hs-CRP, monocyte counts and negatively correlated with hemoglobin, serum phosphorus, HDL-C, serum creatinine, lymphocyte counts and RRF (P < 0.05). However, there was no correlation between NPAR levels and triglyceride, and total Kt/V.
Table 2.
Correlation of NPAR with Clinical Parameters
| Variable | Correlation Coefficient | p-value |
|---|---|---|
| Age (years) | 0.227 | <0.001 |
| Gender (male vs female) | 0.089 | <0.001 |
| Body mass index (kg/m2) | 0.046 | 0.041 |
| Charlson comorbidity index | 0.223 | <0.001 |
| Hemoglobin (g/L) | −0.247 | <0.001 |
| Corrected-calcium (mmol/L) | 0.087 | <0.001 |
| Phosphorus (mmol/L) | −0.051 | 0.029 |
| Triglyceride (mmol/L) | −0.008 | 0.729 |
| Total cholesterol (mmol/L) | 0.047 | 0.037 |
| HDL-C (mmol/L) | −0.076 | 0.001 |
| LDL-C (mmol/L) | 0.083 | <0.001 |
| hs-CRP (mg/L) | 0.193 | <0.001 |
| Lymphocyte (109/L) | −0.292 | <0.001 |
| Monocyte (109/L) | 0.061 | 0.008 |
| Total Kt/V | −0.002 | 0.938 |
| RRF (mL/min/1.73 m2) | −0.083 | 0.001 |
Note: P<0.05 is considered statistically significant.
Abbreviations: LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; hs-CRP, high sensitive C-reactive protein; RRF, residual renal function; Kt/V, urea clearance index.
Association Between NPAR Levels and Mortality
During a median follow-up of 48.1 months (IQR: 21.7–77.2 months), 503 (25.6%) patients died, 460 (23.4%) underwent renal transplantation, 309 (15.7%) were transferred to HD, 75 (3.8%) went to other PD centers, and 71 (3.6%) patients were lost to follow-up. CVD death dominated 50.3% among the causes of death, followed by infection (19.3%).
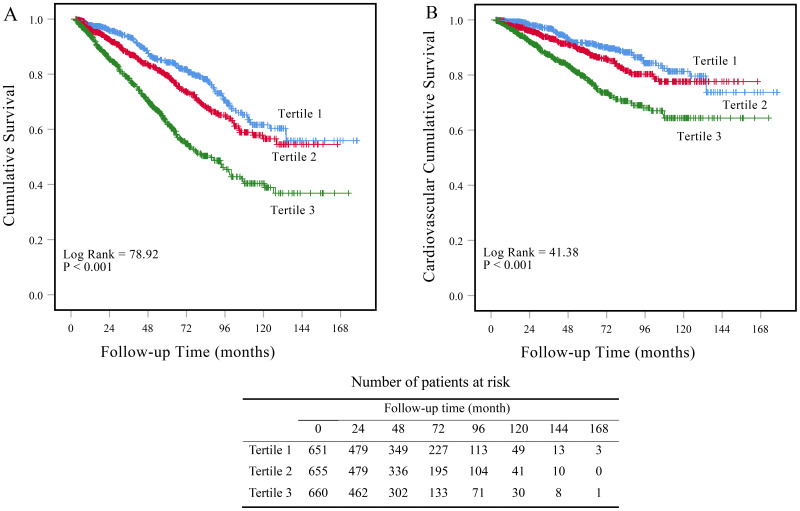
Kaplan-Meier survival curves showed the cumulative rates of overall and cardiovascular survival (Figure 3). At the end of 1, 3, 5 and 10 years, the cumulative survival rates were 97.7%, 93.4%, 84.2% and 60.5% in tertile 1; 96.3%, 87.4%, 79.5% and 56.6% in tertile 2; 93.7%, 78.5%, 62.0% and 39.0% in tertile 3. Cardiovascular survival rates at the end of 1, 3, 5, and 10 years were 99.3%, 96.8%, 91.4% and 80.0% in tertile 1; 97.9%, 93.3%, 88.3% and 77.6% in tertile 2; 96.8%, 87.3%, 78.1% and 64.4% in tertile 3. Patients with higher NPAR levels had higher all-cause (Log Rank = 80.34, P < 0.001) and cardiovascular survival rate (Log Rank = 41.28, P < 0.001) among the three groups.
