Abstract
Background
An abnormal dilatation of the abdominal aorta is referred to as an abdominal aortic aneurysm (AAA). Due to the risk of rupture, surgical repair is offered electively to individuals with aneurysms greater than 5.5 cm in size. Traditionally, conventional open surgical repair (OSR) was considered the first choice approach. However, over the past two decades endovascular aneurysm repair (EVAR) has gained popularity as a treatment option. This article intends to review the role of EVAR in the management of elective AAA.
Objectives
To assess the effectiveness of EVAR versus conventional OSR in individuals with AAA considered fit for surgery, and EVAR versus best medical care in those considered unfit for surgery. This was determined by the effect on short, intermediate and long‐term mortality, endograft related complications, re‐intervention rates and major complications.
Search methods
The Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator (TSC) searched the Specialised Register (January 2013) and the Cochrane Central Register of Controlled Trials (CENTRAL) (2012, Issue 12). The TSC also searched trial databases for details of ongoing or unpublished studies.
Selection criteria
Prospective randomised controlled trials (RCTs) comparing EVAR with OSR in individuals with AAA considered fit for surgery. and comparing EVAR with best medical care in individuals considered unfit for surgery. We excluded studies with inadequate data or using an inadequate randomisation technique.
Data collection and analysis
Three reviewers independently evaluated trials for appropriateness for inclusion and extracted data using pro forma designed by the Cochrane PVD Group. We assessed the quality of trials using The Cochrane Collaboration's 'Risk of bias' tool. We entered collected data in to Review Manager (version 5.2.3) for analysis. Where direct comparisons could be made, we determined odds ratios (OR). We tested studies for heterogeneity and, when present, we used a random‐effects model; otherwise we used a fixed‐effect model. We tabulated data that could not be collated.
Main results
Four high‐quality trials comparing EVAR with OSR (n = 2790) and one high‐quality trial comparing EVAR with no intervention (n = 404) fulfilled the inclusion criteria. In individuals considered fit for surgery, a pooled analysis, including 1362 individuals randomised to EVAR and 1361 randomised to OSR, found short‐term mortality (including 30‐day or inhospital mortality, excluding deaths prior to intervention) with EVAR to be significantly lower than with OSR (1.4% versus 4.2%, OR 0.33, 95% confidence interval (CI) 0.20 to 0.55; P < 0.0001). Using intention‐to‐treat analysis (ITT) there was no significant difference in mortality at intermediate follow‐up (up to four years from randomisation), with 221 (15.8%) and 237 (17%) deaths in the EVAR (n = 1393) and OSR (n = 1390) groups, respectively (OR 0.92, 95% CI 0.75 to 1.12; P = 0.40). There was also no significant difference in long‐term mortality (beyond four years), with 464 (37.3%) deaths in the EVAR and 470 (37.8%) deaths in the OSR group (OR 0.98, 95% CI 0.83 to 1.15; P = 0.78). Similarly, there was no significant difference in aneurysm‐related mortality between groups, either at the intermediate‐ or long‐term follow up.
Studies showed that both EVAR and OSR were associated with similar incidences of cardiac deaths (OR 1.14, 95% CI 0.86 to 1.52; P = 0.36) and fatal stroke rate (OR 0.81, 95% CI 0.42 to 1.55; P = 0.52). The long‐term reintervention rate was significantly higher in the EVAR group than in the OSR group (OR 1.98, 95% CI 1.12 to 3.51; P = 0.02; I2 = 85%). Results of the reintervention analysis should be interpreted with caution due to significant heterogeneity. Operative complications, health‐related quality of life and sexual dysfunction were generally comparable between the EVAR and OSR groups. However, there was a slightly higher incidence of pulmonary complications in the OSR group compared with the EVAR group (OR 0.36, 95% CI 0.17 to 0.75; P = 0.006).
In individuals considered unfit for conventional OSR, the one included trial found no difference between the EVAR and no‐intervention groups with regard to all‐cause mortality at final follow up, with 21.0 deaths per 100 person‐years in the EVAR group and 22.1 deaths per 100 person years in the no‐intervention group (adjusted hazard ratio (HR) with EVAR 0.99, 95% CI 0.78 to 1.27; P = 0.97). Aneurysm‐related deaths were, however, significantly higher in the no‐intervention group than in the EVAR group (adjusted HR 0.53, 95% CI 0.32 to 0.89; P = 0.02). There was no difference in myocardial events (HR 1.07, 95% CI 0.60 to 1.91) between the groups in this study.
Authors' conclusions
In individuals considered fit for conventional surgery, EVAR was associated with lower short‐term mortality than OSR. However, this benefit from EVAR did not persist at the intermediate‐ and long‐term follow ups. Individuals undergoing EVAR had a higher reintervention rate than those undergoing OSR. Most of the reinterventions undertaken following EVAR, however, were catheter‐based interventions associated with low mortality. Operative complications, health‐related quality of life and sexual dysfunction were generally comparable between EVAR and OSR. However, there was a slightly higher incidence of pulmonary complications in the OSR group than in the EVAR group.
In individuals considered unfit for open surgery, the results of a single trial found no overall short‐ or long‐term benefits of EVAR over no intervention with regard to all‐cause mortality, but individuals may differ and individual preferences should always be taken into account.
Plain language summary
Endovascular repair of abdominal aortic aneurysm
The abdominal aorta is a major blood vessel in the body that carries blood from the heart to the major organs in the chest and abdomen. An abdominal aortic aneurysm (AAA) is a balloon‐like bulge (dilation) of the aorta that is greater than 3 cm in diameter. If an AAA ruptures (bursts), this is often fatal. Hence, AAAs that are larger than 5.5 cm are usually treated surgically in order to try to prevent such a rupture. Traditionally, AAAs are treated using an open surgical repair (OSR) technique, in which the abdomen is cut open (referred to as open surgery) and the dilated aorta is repaired using fabric graft material. However, over the past 20 years, a newer, 'key hole' technique has been used, in which the AAA is repaired without the need for open surgery ‐ a thin tube is passed via the blood vessels in the groin to the site of the AAA. Once in the correct position, a sheath is introduced that acts to reline the dilated aorta, acting as an artifical blood vessel through which blood can continue to flow, bypassing the aneurysm. Hence, the risk of further expansion or rupture of the AAA is reduced, This technique is referred to as endovascular aneurysm repair (EVAR). As EVAR is a less invasive technique than OSR, in that there is no need for open surgery, it may have advantages over OSR. In addition, some individuals with other medical illnesses, for whom open surgery may be considered a high‐risk procedure and who are not fit for OSR, can be offered EVAR instead.
The review authors identified four randomised controlled trials (RCTs) of good quality that compared OSR with EVAR, involving a combined total of 2790 participants considered fit for surgery, and one RCT of good quality that compared EVAR with no intervention, involving a total of 404 participants considered unfit for OSR.
The pooled analysis of the four trials comparing OSR with EVAR showed that the death rates within 30 days of operation or during admission for surgery were significantly lower in individuals undergoing EVAR than in those undergoing OSR (1.4% versus 4.2%). However, there was no difference in death rates between the groups up to four years after the operation (intermediate follow up) or beyond four years (long‐term follow up). Participants undergoing EVAR had a significantly higher incidence of a need for an additional intervention to deal with any complications of the procedure undertaken compared with those receiving OSR. Operative complications, health‐related quality of life and sexual dysfunction were generally comparable between the two procedures. However, there was a slightly higher incidence of lung complications in the OSR group than in the EVAR group. The results of the one RCT comparing EVAR with no intervention in individuals considered unfit for OSR showed no difference in overall death rates and complication rates between the groups.
Background
Description of the condition
When the main artery in the body, the aorta, develops a balloon‐like bulge, an abdominal aortic aneurysm (AAA) is said to have developed. The prevalence of AAA (where the aortic diameter exceeds 3 cm) in the UK has been estimated at between 1.2% and 7.6% in populations over 50 years of age, depending on the criteria used to define the AAA (Khoo 1994; Scott 1995). As with most vascular diseases, the prevalence of AAA increases with age and occurs much more frequently in men than in women. In England, treatment of AAA accounts for approximately 3650 elective surgical procedures and 1100 emergency surgical procedures per year (Holt 2009).
Untreated AAAs are likely to increase in size and may eventually rupture, which can lead to death. The decision to repair an AAA is made on an individual basis, balancing the risk of treatment against the risk of aneurysm rupture. There is good evidence that the risk of rupture is related to aneurysm size; hence, whether treatment is necessary or not is generally based on the size of the AAA or the presence of symptoms.
Description of the intervention
Conventional surgical repair of AAA has been practiced since the early 1950s and involves the surgical insertion of a prosthetic (i.e. man‐made) graft. This operation is referred to as open surgical repair (OSR) as it involves a large abdominal incision, and is associated with a significant risk of complications even when individuals are otherwise fit and well. The UK Small Aneurysm Trial found that aneurysms less than 5.5 cm in diameter can be kept under observation using periodic ultrasound scanning, reserving surgical intervention for those that become larger than 5.5 cm or that produce symptoms (UKSAT 1998). The 30‐day mortality associated with surgical repair of AAA in the UK Small Aneurysm Trial was 5.8%, and reported rates range from 2% to 7% in otherwise fit individuals (Feinglass 1995; Mutirangura 1989; Olsen 1991; Steyerberg 1995; Takolander 1998; Zarins 1997). However, if rupture occurs, mortality rises steeply. Inhospital mortality rates are reported to range from 40% to 80%, but as many cases do not reach hospital it is estimated that overall mortality is higher and may reach 95% (Bengtsson 1993; Budd 1989; Campbell 1986; Eskandari 1998; Ingoldby 1986; Johansson 1986; Kantonen 1999; Thomas 1988).
