Abstract
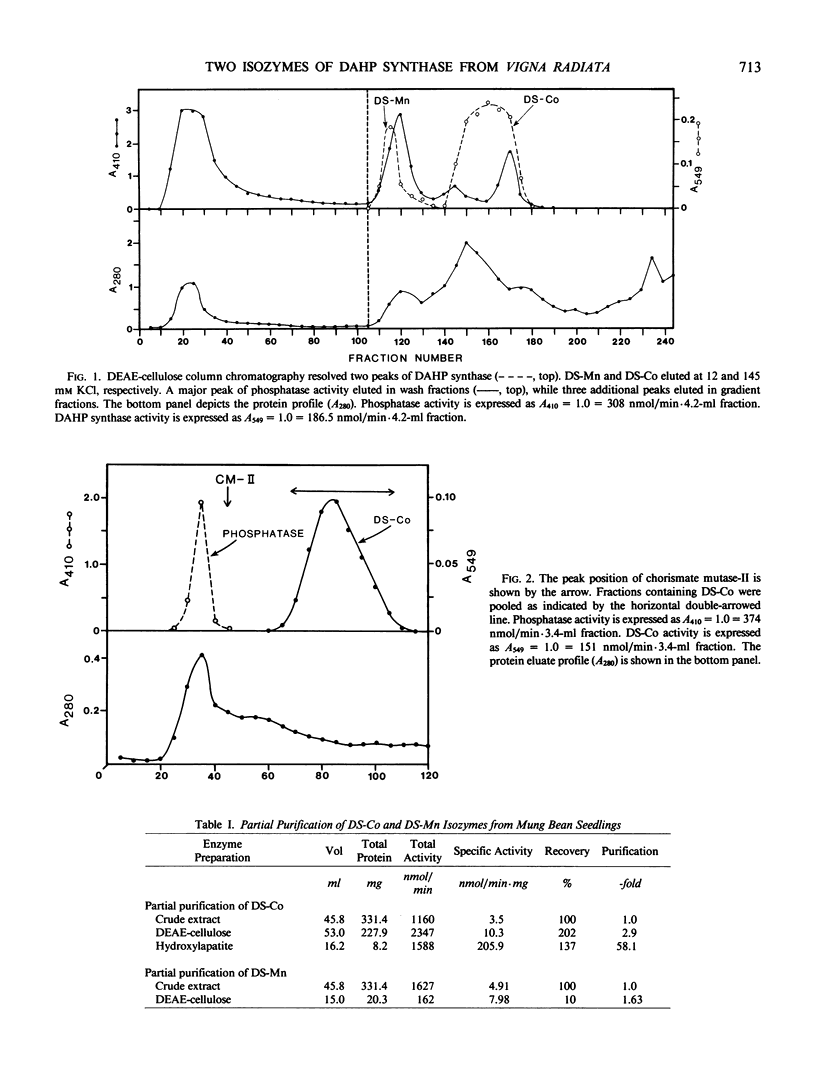
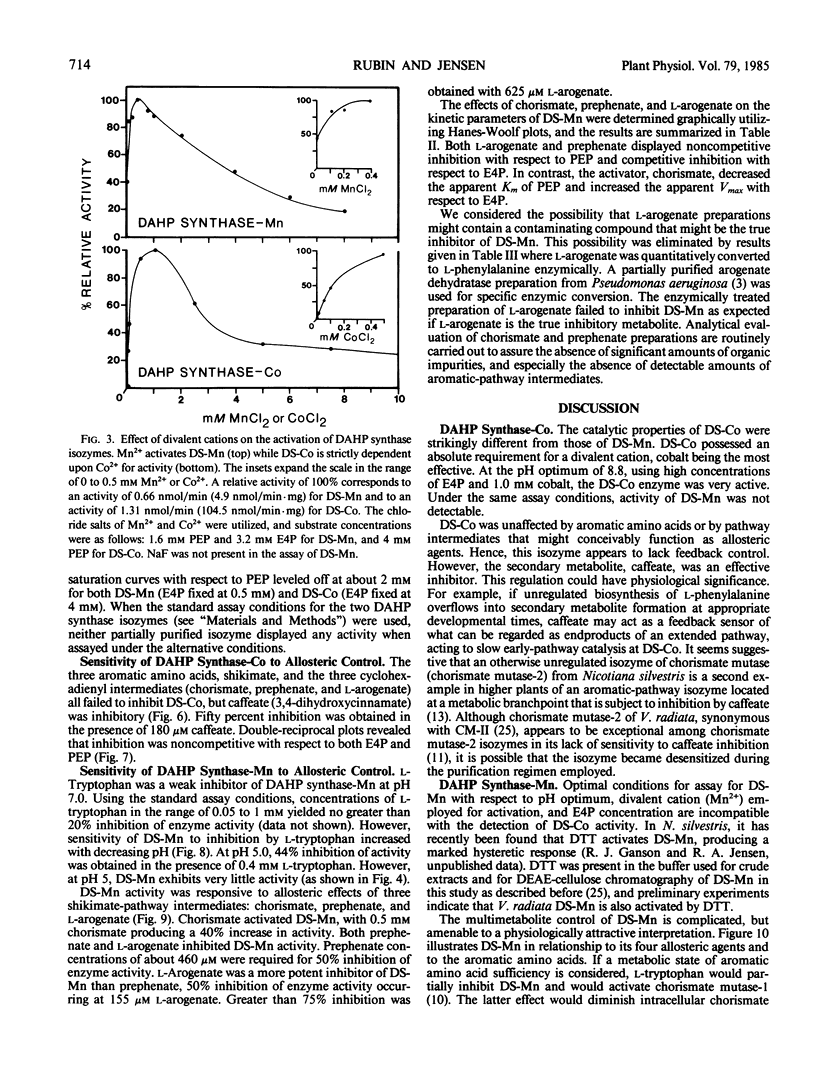
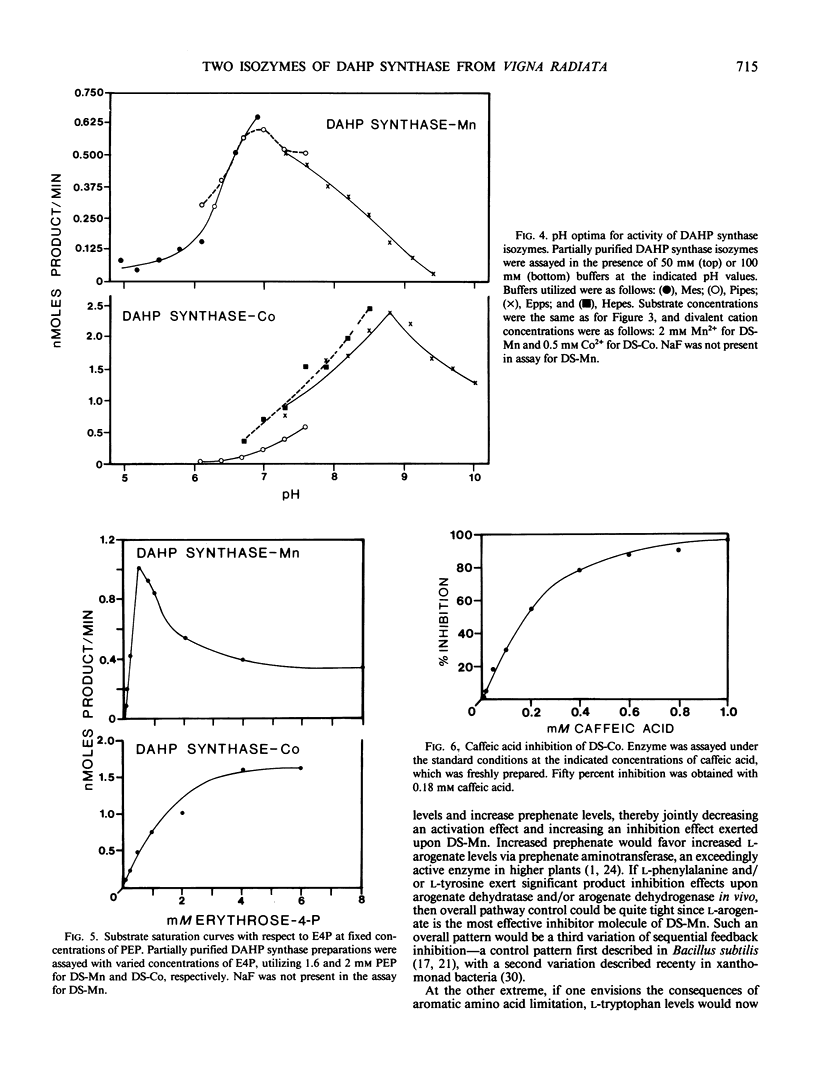
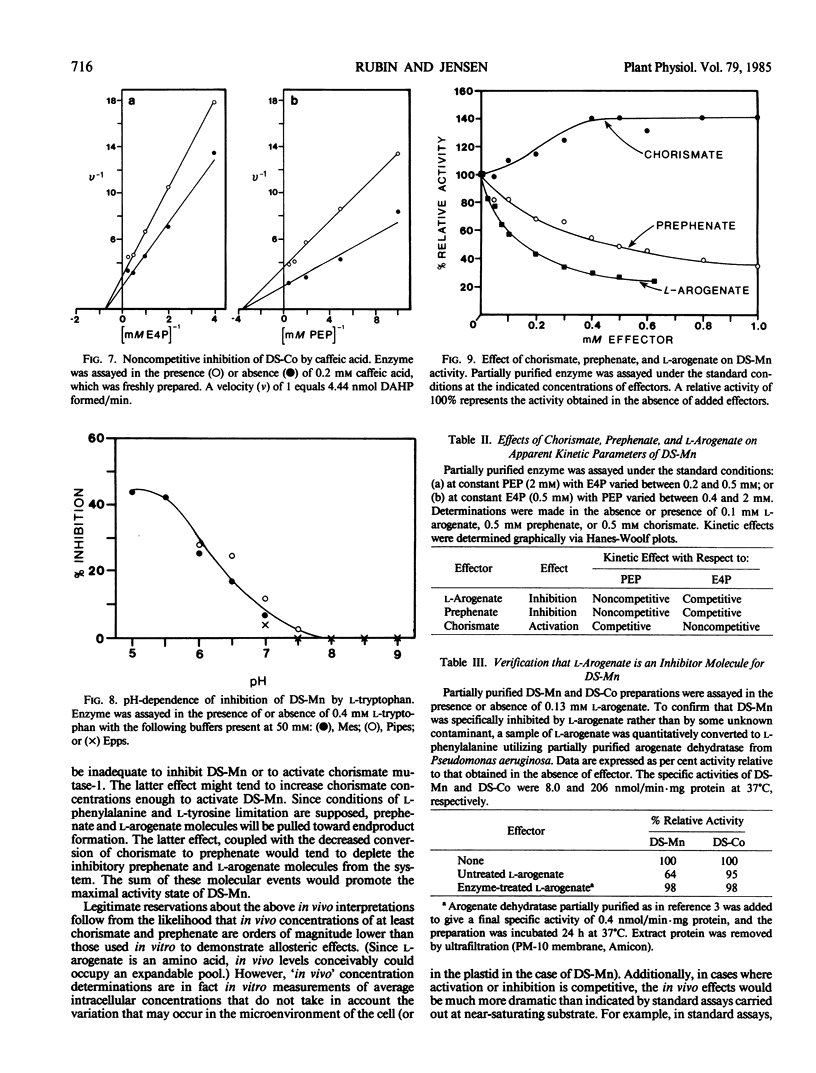
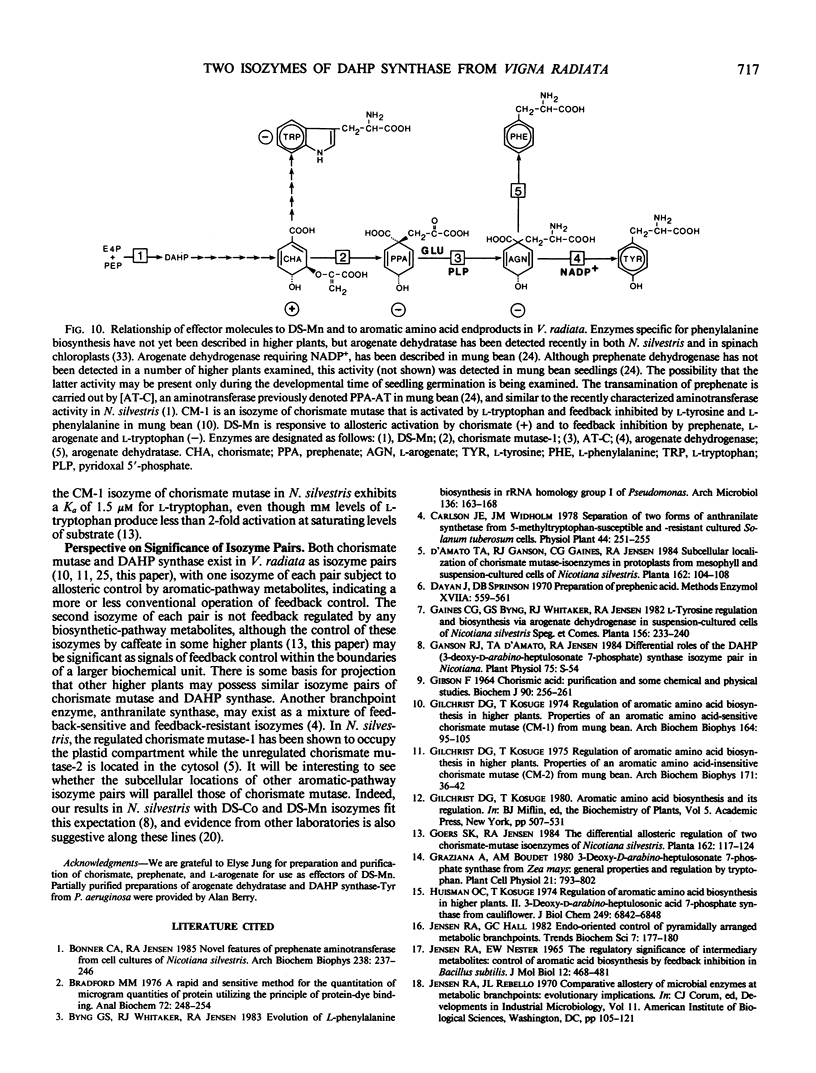
Two isozymes of 3-deoxy-d-arabino-heptulosonate-7-phosphate synthase (EC 4.1.2.15) designated DS-Mn and DS-Co were separated from seedlings of Vigna radiata [L.] Wilczek by DEAE-cellulose column chromatography. DS-Mn was activated 2.6-fold by 0.4 millimolar manganese, had an activity optimum of 7.0, and was substrate inhibited by erythrose-4-phosphate (E4P) concentrations in excess of 0.5 millimolar. In contrast, DS-Co had an activity optimum at pH 8.8, required E4P concentrations of at least 4 millimolar to approach saturation, and exhibited an absolute requirement for divalent cation (cobalt being the best). Regulatory properties of the two isozymes differed dramatically. The activity of DS-Mn was activated by chorismate (noncompetitively against E4P and competitively against phosphoenolpyruvate), and was inhibited by prephenate and l-arogenate (competitively against E4P and noncompetitively against phosphoenolpyruvate