Figure 3.
Kaplan-Meier survival curves for patients with different levels of NPAR. Cumulative mortality curves for (A) all-cause mortality, and (B) cardiovascular mortality according to the tertiles of baseline NPAR levels.
We conducted Cox proportional hazards models to assess the association between NPAR levels with all-cause and cardiovascular mortality (Table 3). After adjustment for age, gender, BMI, CCI, hemoglobin, corrected calcium, phosphorus, total cholesterol, triglyceride, hs-CRP, total Kt/V and RRF, the tertile 3 of NPAR was significantly associated with a higher risk of all-cause and cardiovascular mortality (HR 1.51, 95% CI 1.14–1.98, P = 0.004; HR 1.57, 95% CI 1.07–2.31, P = 0.022; respectively) compared with tertile 1. Similar results could be found in the correlation between NPAR levels and all-cause and cardiovascular mortality when NPAR was considered as a continuous variable. For each 1-unit increase NPAR levels, the risk of all-cause and cardiovascular mortality increased by 56% (HR 1.56, 95% CI 1.18–2.05, P = 0.002) and 57% (HR 1.57, 95% CI 1.07–2.28, P = 0.020), respectively.
Table 3.
The Association of NPAR with All-Cause and Cardiovascular Mortality
| Model 1a | Model 2b | Model 3c | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| All-cause mortality | ||||||
| Continuous NPARd | 2.54 (2.09–3.07) | <0.001 | 1.74 (1.41–2.13) | <0.001 | 1.56 (1.18–2.05) | 0.002 |
| Tertile 1 | 1.0 | 1.0 | 1.0 | |||
| Tertile 2 | 1.35 (1.06–1.73) | 0.015 | 1.21 (0.94–1.54) | 0.136 | 1.14 (0.85–1.53) | 0.369 |
| Tertile 3 | 2.51 (2.01–3.14) | <0.001 | 1.71 (1.36–2.15) | <0.001 | 1.51 (1.14–1.98) | 0.004 |
| P for trend | <0.001 | <0.001 | 0.002 | |||
| Cardiovascular mortality | ||||||
| Continuous NPARd | 2.54 (1.94–3.32) | <0.001 | 1.70 (1.27–2.27) | <0.001 | 1.57 (1.07–2.28) | 0.020 |
| Tertile 1 | 1.0 | 1.0 | 1.0 | |||
| Tertile 2 | 1.37 (0.97–1.94) | 0.073 | 1.21 (0.85–1.71) | 0.294 | 1.17 (0.77–1.78) | 0.452 |
| Tertile 3 | 2.55 (1.86–3.50) | <0.001 | 1.74 (1.26–2.39) | 0.001 | 1.57 (1.07–2.31) | 0.022 |
| P for trend | <0.001 | <0.001 | 0.016 | |||
Notes: aUnadjusted model. bAdjusted for age, gender, body mass index. cAdjusted for model 2 covariates and Charlson comorbidity index, hemoglobin, corrected calcium, phosphorus, total cholesterol, triglycerides, hs-CRP, total Kt/V and residual renal function. dPer unit increment in NPAR levels. P<0.05 is considered statistically significant.
Abbreviations: HR, hazard ratio; CI, confidence interval.