In individuals who have severe coexistent cardiac, respiratory or renal disease, conventional surgical treatment may be considered too dangerous, and individuals considered unfit for surgery may not be offered OSR. In such cases, attempts may be made to lower the risk of rupture by reducing blood pressure or via other pharmacological manipulations, though there is no strong trial evidence that this is effective (Gadowski 1994; Leach 1988; Walton 1999).
The traditional approach to treating individuals with AAA has been challenged by the arrival of a new, minimally invasive technique: endovascular stent grafting of AAA, known as endovascular aneurysm repair, or EVAR. This technique, first described by Parodi in 1991, involves the placement of a tubular graft within the aneurysm sac (bulge), which is anchored in place using metallic stents (Parodi 1991). The aim is to exclude the aneurysm from within, thus preventing further expansion and rupture. The procedure is currently performed via arteriotomies (small holes in an artery to permit access) made in the groin arteries (usually the femoral arteries), using catheters and guidewires, and imaging with arteriography to position the stent‐graft device at the site of the aneurysm. Regional, rather than general, anaesthesia can be used, particularly in individuals for whom general anaesthesia is a relative contraindication.
How the intervention might work
There has been much development of these stent‐grafts in recent years and there are three main configurations of grafts available for use: tube grafts, aorto‐bi‐iliac grafts and aorto‐uni‐iliac grafts. Choice of graft configuration depends on operator experience and the anatomical type of aneurysm to be treated. The aortic tube graft is no longer used because it is unusual for there to be sufficient normal aorta below the infrarenal AAA to which to anchor the lower end of the stent‐graft. In addition, the distal aorta tends to enlarge with these stent‐grafts over time, resulting in the return of blood flow into the aneurysm sac (endoleak) at the distal fixation site. The aorto‐bi‐iliac device is the most commonly used device and is most readily available worldwide. The aorto‐uni‐iliac graft configuration involves the person with AAA having a cross‐over graft to maintain perfusion to both lower limbs. This configuration has the advantage that it can treat a large proportion of aneurysms, but currently it is suitable for use with only approximately 60% of all AAAs (Armon 1998).
Most grafts now in use are commercially produced, either custom made for individuals, or coming in a range of sizes so that a suitable device can be obtained 'off the shelf'. The majority of commercially produced devices are of the aorto‐bi‐iliac configuration, although aorto‐uni‐iliac devices can be produced as custom‐made devices on an individual basis, if required. Previously, some centres used improvised devices constructed from available graft materials and endovascular stents; these were usually of the aorto‐uni‐iliac configuration.
Since Parodi's landmark publication (Parodi 1991) there has been much interest in the EVAR technique. Potential advantages of this 'less invasive' technique over conventional OSR include:
reduced time under general or regional anaesthesia;
avoidance of arterial cross‐clamping, thus decreasing disruption of blood flow to vital organs and the lower extremities;
elimination of the pain and trauma of major abdominal surgery, enabling a shorter recovery time and potentially fewer respiratory complications;
reduced length of hospital stay;
reduced length of stay on an intensive care unit;
reduced blood loss.
All the above have the potential to reduce the morbidity and mortality of AAA repair. The potential cost savings from reduced use of hospital facilities are offset by the cost of the stent‐graft, which, in the UK, is approximately GBP5000, depending on the device used.
Why it is important to do this review
AAA can be treated using OSR or EVAR, and in those individuals considered unfit for surgery, a conservative management option is available in current practice. It is however, unclear as to which is the best option; for example, EVAR has earlier survival advantage compared to OSR but its longevity is unknown. It is therefore necessary to understand clearly the short‐, intermediate‐ and long‐term risks or benefits of the available options as this has implications for clinical practice.
This review draws together the available trial evidence to assess the advantages and disadvantages of EVAR compared with conventional OSR in individuals with AAA considered fit for surgery, and with conservative care (no intervention) in those considered unfit to undergo OSR.
Objectives
To assess the effectiveness of EVAR versus conventional OSR in individuals with AAA considered fit for surgery, and EVAR versus best medical care for those considered unfit for surgery. This was determined by the effect on short, intermediate and long‐term mortality, endograft related complications, re‐intervention rates and major complications.
Methods
Criteria for considering studies for this review
Types of studies
Prospective randomised controlled trials (RCTs) comparing EVAR with OSR in individuals considered fit for surgery, and EVAR with best medical care in individuals considered unfit for surgery. We excluded studies with inadequate data or using an inadequate randomisation technique.
Types of participants
All individuals with an AAA diagnosed by ultrasound or computed tomography (CT) in whom treatment was felt to be indicated. We excluded studies in which the size of aneurysm was not clear. We considered only individuals with asymptomatic AAA undergoing elective aneurysm treatment; we did not consider individuals undergoing emergency repair of an aneurysm.
Types of interventions
The primary intervention was elective EVAR repair of AAA. This can be performed with a variety of devices that fall into two main groups: aorto‐bi‐iliac devices and aorto‐uni‐iliac devices. We considered all device types. We compared EVAR repair with conventional OSR treatment in individuals considered fit for surgery, and with best medical care in those considered unfit for surgery. Complex and hybrid endovascular techniques (including fenestrated EVAR) were not considered in this review.
Types of outcome measures
Primary outcomes
Mortality and aneurysm‐related mortality rates: short term (30‐day or inhospital mortality), intermediate (up to four years from randomisation) and long term (beyond four years).
Endograft‐related complications (e.g. endoleak, reintervention (defined as the rate of any secondary intervention after the primary repair ‐ EVAR or OSR)
Major complications (e.g. those that altered management of the individual (myocardial infarction, stroke, renal failure, bowel ischaemia, pulmonary complications etc.)
Secondary outcomes
Minor complications
Health‐related quality of life (HRQoL): as measured using standardised questionnaires
Economic analysis: based on an analysis of costs, not charges
Search methods for identification of studies
We did not apply any language restrictions or any restrictions regarding publication status.
Electronic searches
The Cochrane Peripheral Vascular Diseases (PVD) Group Trials Search Co‐ordinator (TSC) searched the Specialised Register (January 2013) and the Cochrane Central Register of Controlled Trials (CENTRAL) (2012, Issue 12, part of The Cochrane Library, www.thecochranelibrary.com. See Appendix 1 for details of the search strategy used to search CENTRAL. The Specialised Register is maintained by the TSC and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL and AMED, and through handsearching of relevant journals. The full list of the databases, journals and conference proceedings that were searched, as well as the search strategies used, are described in the Specialised Register section of the Cochrane Peripheral Vascular Diseases Group module in The Cochrane Library (www.thecochranelibrary.com).
The TSC also searched the following trial databases for details of ongoing and unpublished studies using the terms (aneurysm and abdominal and open and endovascular):
World Health Organization International Clinical Trials Registry (http://apps.who.int/trialsearch/);
ClinicalTrials.gov (http://clinicaltrials.gov/);
Current Controlled Trials (http://www.controlled‐trials.com/).
Searching other resources
We searched the reference lists of articles retrieved by electronic searches for additional citations.
In addition, we checked various health service research‐related resources via the internet. These included health economics and Health Technology Assessment organisations and guideline‐producing agencies (e.g. National Institute for Health and Clinical Excellence (NICE) and Scottish Intercollegiate Guidelines Network). We sought further trial reports through examination of the proceedings from the following meetings:
Vascular Society of Great Britain and Ireland (2004 to 2012);
Association of Surgeons of Great Britain and Ireland (2004 to 2012);
British Society of Interventional Radiology (2004 to 2012).
Data collection and analysis
Selection of studies
Three reviewers (SCVP, RJ and RC) independently evaluated trials, considered them for inclusion and assessed trial quality. Any disagreements were resolved by discussion.
Data extraction and management
Three reviewers (SCVP, RJ and RC) independently evaluated trials for appropriateness for inclusion and extracted data using pro forma designed by the Cochrane PVD Group.
Assessment of risk of bias in included studies
Three reviewers (SCVP, RJ and RC) independently assessed the quality of the included studies using the 'Risk of bias' tool developed by The Cochrane Collaboration (Higgins 2011). This tool provides a standard protocol for allowing judgements to be made on sequence generation, allocation methods, blinding, incomplete outcome data, selective outcome reporting and any other relevant biases. For each of these six items we assessed the risk of bias as 'low risk', 'high risk' or 'unclear risk', with the 'unclear risk' of bias category being used to indicate either a lack of information or uncertainty over the potential for bias.
Measures of treatment effect
We used odds ratios (OR) with a 95% confidence interval (CI) as the measure of effect for each dichotomous outcome. Where there were sufficient data, we calculated a summary statistic for each outcome using either a fixed‐effect or random‐effects model, depending on the presence of heterogeneity. We analysed continuous scales of measurement in a continuous form (i.e. mean difference with 95% CI).
Unit of analysis issues
For this review, we considered each participant as an individual unit of analysis.
Dealing with missing data
Where possible, we conducted analyses on an intention‐to‐treat (ITT) basis. The outcome data required were available from all trials. Due to the difficulty in identifying participants who died within the 30 days before the intervention, we did not analyse short‐term mortality on an ITT basis. We used ITT analyses for the intermediate‐ and long‐term outcomes.
Assessment of heterogeneity
We noted heterogeneity in the data and cautiously explored reasons for this using previously identified study characteristics, particularly assessments of quality. We used the I2 statistic to assess heterogeneity, with an I2 statistic of 25% to 50% indicating low heterogeneity, 50% to 75% indicating moderate heterogeneity and over 75% indicating significant heterogeneity. We used a random‐effects model when the I2 statistic was greater than 50%.