in both cases). Under standard assay conditions, l-arogenate inhibited the activity of DS-Mn 50% at a concentration of 155 micromolar and was at least 3 times more potent than prephenate on a molar basis. Weak inhibition of DS-Mn by l-tryptophan was also observed, the magnitude of inhibition increasing with decreasing pH. The pattern of allosteric control found for DS-Mn is consistent with the operation of a control mechanism of sequential feedback inhibition governing overall pathway flux. DS-Co was not subject to allosteric control by any of the molecules affecting DS-Mn. However, DS-Co was inhibited by caffeate (3,4-dihydroxycinnamate), noncompetitively with respect to either substrate. The striking parallels between the isozyme pairs of 3-deoxy-d-arabino-heptulosonate-7-phosphate synthase and chorismate mutase are noted—one isozyme in each case being tightly regulated, the other being essentially unregulated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner C. A., Jensen R. A. Novel features of prephenate aminotransferase from cell cultures of Nicotiana silvestris. Arch Biochem Biophys. 1985 Apr;238(1):237–246. doi: 10.1016/0003-9861(85)90161-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Byng G. S., Whitaker R. J., Jensen R. A. Evolution of L-phenylalanine biosynthesis in rRNA homology group I of Pseudomonas. Arch Microbiol. 1983 Nov;136(3):163–168. doi: 10.1007/BF00409838. [DOI] [PubMed] [Google Scholar]
- Gibson F. Chorismic acid: purification and some chemical and physical studies. Biochem J. 1964 Feb;90(2):256–261. doi: 10.1042/bj0900256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist D. G., Kosuge T. Regulation of aromatic amino acid biosynthesis in higher plants. Properties of an aromatic amino acid-insensitive chorismate mutase (CM-2) from mung bean. Arch Biochem Biophys. 1975 Nov;171(1):36–42. doi: 10.1016/0003-9861(75)90004-1. [DOI] [PubMed] [Google Scholar]
- Gilchrist D. G., Kosuge T. Regulation of aromatic amino acid biosynthesis in higher plants. Properties of an aromatic amino acid-sensitive chorismate mutase (CM-1) from mung bean. Arch Biochem Biophys. 1974 Sep;164(1):95–105. doi: 10.1016/0003-9861(74)90011-3. [DOI] [PubMed] [Google Scholar]