Comparison of Prognostic Values of Biological Biomarkers
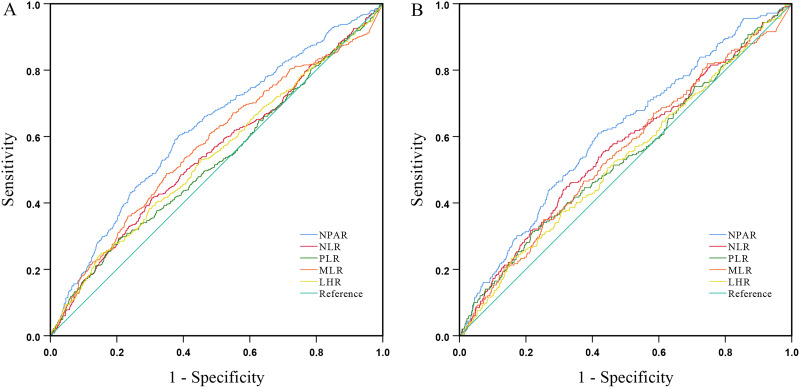
ROC curves and the AUC were shown in Figure 4 and Table 4, respectively. The AUC values of all-cause mortality were 0.62 (95% CI 0.60–0.65, P < 0.001) for NPAR, 0.55 (95% CI 0.52–0.58, P = 0.002) for NLR, 0.53 (95% CI 0.50–0.56, P = 0.088) for PLR, 0.57 (95% CI 0.54–0.60, P < 0.001) for MLR, and 0.54 (95% CI 0.51–0.57, P = 0.006) for LHR. For cardiovascular mortality, AUC were 0.61 (95% CI 0.57–0.65, P < 0.001) for NPAR, 0.56 (95% CI 0.52–0.60, P = 0.002) for NLR, 0.54 (95% CI 0.50–0.58, P = 0.051) for PLR, 0.55 (95% CI 0.51–0.59, P = 0.016) for MLR, and 0.53 (95% CI 0.49–0.57, P = 0.142) for LHR. In the current study, NPAR showed superior predictive value for all-cause and cardiovascular mortality compared to NLR, PLR, LHR and MLR.
Figure 4.
The ROC curves of NPAR, NLR, PLR, LHR, MLR ((A) for all-cause mortality, (B) for cardiovascular mortality) for predicting the all-cause and cardiovascular mortality in PD patients.
Abbreviations: NPAR, neutrophil percentage-to-albumin ratio; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; LHR, LDL-C-to-HDL-C ratio; MLR, monocyte-to-lymphocyte ratio.
Table 4.
Area Under the ROC Curve of Biomarkers for All-Cause and Cardiovascular Mortality
| All-Cause Mortality | Cardiovascular Mortality | |||
|---|---|---|---|---|
| AUC | P-value | AUC | P-value | |
| NPAR | 0.62 | <0.001 | 0.61 | <0.001 |
| NLR | 0.55 | 0.002 | 0.56 | 0.002 |
| PLR | 0.53 | 0.088 | 0.54 | 0.051 |
| LHR | 0.54 | 0.006 | 0.53 | 0.142 |
| MLR | 0.57 | <0.001 | 0.55 | 0.016 |
Note: P<0.05 is considered statistically significant.
Abbreviations: AUC, area under the ROC curve; NPAR, neutrophil percentage-to-albumin ratio; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; LHR, LDL-C –to- HDL-C ratio; MLR, monocyte-to-lymphocyte ratio.
Moreover, given the strong correlation of the NPAR with diabetes and inflammatory markers, we further use the ROC curves and the AUC to evaluate whether this marker is better than simple markers such as neutrophil count, hsCPR, serum albumin, and history of diabetes. As shown in Supplement Figure 1 and Supplement Table 1, the AUC values of all-cause mortality were 0.62 (95% CI 0.60–0.65, P < 0.001) for NPAR, 0.66 (95% CI 0.64–0.70, P < 0.001) for diabetes, 0.64 (95% CI 0.61–0.67, P < 0.001) for hs-CRP, 0.65 (95% CI 0.63–0.68, P < 0.001) for albumin, and 0.59 (95% CI 0.56–0.62, P < 0.001) for neutrophil count. For cardiovascular mortality, AUC were 0.61 (95% CI 0.57–0.65, P < 0.001) for NPAR, 0.65 (95% CI 0.61–0.69, P < 0.001) for diabetes, 0.64 (95% CI 0.60–0.68, P < 0.001) for hs-CRP, 0.63 (95% CI 0.60–0.67, P < 0.001) for albumin, and 0.59 (95% CI 0.55–0.63, P < 0.001) for neutrophil count.
Discussion
As we know, this was the first study to explore the relationship between NPAR and mortality among patients undergoing PD. In this retrospective cohort study, we discovered that elevated NPAR levels were significantly associated with higher all-cause and cardiovascular mortality in PD patients. Furthermore, among the five biomarkers (NPAR, NLR, PLR, MLR and LHR), we demonstrated that NPAR showed the best predictive value for all-cause and cardiovascular mortality.