Assessment of reporting biases
We did not perform a funnel plot to assess reporting bias because we included fewer than 10 studies (Higgins 2011).
Data synthesis
Where direct comparisons could be made, we calculated OR. We tested studies for heterogeneity and, if the I2 statistic was greater than 50%, used a random‐effects model; otherwise we used a fixed‐effect model. We tabulated data that could not be collated. We performed statistical analyses according to the guidelines for reviews outlined in the Cochrane PVD Group's module using RevMan Software (version 5.2.3). With the available results it was not possible to perform time‐to‐event analyses.
Subgroup analysis and investigation of heterogeneity
The two main comparisons were the outcomes between OSR and EVAR in fit (low to moderate risk) patients and the outcomes between EVAR and 'no‐intervention' in unfit (high risk) surgical patients. We undertook no subgroup analyses.
Sensitivity analysis
We undertook no sensitivity analyses as there were no included studies with poor methodological quality.
Results
Description of studies
Results of the search
Figure 1 ‐ PRISMA flow diagram.
1.
Study flow diagram.
Included studies
Four trials comparing EVAR with OSR (ACE; DREAM; EVAR1; OVER) and one trial comparing EVAR with no intervention (EVAR2) fulfilled the inclusion criteria.
The specific details of each included study can be found in the 'Characteristics of included studies’ table.
EVAR1 was a multicentre RCT carried out in 37 UK centres that compared outcomes following EVAR and OSR in individuals considered fit for conventional surgical repair. A total of 1252 individuals aged 60 years or older with an AAA size of at least 5.5 cm in size were recruited between 1 September 1999 and 31 August 2004. A total of 626 individuals each were randomised to receive EVAR or OSR using a permuted block method. Mean (standard deviation (SD)) age was 74.1 (6.1) years and mean AAA size was 6.4 (0.9) cm; the male:female ratio was 10:1. Participants were followed up for a median (interquartile range (IQR)) of 6.0 (3.9 to 7.3) years. After randomisation, 12 individuals in the EVAR group and 19 in the OSR group died before they underwent the procedure. Five participants in the OSR group refused surgery and the procedure was postponed for one participant in each of the EVAR and OSR groups. Eight participants in the OSR group and nine in the EVAR group were lost to follow up. Analysis was ITT. All‐cause mortality was the primary outcome; secondary outcomes included aneurysm‐related mortality, postoperative complications, HRQoL, cost‐effectiveness and durability.
The Dutch Randomised Endovascular Aneurysm Management (DREAM) trial recruited 351 individuals at 26 Dutch and four Belgian centres between 2000 and 2003. A total of 173 participants were randomised to undergo EVAR and 178 to undergo OSR. Four participants declined the procedure postrandomisation (three in the OSR group versus one in the EVAR group) and one participant in each of the OSR and EVAR groups died before surgery. All participants were followed‐up for five years, with an overall 6.4 median years of follow up. All‐cause and aneurysm‐related mortality, procedural complications and reintervention rates were the outcomes assessed. Mean aneurysm size was 7.1 cm and the male:female ratio was 10:1.
Open Versus Endovascular Repair (OVER) was a multicentre RCT conducted at 42 Veteran Affairs Medical centres in the USA. Between October 2002 and October 2008, 881 individuals aged 49 years or older with a AAA of at least 4.5 cm in size were randomised to undergo EVAR (n = 444) or OSR (n = 437). In the EVAR group, two refused repair, two died before surgery, one repair was aborted and 12 participants underwent open repair. In the OSR group, four refused repair, three repairs were aborted, one participant died before repair and 13 underwent EVAR. The intended follow‐up period was until 2011. All‐cause mortality was the primary outcome. Secondary outcomes included procedural failure, short‐term morbidity, inhospital and intensive care stay, HRQoL and erectile dysfunction.
The Anevrysme de l'aorte abdominale, Chirurgie versus Endoprosthese (ACE) trial was a French multicentre trial that randomised individuals with AAA in whom open surgery was considered a low‐to‐medium risk procedure to either EVAR or OSR. Of the 306 individuals recruited between 2003 and 2008, seven withdrew consent and were excluded. A total of 149 participants were randomised to OSR and 150 to EVAR. Seventeen participants in the OSR group underwent EVAR whereas four in the EVAR group underwent OSR, predominantly due to individual preference. One participant in the OSR group did not undergo surgery. ITT analysis was used. All‐cause mortality and major adverse events (myocardial infarction, permanent stroke, permanent haemodialysis, major amputation, paraplegia and bowel infarction) were the main outcome measures. The reintervention rate and minor complications were the secondary outcome measures. Participants were followed up for a median of three years.
Unlike the aforementioned trials, EVAR2 assessed whether EVAR improves outcomes in individuals considered unfit for conventional OSR. Between September 1999 and August 2004, 404 high‐risk surgical individuals with a mean (SD) age of 76.8 (6.5) years and a mean AAA size of 6.7 (1.0) cm were recruited. Participants were allocated to receive no intervention (n = 207) or EVAR (n = 197). In the EVAR group, 18 participants died before surgery, nine due to a ruptured aneurysm. In the no‐intervention group, 70 participants underwent EVAR. The median (IQR) time from randomisation to surgery was 244 (83 to 643) days and 55 (38 to 77) days in the no‐intervention and EVAR groups, respectively. Median (IQR) follow up was 3.6 (1.3 to 5.4) years, with fewer than 1% of participants lost to follow‐up.
Excluded studies
We excluded seven studies from the review and reasons for this are detailed in 'Characteristics of excluded studies'.
Risk of bias in included studies
We included five randomised trials dating from between 1999 and 2008 in the review. We assessed the risk of bias across all included studies using the RevMan 'Risk of bias' assessment tool. All studies were of high quality with good randomisation and allocation concealment, reported all predefined outcomes and used ITT analyses; we therefore considered them at low risk of bias. Further details are shown in Figure 2 and Figure 3.
2.
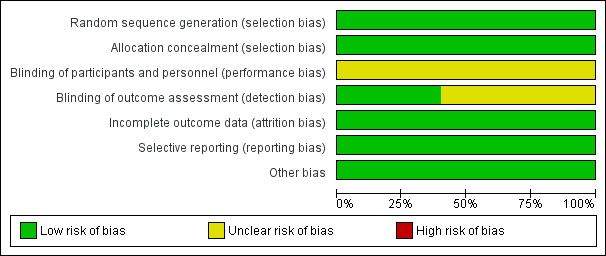
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
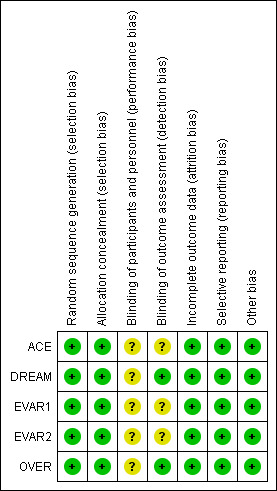
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Allocation in all trials was of acceptable standards, with allocation made only after receiving baseline participant data.
Blinding
Due to the nature of the intervention, participants and operators were not blinded to the treatment; however, individuals who assessed outcomes were blinded in the DREAM and OVER trials.
Incomplete outcome data
All studies used ITT analysis and reported on all recruited participants.
Selective reporting
All studies reported their predefined outcomes and hence we assessed the risk of reporting bias as minimal. However, not all trials reported the incidence of incisional hernia following OSR and the interventions needed to repair those hernias. This information would be useful in determining the reintervention rate after OSR.
Other potential sources of bias
The EVAR1 and DREAM trials recruited participants between 1999 and 2004, whereas the OVER and ACE trials recruited participants between 2002 and 2008. There may, therefore, be some bias with regards to the EVAR devices used in these studies, as advanced devices were available for use in the later OVER and ACE trials.
Effects of interventions
EVAR versus OSR in the management of surgically fit participants
All‐cause mortality
All four RCTs reported short‐term (30‐day or inhospital) and intermediate mortality, whereas three trials (EVAR1, DREAM and OVER) reported long‐term mortality.
Short‐term (30‐day or inhospital) mortality (comparison 1.1)
All four RCTs reported short‐term mortality. Some participants died before surgery or did not undergo the intervention. Hence, we excluded these individuals from the analysis of short‐term mortality. Intention‐to‐treat analysis was performed for intermediate and long‐term mortality.
For this analysis we used inhospital mortality data. There was no significant heterogeneity among trials. Short‐term mortality was significantly lower in the EVAR group (1.4%) than in the OSR group (4.2%) (P < 0.0001) (OR 0.33, 95% CI 0.20 to 0.55) (Analysis 1.1).
1.1. Analysis.
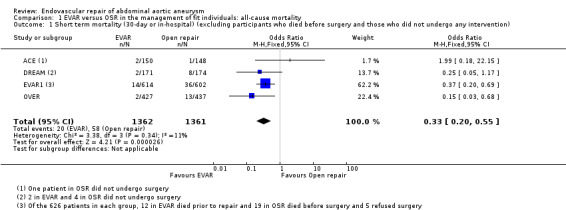
Comparison 1 EVAR versus OSR in the management of fit individuals: all‐cause mortality, Outcome 1 Short term mortality (30‐day or in‐hospital) (excluding participants who died before surgery and those who did not undergo any intervention).
Intermediate (up to four years) mortality (comparison 1.2)
Mortality at intermediate follow up (up to four years, including those who died prior to intervention; ITT analysis) was reported by all trials. EVAR1 and DREAM reported four‐year outcomes, OVER reported two‐year outcomes and ACE reported three‐year follow up data. Intermediate follow‐up data was available for 1393 participants randomised to EVAR and 1390 randomised to OSR. There was no significant heterogeneity among trials (I2 = 7%). There was no significant difference in mortality at follow up, with 221 (15.8%) and 237 (17%) deaths in the EVAR and OSR groups, respectively (OR 0.92, 95% CI 0.75 to 1.12; P = 0.40) (Analysis 1.2).