- Huisman O. C., Kosuge T. Regulation of aromatic amino acid biosynthesis in higher plants. II. 3-Deoxy-arabino-heptulosonic acid 7-phosphate synthetase from cauliflower. J Biol Chem. 1974 Nov 10;249(21):6842–6848. [PubMed] [Google Scholar]
- JENSEN R. A., NESTER E. W. THE REGULATORY SIGNIFICANCE OF INTERMEDIARY METABOLITES: CONTROL OF AROMATIC ACID BIOSYNTHESIS BY FEEDBACK INHIBITION IN BACILLUS SUBTILIS. J Mol Biol. 1965 Jun;12:468–481. doi: 10.1016/s0022-2836(65)80270-4. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Zamir L., Saint Pierre M., Patel N., Pierson D. L. Isolation and preparation of pretyrosine, accumulated as a dead-end metabolite by Neurospora crassa. J Bacteriol. 1977 Dec;132(3):896–903. doi: 10.1128/jb.132.3.896-903.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E. W., Jensen R. A. Control of aromatic acid biosynthesis in Bacillus subtilis: sequenial feedback inhibition. J Bacteriol. 1966 Apr;91(4):1594–1598. doi: 10.1128/jb.91.4.1594-1598.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J. L., Gaines C. G., Jensen R. A. Enzymological basis for herbicidal action of glyphosate. Plant Physiol. 1982 Sep;70(3):833–839. doi: 10.1104/pp.70.