In recent years, NPAR has been proposed as a novel marker for systemic inflammation, based on its combination with two clinical inflammatory evaluation parameters. In the current study, we discovered that NPAR levels was positively correlated with hs-CRP, LDL-C levels and negatively correlated with hemoglobin and RRF. A retrospective cohort study, which included 277 PD patients, indicated that individuals with CRP levels above 16.8 mg/L had a 2.62 times higher risk of all-cause death and a 2.60 times higher risk of cardiovascular events than the patients with CRP levels below 1.6 mg/L.16 In our study, we found a positive correlation between NPAR levels and hs-CRP levels, suggesting that increased NPAR levels may indicate the exacerbation of inflammation in PD patients, and NPAR can potentially be used as a marker to evaluate the inflammatory status in PD patients. LDL-C is a key contributor in the development of atherosclerosis and is strongly associated with cardiovascular disease.17 The positive correlation between NPAR and LDL-C indicated that PD patients with higher NPAR levels may suffer a greater risk of cardiovascular disease. Anemia is a common complication in patients with chronic kidney disease (CKD), and chronic inflammation is one of the factors that contributes to and exacerbates anemia, which could partially explain the negative correlation between NPAR and hemoglobin observed in our study.18,19 Patients with elevated NPAR levels often experienced hypoalbuminemia, which led to renal ischemia and hypoxia by reducing circulatory volume and renal perfusion. Heyman et al demonstrated that ischemia and hypoxia in renal tissue induced the production of toxic mediators, which ultimately contribute to the decline of RRF in CKD patients.20 This may be one of the explanations for the negative correlation between NPAR levels and RRF.
Cardiovascular disease is the primary cause of death in PD patients, accounting for 52.7% of cases.21 Similar to previous studies, we found that 50.3% of patients died from cardiovascular disease. Numerous studies have explored the association between elevated NPAR levels and mortality in various populations, including myocardial infarction, heart failure, septic shock and acute kidney injury.22–25 In a retrospective study of 2942 critically ill patients with heart failure, it was found that those with elevated NPAR levels had significantly higher mortality rates at 30, 90, and 365 days compared to those with lower NPAR levels.24 Another cohort study of patients with ST-segment elevation myocardial infarction revealed that the risk of in-hospital mortality increased by 4.928 times for each unit increase in baseline NPAR levels.26 Our findings showed that the risk of all-cause and cardiovascular mortality was, respectively, 1.51 and 1.57 times higher in the highest tertile of NPAR than in the lowest tertile. The association could be explained by the fact that increased neutrophil percentage and decreased albumin levels result in elevated NPAR levels, which may be able to reflect the existence of both the systemic inflammatory states and worsening nutritional status in PD patients. Our findings suggested that PD patients with elevated NPAR levels are more likely to have poor outcomes and require special attention and medical intervention in clinical practice.
Emerging evidences demonstrated that neutrophils contribute to various stages of atherosclerosis, mainly by stimulating platelet adhesion, promoting the activation of endothelial cells and macrophages, boosting monocyte recruitment, and touching off the formation of atherosclerotic plaque.27–29 Besides, elevated neutrophil count increases the risk of adverse cardiovascular events through releasing inflammatory mediators and promoting endothelial regeneration and angiogenesis.30,31 Serum albumin has been found to maintain plasma colloid osmotic pressure and circulation volume, increase renal perfusion and urine volume, as well as exert antioxidant, anti-inflammatory, anticoagulant and antiplatelet aggregation effects.32–34 PD patients often experienced hypoalbuminemia, which is caused by a variety of factors, such as albumin loss during dialysis, decreased albumin synthesis and increased degradation due to chronic inflammation, insufficient protein intake, and albumin loss in the urine. There was a significant correlation between decreased serum albumin levels and the loss of RRF, as well as an increased risk of all-cause mortality in PD patients.11,35 In conclusion, higher NPAR levels due to elevated neutrophil percentage and/or decreased serum albumin, to some extent, indicated an increased risk of cardiovascular adverse events or death in PD patients.