1.2. Analysis.
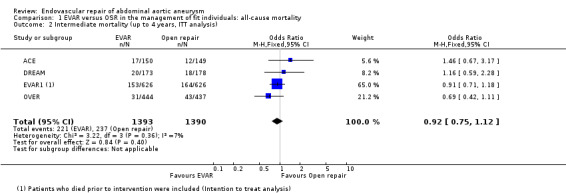
Comparison 1 EVAR versus OSR in the management of fit individuals: all‐cause mortality, Outcome 2 Intermediate mortality (up to 4 years, ITT analysis).
Long‐term mortality (comparison 1.3)
Long‐term mortality data (ITT analysis) were reported for the EVAR1, DREAM and OVER trials for 1243 participants randomised to EVAR and 1241 randomised to OSR. Heterogeneity among trials was minimal (I2 = 0%). There was no significant difference in long‐term mortality between groups, with 464 (37.3%) deaths in the EVAR group and 470 (37.8%) deaths in the OSR group (OR 0.98, 95% CI 0.83 to 1.15; P = 0.78) (Analysis 1.3).
1.3. Analysis.
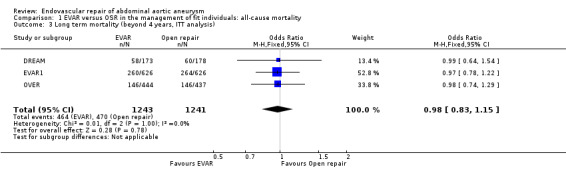
Comparison 1 EVAR versus OSR in the management of fit individuals: all‐cause mortality, Outcome 3 Long term mortality (beyond 4 years, ITT analysis).
Aneurysm‐related mortality
Short‐term aneurysm‐related mortality was not reported by all studies; intermediate‐ and long‐term results are presented only.
Intermediate AAA‐related mortality (comparison 2.1)
Intermediate (up to four years) AAA‐related mortality outcomes were reported by all four trials. There was moderate‐to‐high heterogeneity among trials (I2 = 53%); hence, a random‐effects model was used. At intermediate follow up, aneurysm‐related mortality was slightly lower in the EVAR group with 40 deaths (n = 1393) compared to 60 deaths in the OSR group (n = 1390) (OR 0.64, 95% CI 0.29 to 1.44; P = 0.28) (Analysis 2.1).
2.1. Analysis.
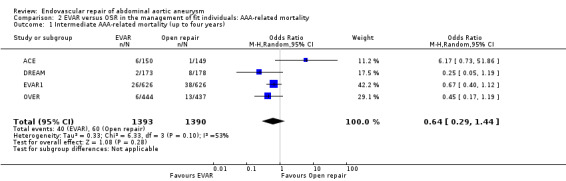
Comparison 2 EVAR versus OSR in the management of fit individuals: AAA‐related mortality, Outcome 1 Intermediate AAA‐related mortality (up to four years).
Long‐term AAA‐related mortality (comparison 2.2)
Long‐term follow‐up of the EVAR1, DREAM and OVER trials found no significant difference between the groups with regard to long‐term AAA‐related mortality (OR 0.74, 95% CI 0.50 to 1.08; P = 0.12) (Analysis 2.2). It is noteworthy that there were no deaths in the DREAM trial between the intermediate and final follow ups.
2.2. Analysis.
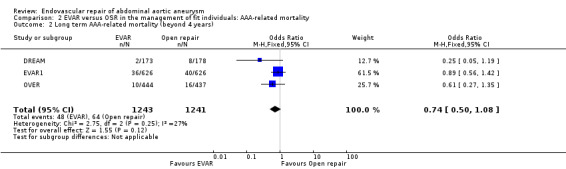
Comparison 2 EVAR versus OSR in the management of fit individuals: AAA‐related mortality, Outcome 2 Long term AAA‐related mortality (beyond 4 years).
AAA‐related reintervention rate
Reintervention rates were reported in all four trials. In the DREAM trial, the reintervention rate, up to nine months after randomisation, was almost three times higher in the EVAR group than in the OSR group (HR 2.9, 95% CI 1.1 to 6.2, P = 0.03); thereafter there was no difference between groups (HR 1.1, 95% CI 0.1 to 9.3, P = 0.95).
Reintervention at intermediate follow up (comparison 3.1)
Reintervention data at intermediate follow up were available for the EVAR1, OVER and ACE trials. There was significant heterogeneity among trials (I2 = 89%). The pooled estimate, using a random‐effects model, showed a significantly higher reintervention rate in the EVAR group than in the OSR group (OR 2.56, 95% CI 1.04 to 6.33; P = 0.04) (Analysis 3.1).
3.1. Analysis.
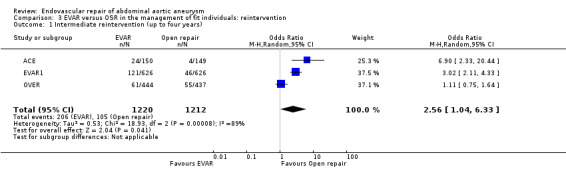
Comparison 3 EVAR versus OSR in the management of fit individuals: reintervention, Outcome 1 Intermediate reintervention (up to four years).
Reintervention at long‐term follow up (comparison 3.2)
Long‐term follow up data on reinterventions was available from the DREAM, EVAR1 and OVER trials. There was moderate‐to‐high heterogeneity among trials (I2 = 85%). The pooled estimate, using a random‐effects model, found that the reintervention rate was significantly higher in the EVAR group (23.4%) than in the OSR group (13.1%) (OR 1.98, 95% CI 1.12 to 3.51; P = 0.02) (Analysis 3.2).
3.2. Analysis.
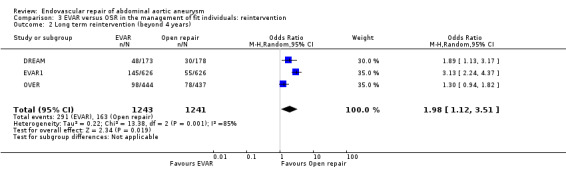
Comparison 3 EVAR versus OSR in the management of fit individuals: reintervention, Outcome 2 Long term reintervention (beyond 4 years).
It is important to note that the numbers presented are the numbers of individuals who needed reintervention rather than the numbers of reintervention procedures, as few individuals needed more than one intervention. In the OVER trial, 148 and 105 procedures were performed in 98 EVAR and 78 OSR participants, respectively.
Endograft‐related complications
See Analysis 4.1.
4.1. Analysis.
Comparison 4 EVAR versus OSR in the management of fit individuals: endograft‐related complications, Outcome 1 Endograft‐related complications.
| Endograft‐related complications | |||
|---|---|---|---|
| Study | Any complication | Endoleaks | Graft migration |
| ACE | 41 (N = 150) | (N = 150) Type I = 10 (7%) Type II = 31 (21%) |
Not reported |
| DREAM | 48 (N = 173) | (N = 173) Type I = 12 (7%) Type II = 8 (5%) |
7 (4%) |
| EVAR1 | 282 (N = 626) | (N = 529) Type I = 27 (5%) Type II = 79 (15%) Type III = 8 (1.5%) Unspecified = 4 (0.75%) |
12 (2%) |
| OVER | 110 (N = 444) | (N = 444) Overall = 110 (25%) |
Not reported |
The overall incidence of any endograft‐related complication, reported in all four trials, was 34.5% (n/N = 481/1393). The OVER and ACE trials reported only the number of endoleaks (leakage of blood in to the aneurysm sac) . The incidence of endoleak was reported in all four trials. Whereas the DREAM trial reported long‐term follow up data on endoleak, the EVAR1, OVER and ACE studies presented endoleak data at intermediate follow up. The OVER trial presented the overall number of endoleaks, whereas EVAR1, DREAM and ACE trials presented data on different types of endoleak.
Combining data from the DREAM, EVAR1 and ACE trials (n = 852), the incidences of type I and type II endoleaks were 6% (n = 49) and 14% (n = 118), respectively. Intervention was required in 80% (n = 39) and 28% (n = 33) of individuals with type I and type II endoleaks, respectively. In the OVER trial, the incidence of endoleaks was 25% (110 of 444 participants) and intervention was needed in only 16% (n = 18) of those with an endoleak.
Stent‐graft migration was reported in seven participants in DREAM and 12 participants in EVAR1. The overall known incidence of migration of the endovascular stent‐grafts is therefore 3% (n/N = 19/702).
Conversion to OSR after EVAR was reported in three participants in DREAM and seven participants in OVER trial. OSR after unsuccessful deployment of endovascular stent was required in 14 participants in the EVAR1 trial (n = 529) and two participants in the ACE trial (n = 150).
Major complications
Myocardial complications (comparison 5.1)
Cardiac deaths following EVAR (n = 1393) and OSR (n = 1390) were reported in the EVAR1, DREAM, ACE and OVER trials. There was no significant heterogeneity among trials (I2 = 0%). The pooled estimate showed no significant difference in the incidence of myocardial deaths between groups (OR 1.14, 95% CI 0.86 to 1.52; P = 0.36) (Analysis 5.1).
5.1. Analysis.
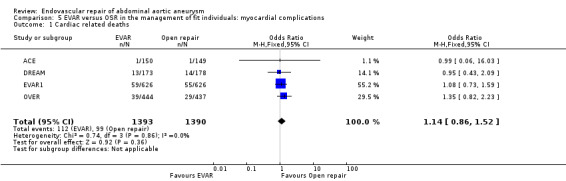
Comparison 5 EVAR versus OSR in the management of fit individuals: myocardial complications, Outcome 1 Cardiac related deaths.