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J. L., Jensen R. A. Enzymology of l-Tyrosine Biosynthesis in Mung Bean (Vigna radiata [L.] Wilczek). Plant Physiol. 1979 Nov;64(5):727–734. doi: 10.1104/pp.64.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRINIVASAN P. R., SPRINSON D. B. 2-Keto-3-deoxy-D-arabo-heptonic acid 7-phosphate synthetase. J Biol Chem. 1959 Apr;234(4):716–722. [PubMed] [Google Scholar]
- Suzich J. A., Ranjeva R., Hasegawa P. M., Herrmann K. M. Regulation of the shikimate pathway of carrot cells in suspension culture. Plant Physiol. 1984 Jun;75(2):369–371. doi: 10.1104/pp.75.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker R. J., Berry A., Byng G. S., Fiske M. J., Jensen R. A. Clues from Xanthomonas campestris about the evolution of aromatic biosynthesis and its regulation. J Mol Evol. 1984;21(2):139–149. doi: 10.1007/BF02100088. [DOI] [PubMed] [Google Scholar]
- Whitaker R. J., Fiske M. J., Jensen R. A. Pseudomonas aeruginosa possesses two novel regulatory isozymes of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase. J Biol Chem. 1982 Nov 10;257(21):12789–12794. [PubMed] [Google Scholar]