Previous studies have indicated that there have been similar biological biomarkers verified to predict mortality in PD patients, such as NLR, PLR, MLR and LHR.36–39 In the current study, we compare the predictive values of NPAR with NLR, PLR, MLR and LHR. Our findings revealed that NPAR had a higher AUC value compared with other four biomarkers and showed superior and more reliable predictive efficiency. One possible explanation for the superior predictive value of NPAR was that, being a combination of hematological and biochemical parameters, NPAR represented more comprehensive evaluation function, whereas the other biomarkers contained only one aspect. Additionally, we compared the predictive value of NPAR with neutrophil count, serum albumin, hs-CRP, and history of diabetes in predicting all-cause and cardiovascular mortality of PD patients. The predictive value of NPAR was found to be better than neutrophil count but not superior to serum albumin, hs-CRP, and history of diabetes. In previous studies, history of diabetes and albumin had already been proved to be associated with elevated risk of all-cause or cardiovascular mortality in PD patients.13,40 As a new inflammatory biomarker, NPAR did not show the most excellent predictive value, while it did provide a new strategy for predicting the prognosis of PD patients beyond the classical biomarkers. Therefore, NPAR has the potential to become a novel effective prognostic predictor that can be applied across various medical institutions, which can help clinicians to identify PD patients at higher risk of mortality, enabling them to administer timely medical interventions and ultimately improve the patients’ survival.
Conclusion
In conclusion, the present study suggested that high NPAR level was an independent risk factor for all-cause and cardiovascular mortality in PD patients. Moreover, NPAR showed superior predictive value for mortality compared with NLR, PLR, MLR and LHR. NPAR could become a novel effective prognostic predictor widely applicable in outcome prediction for PD patients.
Acknowledgments
We appreciate all the nephrologists and nurses in our PD center for their cooperation.
Funding Statement
This study was supported by the Guangzhou science and technology planning project (2023A04J2183), National Natural Science Foundation of China (82000677), Guangdong Basic and Applied Basic Research Foundation (2022A1515012532), Guang dong Provincial Key Laboratory of Nephrology (2020B1212060028), and NHC Key Laboratory of Clinical Nephrology (Sun Yat-sen University).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors have declared no conflicts of interest.
References
- 1.Teitelbaum I, Ingelfinger JR. Peritoneal Dialysis. New Engl J Med. 2021;385(19):1786–1795. doi: 10.1056/NEJMra2100152 [DOI] [PubMed] [Google Scholar]
- 2.Li PK, Chow KM, Van de Luijtgaarden MW, et al. Changes in the worldwide epidemiology of peritoneal dialysis. Nat Rev Nephrol. 2017;13(2):90–103. doi: 10.1038/nrneph.2016.181 [DOI] [PubMed] [Google Scholar]
- 3.Kiebalo T, Holotka J, Habura I, Pawlaczyk K. Nutritional status in peritoneal dialysis: nutritional guidelines, adequacy and the management of malnutrition. Nutrients. 2020;12(6):1715. doi: 10.3390/nu12061715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cobo G, Lindholm B, Stenvinkel P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol Dial Transplant. 2018;33(suppl_3):iii35–iii40. doi: 10.1093/ndt/gfy175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li PK, Ng JK, McIntyre CW. Inflammation and Peritoneal Dialysis. Semin Nephrol. 2017;37(1):54–65. doi: 10.1016/j.semnephrol.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 6.Cai L, Yu J, Yu J, et al. Prognostic value of inflammation-based prognostic scores on outcome in patients undergoing continuous ambulatory peritoneal dialysis. BMC Nephrol. 2018;19(1):297. doi: 10.1186/s12882-018-1092-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shahab I, Nolph KD. MIA syndrome in peritoneal dialysis: prevention and treatment. Contrib Nephrol. 2006;150:135–143. doi: 10.1159/000093513 [DOI] [PubMed] [Google Scholar]