Stroke (comparisons 6.1 and 6.2)
All four trials reported the incidence of stroke; DREAM reported fatal strokes, whereas OVER and ACE reported non‐fatal strokes. EVAR 1 reported both fatal and non‐fatal strokes.
For non‐fatal strokes, there was no significant heterogeneity among trials (I2 = 0%). The pooled estimate, using a fixed‐effect model, showed a similar incidence of stroke in both groups (OR 0.81, 95% CI 0.50 to 1.31; P = 0.39) (Analysis 6.1).
6.1. Analysis.
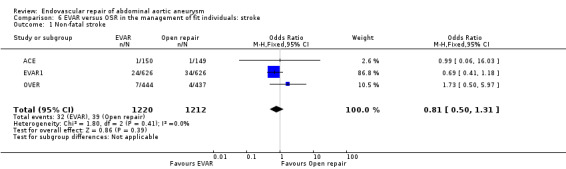
Comparison 6 EVAR versus OSR in the management of fit individuals: stroke, Outcome 1 Non‐fatal stroke.
There was no significant heterogeneity among trials reporting fatal strokes (I2 = 0%). The pooled estimate, using a fixed‐effect model, showed a similar incidence of stroke in both groups (OR 0.81, 95% CI 0.42 to 1.55; P = 0.52) (Analysis 6.2).
6.2. Analysis.
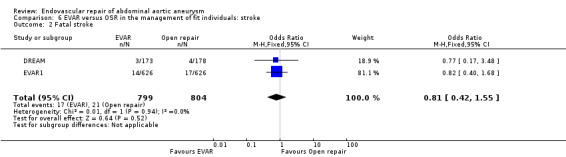
Comparison 6 EVAR versus OSR in the management of fit individuals: stroke, Outcome 2 Fatal stroke.
Pulmonary complications (comparisons 7.1 and 7.2)
The DREAM, EVAR1 and ACE trials reported pulmonary complications. DREAM and ACE reported significantly higher pulmonary complications in the OSR group than in the EVAR group (OR 0.36, 95% CI 0.17 to 0.75; P = 0.006). A similar trend (statistically insignificant) was noted when pulmonary‐related deaths were analysed (reported in the DREAM, EVAR1 and OVER trials) (OR 0.69, 95% CI 0.45 to 1.05; P = 0.08) (Analysis 7.1; Analysis 7.2).
7.1. Analysis.
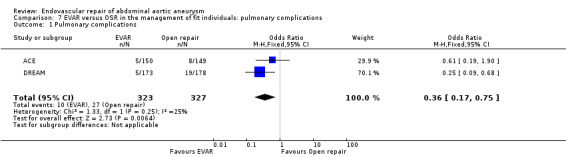
Comparison 7 EVAR versus OSR in the management of fit individuals: pulmonary complications, Outcome 1 Pulmonary complications.
7.2. Analysis.
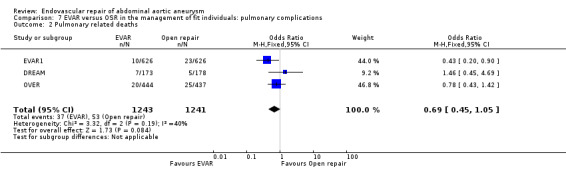
Comparison 7 EVAR versus OSR in the management of fit individuals: pulmonary complications, Outcome 2 Pulmonary related deaths.
Renal complications (comparison 8.1)
The EVAR1, OVER and ACE trials reported periprocedural renal complications: there was no significant difference in the number of participants requiring postoperative dialysis (OR 1.23, 95% CI 0.60 to 2.55; P = 0.57; Analysis 8.1). EVAR1 also reported renal‐related deaths, which were slightly higher in the EVAR group (10/626) than in the OSR group (3/626). The DREAM trial reported perioperative renal function showing there was no significant difference in change in creatinine levels between the EVAR and OSR groups (a 20% or greater increase in creatinine was noted in 13% (IQR 8% to 19%) and 13% (IQR 8% to 20%) of participants, respectively; P = 1.0). In the DREAM trial, two participants each in the EVAR and OSR groups experienced renal complications. To estimate long‐term renal function, EVAR1 trialists reported renal outcomes in those participants for whom baseline and at least one‐year follow‐up renal function data were available. Using per‐protocol analyses, 509 participants randomised to EVAR and 463 randomised to OSR were included in these analyses. Results showed no significant change in the estimated glomerular filtration rate (eGFR) between groups, with a mean (SD) difference from baseline of ‐1.13 (1.43) and ‐1.00 (1.43) mL/min/1.732/year in the EVAR and OSR groups, respectively (P = 0.275). The rate of deterioration in renal function following EVAR was estimated to be only 0.13 mL/min/1.732/year faster than that following OSR, and this difference was not statistically significant (EVAR1).
8.1. Analysis.
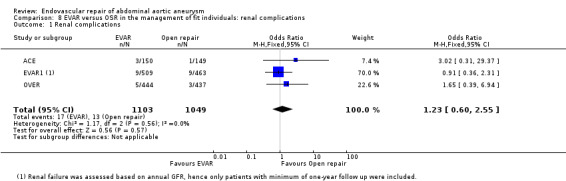
Comparison 8 EVAR versus OSR in the management of fit individuals: renal complications, Outcome 1 Renal complications.
Incisional hernia
A total of 14 (7.8%) participants in DREAM trial developed an incisional hernia after OSR; 48 (10.9%) participants required surgical repair of an incisional hernia in the OVER trial. The ACE trial reported incisional complications, which included wall dehiscence and large abdominal wall palsy, in 38participants undergoing OSR (35.5%). However, the study authors do not specify whether all such complications were hernias; further, they do not record the interventions required to address them. The EVAR1 trial also does not report the incidence of incisional hernias following OSR.
Bowel complications
In the DREAM trial, bowel ischaemia was noted in two participants in the OSR group and one participant in the EVAR group; both participants in the OSR group had severe ischaemia requiring bowel resection. A further participant in the OSR group underwent bowel resection for intestinal obstruction. In the OVER trial, 11 participants in the OSR group underwent laparotomy for bowel ischaemia or obstruction.
Minor complications
Two wound infections in the OSR group and one in the EVAR group were reported in the DREAM trial. The OVER trial reported 11 wound‐related complications in the EVAR group and four in the OSR group.
Sexual dysfunction
See Analysis 9.1.
9.1. Analysis.
Comparison 9 EVAR versus OSR in the management of fit individuals: sexual dysfunction, Outcome 1 Erectile dysfunction.
| Erectile dysfunction | |||
|---|---|---|---|
| Study | Erectile dysfunction measure | EVAR | OSR |
| ACE | Basic assessment ‐‐ no questionnaire used | 7 (4.7%) patients | 11 (7.4%) patients |
| OVER | 5‐item International Index of Erectile dysfunction (IIEF) (Follow up data presented a mean difference from baseline) |
Baseline: 11.4 (8.7) 1 year: ‐2.5 (8.3) 2 years: ‐3.0 (8.5) |
Baseline: 10.3 (8.8) 1 year: ‐2.3 (7.8) 2 year: ‐2.9 (8.5) |
Both the DREAM and OVER trials reported sexual function after surgery by estimating desire, erection, orgasm, engagement, and pleasure using the five‐item international index of erectile dysfunction (IIEF). Whereas DREAM reported scores for each domain at baseline, 3, 6, 13, 26 and 52 weeks, OVER reported total IIEF score at baseline, and one and two years postsurgery. Participants in the OSR and EVAR groups in both trials had comparable baseline scores. Both trials reported a slight, but not significant, deterioration in sexual function after both EVAR and OSR. The DREAM trial found such deterioration to be only transient and that function was restored to preoperative status in both the EVAR and OSR groups after three weeks and three months, respectively. The OVER trial reported the persistence of slightly, but not significantly, lower IIEF scores two years postsurgery in both groups. The ACE trial found no difference in sexual dysfunction between groups; however, this trial did not use sexual dysfunction scores.
Health‐related quality of life
See Analysis 10.1.
10.1. Analysis.
Comparison 10 EVAR versus OSR in the management of fit individuals: health‐related quality of life, Outcome 1 Quality of Life using SF‐36 and EQ‐5D.
| Quality of Life using SF‐36 and EQ‐5D | |||||
|---|---|---|---|---|---|
| Study |
QOL Measure |
Components | Baseline score EVAR | Baseline score OSR | Follow up scores |
| EVAR1 | SF‐36 | Mental Component Score (MCS) (Mean (SD)) |
EVAR: 43.59 (6.79) | OSR: 43.95 (6.73) |
EVAR 0‐3 months: 43.86 (7.02) EVAR 3‐12 months: 44.64 (6.67) EVAR 12‐24 months: 44.54 (6.43) OSR 0‐3 months: 44.04 (7.31) OSR 3‐12 months: 44.18 (6.81) OSR 12‐24 months: 44.76 (6.81) |
| EVAR1 | SF‐36 | Physical Component Score (PCS) (Mean (SD)) |
EVAR: 39.92 (5.92) | OSR: 39.83 (5.90) |
EVAR 0‐3 months: 37.82 (5.92) EVAR 3‐12 months: 37.77 (5.73) EVAR 12‐24 months: 38.17 (5.83) OSR 0‐3 months: 36.14 (5.45) OSR 3‐12 months: 37.81 (5.84) OSR 12‐24 months: 38.33 (5.78) |
| EVAR1 | EQ‐5D | Index score (Mean (SD)) |
EVAR: 0.75 (0.22) | OSR: 0.77 (0.23) |
EVAR 0‐3 months: 0.73 (0.21) EVAR 3‐12 months: 0.71 (0.25) EVAR 12‐24 months: 0.74 (0.24) OSR 0‐3 months: 0.67 (0.25) OSR 3‐12 months: 0.73 (0.23) OSR 12‐24 months: 0.75 (0.25) |
| OVER | SF‐36 | Mental Component Score (MCS) | EVAR: 50.6 (10.9) | OSR: 51.7 (10.4) |
EVAR 1 year: ‐0.77 (10.2) EVAR 2 years: ‐0.01 (10.0) OSR 1 year: ‐0 (10.0) OSR 2 years: ‐0.93 (9.8) (mean differences from baseline) |
| OVER | SF‐36 | Physical Component Score (PCS) | EVAR: 40.5 (10.4) | OSR: 40.1 (10.5) |
EVAR 1 year: ‐1.2 (9.8) EVAR 2 years: ‐2.2 (10.2) OSR 1 year: ‐1.2 (10.1) OSR 2 years: ‐2.0 (10.8) (mean differences from baseline) |
| OVER | EQ‐5D | Index score (Mean (SD)) |
EVAR: 0.79 (0.16) | OSR: 0.79 (0.16) |
EVAR 1 year: ‐0.02 (0.16) EVAR 2 years: ‐0.01 (0.19) OSR 1 year: ‐0 (0.17) OSR 2 years: ‐0.02 (0.16) (mean differences from baseline) |
HRQoL data were presented in three trials. Whereas DREAM presented data for HRQoL from baseline to one year postprocedure, the EVAR1 and OVER trials presented HRQoL data for two years postprocedure. Short‐Form 36 (SF‐36) and EuroQol 5 D (EQ‐5D) questionnaires were used to assess HRQoL. Whereas the DREAM trial presented data for individual SF‐36 domains, the EVAR1 and OVER trials presented mental component summary (MCS) and physical component summary (PCS) scores. Only the EVAR1 trial presented complete data for SF‐36 and EQ‐5D at all stages of follow up; in contrast,the DREAM and OVER trials presented the mean difference in scores at various stages of follow up compared with baseline. We were therefore unable to calculate a pooled estimate. In all the trials, baseline scores were comparable between both groups. Results from DREAM and OVER found EVAR to have a slight advantage over OSR in the first few weeks after surgery, with no difference noted after three months. All three trials showed no significant difference in HRQoL between EVAR and OSR at one year of follow up.
Economic analysis
In all the trials, operative time, blood loss, intensive care unit and total hospital stay were significantly lower in the EVAR group compared with the OSR group (ACE; DREAM; EVAR1; OVER). See Analysis 11.1 for specific data on length of hospital stay (P < 0.001). The EVAR1 trial reported the mean cost of primary aneurysm repair to be GBP13,019 for EVAR and GBP11,482 for OSR; mean aneurysm‐related readmission costs were GBP2283 for EVAR and GBP442 for OSR. The overall estimated costs over an eight‐year period were higher for EVAR than for OSR (mean difference GBP3019 in favour of OSR). More recently, a cost‐effectiveness analysis of the OVER trial at two years from intervention showed no significant difference in costs for EVAR (OR ‐USD5019, 95% CI ‐USD16,720 to USD4928; P = 0.35).
11.1. Analysis.
Comparison 11 EVAR versus OSR in the management of fit individuals: length of hospital stay, Outcome 1 Length of hospital stay.
| Length of hospital stay | |||
|---|---|---|---|
| Study | EVAR | OSR | P value |
| ACE | 5.8 ± 5.5 days (mean ± SD) | 10.4 ± 8.3 days (mean ± SD) | < 0.0001 |
| DREAM | 6 days (mean) | 13 days (mean) | < 0.001 |
| EVAR1 | 10.3 ± 17.8 days (mean ± SD) | 15.7 ± 16.9 days (mean ± SD) | < 0.001 |
| OVER | 3.0 days (2.0 to 5.0) (mean/range) | 7.0 days (6.0 ‐ 10.0) (mean/range) | < 0.001 |
EVAR versus no intervention in participants unfit for surgery
Only one RCT (EVAR2) evaluated the role of EVAR in individuals considered unfit for conventional OSR of AAA. Participants were allocated to EVAR or to no intervention. As a meta‐analysis was not performed, trial results are presented as hazard ratios (HR).
Short‐ and long‐term mortality
Among participants randomised to EVAR, short‐term mortality was 8.4% (15/175). A total of 64 participants randomised to the no‐intervention group underwent surgical repair, with short‐term mortality rate of 4.3% (3/64). At final follow up, 145 participants in the EVAR group and 160 participants in the no‐intervention group had died.
There was no difference between groups in all‐cause mortality at final follow‐up, with 21.0 deaths per 100 person‐years in the EVAR group and 22.1 deaths per 100 person years in the no‐intervention group (adjusted HR with EVAR 0.99, 95% CI 0.78 to 1.27; P = 0.97). Aneurysm‐related deaths were, however, significantly higher in the no‐intervention group than in the EVAR group (adjusted HR 0.53, 95% CI 0.32 to 0.89; P = 0.02) (EVAR2).
Endograft‐related complications and reinterventions
Some 158 graft‐related complications, such as endoleak, infection, stenosis, migration, thrombosis, rupture and kinking, were reported in 97 participants, for which reintervention was performed in 55 participants.
Major complications
There were no significant differences in myocardial or stroke‐related complications between the EVAR and no‐intervention groups. Of the 319 participants who complied with the randomisation allocation, a cardiovascular event occurred in 19% (n = 33) and 15% (n = 22) in the EVAR and no‐intervention groups, respectively (HR 1.07, 95% CI 0.60 to 1.91; EVAR2). Under ITT analysis, there were 14 fatal and 10 non‐fatal myocardial events in the EVAR group (n = 197), compared with 20 fatal and 2 non‐fatal myocardial infarctions in the no‐intervention group. Twelve participants in the EVAR group and nine in the no‐intervention group experienced a stroke, which was fatal in five and three participants, respectively.
Health‐related quality of life
SF‐36 and EQ‐5D questionnaires were used to assess HRQoL at baseline, zero to three, three to 12 and 12 to 24 months. Both groups had comparable scores at baseline and at 12 to 24 months of follow up. Participants in the no‐intervention group had slightly better values for the SF‐36 PCS (P = 0.04) at zero to three months, but by 12 months there was no significant difference between groups (EVAR2).
Economic analysis
The mean cost of AAA repair was GBP13,301 in those undergoing EVAR and GBP4467 in the no‐intervention group (according to the study authors fewer participants underwent repair therefore mean costs were lower). Overall eight‐year aneurysm‐related admission costs were GBP14,995 in the EVAR group and GBP5169 in the no‐intervention group (mean difference GBP9826, 95% CI 7638 to 12,013; EVAR2).
Discussion
Summary of main results
The results of this meta‐analysis of high‐quality randomised trials found EVAR to be associated with a significantly lower short‐term mortality than OSR (OR 0.33, 95% CI 0.20 to 0.55; P < 0.0001) in individuals considered fit for conventional surgery. Participants who died before surgery or those who did not undergo surgery were excluded from the analysis of short‐term outcomes as we could not determine the timing of the deaths prior to intervention; these individuals were considered in the analyses of intermediate‐ and long‐term outcomes. The short‐term mortality rate reported for the EVAR group is similar to the 1.3% noted in the Registry of Endovascular Treatment of Aneurysms (RETA), a large UK‐based endovascular registry containing data on 3159 individuals (Thomas 2005). Although EVAR was associated with a significantly lower short‐term mortality than OSR, this benefit did not persist at intermediate‐ and long‐term follow up. ITT analyses found no difference in all‐cause mortality at intermediate‐ and long‐term follow up between the two groups (P = 0.40 and 0.78, respectively). The early benefit from EVAR is annulled by the number of late deaths from cardiac and other unrelated causes. Although the EVAR1 trialists, in an interim publication (EVAR1 2005), noted a significantly lower aneurysm‐related mortality at intermediate follow up with EVAR than with OSR (HR 0.55, 95% CI 0.31 to 0.96; P = 0.04), such a difference was not reported in their final analysis. Similarly, we found no significant difference in aneurysm‐related mortality between EVAR and OSR. One possible reason for this could be the occurrence of late ruptures after EVAR, as reported in the EVAR1 trial.
Although an initial operative survival benefit was apparent with EVAR compared with OSR, we found no difference in the observed rate of complications between the EVAR and OSR groups. Both groups had similar incidences of strokes and renal impairment. Although we found no significant difference in the overall incidence of cardiac deaths between the EVAR and OSR groups, data from the EVAR1 trial found EVAR to be associated with a lower rate of cardiovascular deaths within six months of randomisation compared with OSR (adjusted HR 0.52, 95% CI 0.30 to 0.91; P = 0.021). This finding suggests that EVAR is associated with less cardiac stress in the early peri‐ and postoperative periods than OSR. No trial found a significant difference in HRQoL or sexual dysfunction between groups. Although there was a slightly higher incidence of pulmonary complications in the OSR group than in the EVAR group (P = 0.006), death from pulmonary causes did not differ significantly between groups (P = 0.08).
Endovascular stent‐graft‐related complications led to a higher rate of reintervention in the EVAR group compared with the OSR group, and laparotomy‐related reinterventions were the most common type of reintervention. However, not all trials recorded reintervention outcomes, and in most trials, participants randomised to OSR were not regularly followed up, which may have led to bias. The results of a recent Medicare study (Giles 2011) support the findings of our meta‐analysis. The authors of this case‐matched study involving 45,652 individuals concluded that the number of AAA‐related reinterventions over a six‐year follow up was significantly higher with EVAR than with OSR.