- 8.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. doi: 10.1038/nri3399 [DOI] [PubMed] [Google Scholar]
- 9.Johnson DW, Wiggins KJ, Armstrong KA, Campbell SB, Isbel NM, Hawley CM. Elevated white cell count at commencement of peritoneal dialysis predicts overall and cardiac mortality. Kidney Int. 2005;67(2):738–743. doi: 10.1111/j.1523-1755.2005.67135.x [DOI] [PubMed] [Google Scholar]
- 10.Reddan DN, Klassen PS, Szczech LA, et al. White blood cells as a novel mortality predictor in haemodialysis patients. Nephrol Dial Transplant. 2003;18(6):1167–1173. doi: 10.1093/ndt/gfg066 [DOI] [PubMed] [Google Scholar]
- 11.Yamada S, Kawai Y, Tsuneyoshi S, et al. Lower serum albumin level is associated with an increased risk for loss of residual kidney function in patients receiving peritoneal dialysis. Therapeutic Apheresis Dialysis. 2020;24(1):72–80. doi: 10.1111/1744-9987.12861 [DOI] [PubMed] [Google Scholar]
- 12.de Mutsert R, Grootendorst DC, Indemans F, Boeschoten EW, Krediet RT, Dekker FW. Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J Ren Nutr. 2009;19(2):127–135. doi: 10.1053/j.jrn.2008.08.003 [DOI] [PubMed] [Google Scholar]
- 13.Huang N, Li H, Fan L, et al. Serum phosphorus and albumin in patients undergoing peritoneal dialysis: interaction and association with mortality. Front Med. 2021;8:760394. doi: 10.3389/fmed.2021.760394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 15.Zhong Z, Luo D, Luo N, et al. Serum Hepcidin-25 and risk of mortality in patients on peritoneal dialysis. Front Med. 2021;8:684548. doi: 10.3389/fmed.2021.684548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen T, Hassan HC, Qian P, Vu M, Makris A. High-sensitivity troponin T and C-reactive protein have different prognostic values in hemo- and peritoneal dialysis populations: a cohort study. J Am Heart Assoc. 2018;7(5):e007876. doi: 10.1161/jaha.117.007876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruscica M, Tokgözoğlu L, Corsini A, Sirtori CR. PCSK9 inhibition and inflammation: a narrative review. Atherosclerosis. 2019;288:146–155. doi: 10.1016/j.atherosclerosis.2019.07.015 [DOI] [PubMed] [Google Scholar]
- 18.Kuo KL, Liu JS, Lin MH, Hsu CC, Tarng DC. Association of anemia and iron parameters with mortality among prevalent peritoneal dialysis patients in Taiwan: the AIM-PD study. Sci Rep. 2022;12(1):1269. doi: 10.1038/s41598-022-05200-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gluba-Brzózka A, Franczyk B, Olszewski R, Rysz J. The influence of inflammation on anemia in CKD patients. Int J Mol Sci. 2020;21(3):725. doi: 10.3390/ijms21030725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heyman SN, Khamaisi M, Rosen S, Rosenberger C. Renal parenchymal hypoxia, hypoxia response and the progression of chronic kidney disease. Am j Nephrol. 2008;28(6):998–1006. doi: 10.1159/000146075 [DOI] [PubMed] [Google Scholar]
- 21.Bello AK, Okpechi IG, Osman MA, et al. Epidemiology of peritoneal dialysis outcomes. Nat Rev Nephrol. 2022;18(12):779–793. doi: 10.1038/s41581-022-00623-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang B, Li D, Cheng B, Ying B, Gong Y. The neutrophil percentage-to-albumin ratio is associated with all-cause mortality in critically ill patients with acute kidney injury. Biomed Res Int. 2020;2020:5687672. doi: 10.1155/2020/5687672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin Y, Lin Y, Yue J, Zou Q. The neutrophil percentage-to-albumin ratio is associated with all-cause mortality in critically ill patients with acute myocardial infarction. BMC Cardiovasc Disord. 2022;22(1):115. doi: 10.1186/s12872-022-02559-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu Z, Wang J, Xue Y, et al. The neutrophil-to-albumin ratio as a new predictor of all-cause mortality in patients with heart failure. J Inflamm Res. 2022;15:701–713. doi: 10.2147/jir.S349996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong Y, Li D, Cheng B, Ying B, Wang B. Increased neutrophil percentage-to-albumin ratio is associated with all-cause mortality in patients with severe sepsis or septic shock. Epidemiol Infect. 