EVAR was associated with a shorter operative time and intensive care stay, and less blood loss than OSR, which may have a bearing on any cost‐effectiveness analyses. It would seem that any costs of reintervention following EVAR could be balanced by the initial savings from a reduced operative time, less need for transfusion and a shorter hospital stay, as well as the costs of reinterventions for the treatment of incisional hernias following OSR. The OVER trial showed that at two years there was no significant difference in costs between the two interventions. However, the EVAR1 trial, which reported long‐term cost‐effectiveness, found EVAR to be more expensive than OSR at an eight‐year follow up.
Evidence for the use of EVAR in individuals considered unfit for surgery could only be drawn from a single RCT ‐ the EVAR2 trial. Though there was no significant difference in long‐term all‐cause mortality between the EVAR and no‐intervention groups, higher numbers of aneurysm‐related deaths were noted in the no‐intervention group compared with the EVAR group, which could be attributed to the higher number of ruptures in this group before undergoing repair. The trial found no significant differences in myocardial or stroke‐related complications between groups. Both groups also had comparable HRQoL scores at baseline and at 12 to 24 months of follow up. However, EVAR was associated with a higher reintervention rate, making it more expensive than no intervention.
However, the EVAR2 trial has a number of limitations. First, the assessment of individuals as fit or unfit for surgery was based on factors such as their cardiac, respiratory and renal function (EVAR2). Participants were considered for the EVAR2 trial if they were not suitable for open surgery because of cardiac conditions, such as severe cardiac valve disease, significant arrhythmia or uncontrolled congestive cardiac failure; respiratory symptoms, an inability to walk a flight of stairs, a forced expiratory volume (FEV1) less than 1.0 L, a PO2 less than 8.0 kPa or a PCO2 greater than 6.5 kPa; or renal impairment, with serum creatinine levels greater than 200 µmol/L (EVAR2). Despite this guidance, participant recruitment was left entirely to the discretion of the individual participating centre, which could have introduced significant bias in the study cohort. Further, in current UK practice, a preoperative checklist is used to record major comorbidities or symptoms, which are graded in a manner similar to the UK traffic light system as red, amber or green. For those individuals falling in the red or amber categories, the appropriate specialty opinion is sought and considered for the preoperative optimisation of their comorbidities. Individuals in the green category will undergo cardiac and respiratory function tests, either individual tests or as part of cardiopulmonary exercise testing, to determine their fitness for surgery (AAAQIP 2011). As current practice for the assessment of individual fitness differs significantly from that used when the EVAR2 trial was conducted (1994 to 2004), the participants selected for the EVAR2 trial may not be representative of individuals who would currently be considered unfit for surgery. Secondly, there was a notable delay in participants undergoing an intervention after randomisation in EVAR2, during which some died due to rupture of the aneurysm. This contributed to an increased early mortality rate in the EVAR group. Finally, a notable number of participants in the no‐intervention group underwent surgical intervention, which could have balanced the odds between the two group; hence the NICE guideline on endovascular stent‐grafts considered the evidence from the EVAR2 trial as "not definitive" (NICE EVAR 2012).
Overall, the results of this meta‐analysis show an early survival benefit for EVAR compared with OSR and 'no‐intervention', with no significant long‐term benefit and higher costs for EVAR. The results of this analysis should be interpreted with caution, however, especially the need for reintervention, due to the identified heterogeneity. Although a higher reintervention rate was seen with EVAR than with OSR, most such reinterventions were minor catheter‐based interventions associated with low mortality. Further, incisional hernia repair post‐OSR was not recorded in the EVAR1 and ACE trials. Hence, reintervention rates reported for the OSR group may not be accurate. In contrast, in the OVER trial, the incidence of incisional hernias and the interventions to correct them did not differ significantly between groups (P = 0.26). Most reinterventions in the EVAR and OSR groups were secondary to endoleaks and incisional hernias, respectively. It may be argued that reintervention following EVAR may partially be due to the type of stent‐grafts used. However, both the EVAR1 and DREAM trials used second‐ and third‐generation devices, whereas the OVER and ACE trials used third‐ and fourth‐generation devices.
Overall completeness and applicability of evidence
This review addressed the role of EVAR as an alternative to OSR in individuals considered fit for surgery (i.e. individuals in whom surgery is considered a low‐to‐medium‐risk procedure) and as an alternative to conservative management in those considered unfit (i.e. individuals in whom surgery is considered a high‐risk procedure) for surgery. Most outcome measures assessing morbidity and mortality were available from the trials included in the review. However, not all trials provided data on cost‐effectiveness, sexual dysfunction and reintervention after OSR. Taking these minor elements into consideration, this review shows that there is good‐quality evidence available to assess the role of EVAR.
Quality of the evidence
This review included five high‐quality RCTs that followed strict randomisation techniques and maintained allocation concealment. All trials reported on an ITT basis and took care to minimise the risk of reported or outcome bias. Therefore, the results from the meta‐analysis of the above trials can be considered valid and reliable.
Potential biases in the review process
Three reviewers (SCVP, RJ, RC) independently assessed the articles for inclusion and exclusion criteria, thereby minimising the risk of any potential selection bias. All data were extracted using pro forma developed by the Cochrane PVD Group. Two authors (SCVP and RJ) performed data analyses and the senior author (ST) independently checked all the results before final assessment and drafting of the manuscript.
Agreements and disagreements with other studies or reviews
The results of this meta‐analysis show that EVAR is associated with a significant lower short‐term mortality than OSR, a finding supported by data from RETA (Thomas 2005). A recent meta‐analysis has also reported EVAR to have a short‐term advantage over OSR (Dangas 2012); however this meta‐analysis also found EVAR to have an intermediate‐term advantage with regard to AAA‐related mortality compared with OSR, a finding not supported by the current meta‐analysis; this finding may be attributed to differences in the definitions used to describe an intermediate follow up in these two studies (two years in the Dangas 2012 meta‐analysis compared with four years in our review). In line with the results of a large Medicare study involving 45,652 individuals (Giles 2011), we also found reintervention rates to be significantly higher after EVAR than after OSR. Hence, the results of our meta‐analysis are a reflection of clinical practice.
More recently, a meta‐analysis comparing the perioperative and long‐term mortality of OSR versus EVAR has been published and these results concur with those of this review in that EVAR has a short‐term benefit over OSR, with no difference in outcomes in the longer term (Karthikesalingam 2013).
Authors' conclusions
Implications for practice.
In individuals considered fit for conventional surgery, EVAR was associated with lower short‐term mortality than OSR. All‐cause mortality with EVAR was significantly lower than that with OSR in the short term, with the benefit disappearing in the intermediate and long term. Operative complications, HRQoL and sexual dysfunction were generally comparable between EVAR and OSR. However, there was a slightly higher incidence of pulmonary complications in the OSR group compared with the EVAR group. EVAR was associated with a higher reintervention rate than OSR. Most reinterventions with EVAR, however, were catheter‐based interventions associated with low mortality.
In individuals considered unfit for open surgery, the results of a single trial found no short‐ or long‐term benefits of EVAR over no intervention with regard to all‐cause mortality, but individual outcomes may differ and individual preferences should always be taken into account.
Implications for research.
Although EVAR showed an early survival benefit over OSR, stent‐graft durability is an area of interest and studies presenting long‐term results of newer devices would be useful in addressing this issue. Estimating the cost‐effectiveness of EVAR is another key issue; hence, studies looking at the long‐term outcomes of EVAR are essential. Although further randomised trials may not reveal more about the short‐term benefit of EVAR over OSR, controlled trials evaluating the role of new‐generation stent‐grafts may prove useful.
Follow up of EVAR is an area of significant interest. Currently, different investigative modalities use varying follow‐up strategies. Research into identifying the most reliable follow‐up strategy and identifying the right investigative modality may help determine the benefit of EVAR in the long term. These findings will be of great value when calculating the cost‐effectiveness of EVAR.
History
Protocol first published: Issue 2, 2003 Review first published: Issue 1, 2014
| Date | Event | Description |
|---|---|---|
| 10 November 2008 | Amended | Converted to new review format. |
Acknowledgements
The authors would like to thank Professor Stephen Walters, Professor of Medical Statistics and Clinical trials, School of Health and Related Research (ScHARR), University of Sheffield, for his invaluable statistical support.
The authors would like to thank Dr Karen Welch, Trials Search Co‐ordinator, Cochrane PVD Group, and Dr Cathryn Broderick, Assistant Managing Editor, Cochrane PVD Group, for their invaluable support.