2020;148:e87. doi: 10.1017/s0950268820000771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui H, Ding X, Li W, Chen H, Li H. The neutrophil percentage to albumin ratio as a new predictor of In-Hospital mortality in patients with ST-segment elevation myocardial infarction. Med Sci Monit. 2019;25:7845–7852. doi: 10.12659/msm.917987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balta S, Celik T, Mikhailidis DP, et al. The relation between atherosclerosis and the neutrophil-lymphocyte ratio. Clin App Thromb Hemost. 2016;22(5):405–411. doi: 10.1177/1076029615569568 [DOI] [PubMed] [Google Scholar]
- 28.Döring Y, Soehnlein O, Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circulat Res. 2017;120(4):736–743. doi: 10.1161/circresaha.116.309692 [DOI] [PubMed] [Google Scholar]
- 29.Döring Y, Libby P, Soehnlein O. Neutrophil extracellular traps participate in cardiovascular diseases: recent experimental and clinical insights. Circulat Res. 2020;126(9):1228–1241. doi: 10.1161/circresaha.120.315931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silvestre-Roig C, Braster Q, Ortega-Gomez A, Soehnlein O. Neutrophils as regulators of cardiovascular inflammation. Nat Rev Cardiol. 2020;17(6):327–340. doi: 10.1038/s41569-019-0326-7 [DOI] [PubMed] [Google Scholar]
- 31.Bonaventura A, Montecucco F, Dallegri F, et al. Novel findings in neutrophil biology and their impact on cardiovascular disease. Cardiovasc Res. 2019;115(8):1266–1285. doi: 10.1093/cvr/cvz084 [DOI] [PubMed] [Google Scholar]
- 32.Lee EH, Kim WJ, Kim JY, et al. Effect of exogenous albumin on the incidence of postoperative acute kidney injury in patients undergoing off-pump coronary artery bypass surgery with a preoperative albumin level of less than 4.0 g/dl. Anesthesiology. 2016;124(5):1001–1011. doi: 10.1097/aln.0000000000001051 [DOI] [PubMed] [Google Scholar]
- 33.Huang CY, Liou SY, Kuo WW, Wu HC, Chang YL, Chen TS. Chemiluminescence analysis of antioxidant capacity for serum albumin isolated from healthy or uremic volunteers. Luminescence. 2016;31(8):1474–1478. doi: 10.1002/bio.3132 [DOI] [PubMed] [Google Scholar]
- 34.Arques S. Human serum albumin in cardiovascular diseases. Eur J Internal Med. 2018;52:8–12. doi: 10.1016/j.ejim.2018.04.014 [DOI] [PubMed] [Google Scholar]
- 35.Shi Y, Cai J, Shi C, Liu C, Zhou J, Li Z. Low serum albumin is associated with poor prognosis in patients receiving peritoneal dialysis treatment. J Healthcare Eng. 2022;2022:7660806. doi: 10.1155/2022/7660806 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Lin T, Xia X, Yu J, et al. The predictive study of the relation between elevated low-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio and mortality in peritoneal dialysis. Lipids Health Dis. 2020;19(1):51. doi: 10.1186/s12944-020-01240-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu X, Wang S, Zhang G, Xiong R, Li H. High neutrophil-to-lymphocyte ratio is a significant predictor of cardiovascular and all-cause mortality in patients undergoing peritoneal dialysis. Kidney Blood Pressure Res. 2018;43(2):490–499. doi: 10.1159/000488696 [DOI] [PubMed] [Google Scholar]
- 38.Sheng H, Qiu Y, Xia X, et al. Sexual effect of platelet-to-lymphocyte ratio in predicting cardiovascular mortality of peritoneal dialysis patients. Mediators Inflammation. 2022;2022:8760615. doi: 10.1155/2022/8760615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wen Y, Zhan X, Wang N, Peng F, Feng X, Wu X. Monocyte/lymphocyte ratio and cardiovascular disease mortality in peritoneal dialysis patients. Mediators Inflammation. 2020;2020:9852507. doi: 10.1155/2020/9852507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J, Lu X, Li H, Wang S. Risk factors for mortality in patients undergoing peritoneal dialysis: a systematic review and meta-analysis. Renal Failure. 2021;43(1):743–753. doi: 10.1080/0886022x.2021.1918558 [DOI] [PMC free article] [PubMed] [Google Scholar]