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MeSH descriptor: [Endovascular Procedures] explode all trees | 5267 |
| #2 | MeSH descriptor: [Stents] explode all trees | 2939 |
| #3 | MeSH descriptor: [Vascular Surgical Procedures] this term only | 617 |
| #4 | MeSH descriptor: [Blood Vessel Prosthesis] explode all trees | 432 |
| #5 | MeSH descriptor: [Blood Vessel Prosthesis Implantation] this term only | 456 |
| #6 | endovasc*:ti,ab,kw | 688 |
| #7 | endostent*:ti,ab,kw | 1 |
| #8 | stent*:ti,ab,kw | 4355 |
| #9 | EVAR:ti,ab,kw | 63 |
| #10 | EVRAR:ti,ab,kw | 1 |
| #11 | percutaneous:ti,ab,kw | 5781 |
| #12 | TEVAR:ti,ab,kw | 9 |
| #13 | (endoprosthe* or endograft*):ti,ab,kw | 206 |
| #14 | Palmaz:ti,ab,kw | 91 |
| #15 | Zenith or Dynalink or Hemobahn or Luminex* or Memotherm or Wallstent:ti,ab,kw | 105 |
| #16 | Viabahn or Nitinol or Hemobahn or Intracoil or Tantalum:ti,ab,kw | 116 |
| #17 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 | 12362 |
| #18 | MeSH descriptor: [Aortic Aneurysm] explode all trees | 693 |
| #19 | aneurysm* near/4 (abdom* or thoracoabdom* or thoraco‐abdom* or aort*) | 996 |
| #20 | (aort* near/3 (ballon* or dilat* or bulg*)) | 50 |
| #21 | AAA | 389 |
| #22 | #18 or #19 or #20 or #21 | 1151 |
| #23 | #17 and #22 in Trials | 259 |
Data and analyses
Comparison 1. EVAR versus OSR in the management of fit individuals: all‐cause mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short term mortality (30‐day or in‐hospital) (excluding participants who died before surgery and those who did not undergo any intervention) | 4 | 2723 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.20, 0.55] |
| 2 Intermediate mortality (up to 4 years, ITT analysis) | 4 | 2783 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.75, 1.12] |
| 3 Long term mortality (beyond 4 years, ITT analysis) | 3 | 2484 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.83, 1.15] |
Comparison 2. EVAR versus OSR in the management of fit individuals: AAA‐related mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Intermediate AAA‐related mortality (up to four years) | 4 | 2783 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.29, 1.44] |
| 2 Long term AAA‐related mortality (beyond 4 years) | 3 | 2484 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.50, 1.08] |
Comparison 3. EVAR versus OSR in the management of fit individuals: reintervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Intermediate reintervention (up to four years) | 3 | 2432 | Odds Ratio (M‐H, Random, 95% CI) | 2.56 [1.04, 6.33] |
| 2 Long term reintervention (beyond 4 years) | 3 | 2484 | Odds Ratio (M‐H, Random, 95% CI) | 1.98 [1.12, 3.51] |
Comparison 4. EVAR versus OSR in the management of fit individuals: endograft‐related complications.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Endograft‐related complications | Other data | No numeric data |
Comparison 5. EVAR versus OSR in the management of fit individuals: myocardial complications.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cardiac related deaths | 4 | 2783 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.86, 1.52] |
Comparison 6. EVAR versus OSR in the management of fit individuals: stroke.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐fatal stroke | 3 | 2432 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.50, 1.31] |
| 2 Fatal stroke | 2 | 1603 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.42, 1.55] |
Comparison 7. EVAR versus OSR in the management of fit individuals: pulmonary complications.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pulmonary complications | 2 | 650 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.17, 0.75] |
| 2 Pulmonary related deaths | 3 | 2484 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.45, 1.05] |
Comparison 8. EVAR versus OSR in the management of fit individuals: renal complications.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Renal complications | 3 | 2152 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.60, 2.55] |
Comparison 9. EVAR versus OSR in the management of fit individuals: sexual dysfunction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Erectile dysfunction | Other data | No numeric data |
Comparison 10. EVAR versus OSR in the management of fit individuals: health‐related quality of life.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of Life using SF‐36 and EQ‐5D | Other data | No numeric data |
Comparison 11. EVAR versus OSR in the management of fit individuals: length of hospital stay.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length of hospital stay | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ACE.
| Methods |
Study design: Multicentre RCT Method of randomisation: Stratified by centre Exclusions post randomisation: 7 (due to withdrawal from consent) Losses to follow up: 8 (EVAR = 3, OSR = 5) Intention‐to‐treat analysis: Yes |
|
| Participants |
Country: France Setting: Hospital (25 centres) Recruitment: 2003 to 2008 Number: 306 (149 OSR, 150 EVAR) Age: 69 ± 7 years (mean) Sex: 296 male / 3 female Inclusion criteria: Consisted of both anatomical criteria and clinical assessment. ‐ Anatomical criteria (based on CT scan findings):
‐ Clinical assessment:
Exclusion criteria:
|
|
| Interventions |
Treatment: EVAR Control: OSR |
|
| Outcomes |
Primary: All‐cause mortality, major adverse events (myocardial infarction, permanent stroke, permanent haemodialysis, major amputation, paraplegia and bowel infarction) Secondary: Vascular reinterventions and minor complications |
|
| Notes | Trial was conducted in individuals considered to be at low‐to‐medium risk of surgery Reinterventions for incisional hernia repair were not recorded In the EVAR group, 4 participants each had surgery under local and epidural anaesthesia. Remainder all had surgery under general anaesthesia |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation done based on centre |
| Allocation concealment (selection bias) | Low risk | Allocation only notified < 24 hours |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and operating surgeons not feasible in such a study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear data available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Presented results based on intention‐to‐treat and presented final follow up results. All participants accounted for |
| Selective reporting (reporting bias) | Low risk | Reported on all predefined outcomes |
| Other bias | Low risk | None identified |
DREAM.
| Methods |
Study design: Multicentre RCT Method of randomisation: Computer‐generated permuted block sequence; in blocks of four Exclusions post randomisation: 6 participants did not undergo aneurysm repair following randomisation. 4 declined procedure (3 OSR vs 1 EVAR), 1 died from ruptured AAA before repair (OSR) and 1 died from pneumonia (EVAR) Losses to follow up: None Intention‐to‐treat analysis: Yes |
|
| Participants |
Country: The Netherlands (26 centres) and Belgium (4 centres) Setting: Hospital Recruitment: November 2000 to December 2003 Number: 351 (EVAR = 173; OSR = 178) Age: Mean 70.1 years Sex: Male:female 10:1 Inclusion criteria: AAA of at least 5 cm in diameter Exclusion criteria: Participants requiring emergency repair, or participants with inflammatory aneurysms, presence of anatomical variations, connective tissue disease, history of organ transplant, or life expectancy < 2 years |
|
| Interventions |
Treatment: EVAR Control: OSR |
|
| Outcomes | All‐cause mortality; aneurysm‐related mortality; complications; reintervention rate | |
| Notes | Participants in both groups were followed up regularly for two years and were subsequently sent questionnaires about health. During year 3 and 4, only EVAR participants had a follow‐up visit organised, whereas OSR group participants were advised to see their respective physicians. Five years post randomisation, all participants were contacted by telephone | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization carried out centrally with the use of a computer‐generated permuted‐block sequence and stratified according to study centre in blocks of four patients." |
| Allocation concealment (selection bias) | Low risk | Adequate randomisation technique |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and operating surgeons not feasible in such a study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis was intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | Reported on all predefined outcomes |
| Other bias | Low risk | None identified |
EVAR1.
| Methods |
Study design: Multicentre RCT Method of randomisation: Permuted block randomisation Exclusions post randomisation: 37 participants excluded. 31 died before surgery (EVAR = 12; OSR = 19); 5 refused surgery (all OSR), 2 postponed surgery (EVAR = 1; OSR = 1) Losses to follow up: 17 (EVAR = 9; OSR = 8) Intention‐to‐treat analysis: Yes |
|
| Participants |
Country: UK Setting: Hospital (37 centres) Recruitment: 1 September 1999 to 31 August 2004 Number: 1252 (EVAR = 626; OSR = 626) Age: Mean (SD) = 74.1 (6.1) years Sex: Male:female = 10:1 Inclusion criteria: Aged ≥ 60 years with AAA ≥ 5.5 cm in any plane, assessed by CT Exclusion criteria: Participants unsuitable for EVAR or unfit for operation |
|
| Interventions |
Treatment: EVAR Control: OSR |
|
| Outcomes |
Primary: All‐cause mortality Secondary: Aneurysm‐related mortality; incidence of postoperative complications; secondary interventions; HRQoL and hospital costs and durability |
|
| Notes | Results from this trial were published at different stages of recruitment and follow up. Data for outcomes were taken from the most relevant publication and this is reflected in the total number of participants for some outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation is performed for each trial using a 50:50 ratio randomly permuted block sizes constructed by the STATA package. Randomisation is stratified by centre and is performed centrally by the trial manager only when all necessary baseline data had been received at the trial co‐ordinating centre" |
| Allocation concealment (selection bias) | Low risk | Allocation was done only after all baseline data were recorded |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and operating surgeons not feasible in such a study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear data available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis was intention‐to‐treat basis; all participants accounted for |
| Selective reporting (reporting bias) | Low risk | Reported on all predefined outcomes |
| Other bias | Low risk | None identified |
EVAR2.
| Methods |
Study design: Multicentre RCT Method of randomisation: Permuted block randomisation Exclusions post randomisation: EVAR = 18 (died before undergoing repair) Within EVAR randomisation group: 7 died, 8 became ineligible, 1 refused surgery and 2 for unknown reasons Within the no‐intervention group: 70 participants had EVAR Losses to follow up: 1 (no‐intervention group) Intention‐to‐treat analysis: Yes |
|
| Participants |
Country: UK Setting: Hospital (33 centres) Recruitment: 1 September 1999 to 31 August 2004 Number: 404 (EVAR = 197; no intervention = 207) Age: Mean (SD) = 76.8 (6.5) years Sex: Male:female = 6:1 Inclusion criteria: Aged ≥ 60 years with AAA ≥ 5.5 cm, assessed by CT scan and deemed unfit for surgery locally by surgeon, radiologist, anaesthetist and cardiologist Exclusion criteria: MI within last 3 months, onset of angina within last 3 months, unstable angina at night or at rest |
|
| Interventions |
Treatment: EVAR Control: No intervention |
|
| Outcomes |
Primary: All‐cause mortality Secondary: Aneurysm‐related death; HRQoL; postoperative complications; hospital costs |
|
| Notes | Results from this trial were published at different stages of recruitment and follow up. Data for outcomes were taken from the most relevant publication and this is reflected in the total number of participants for outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation is performed for each trial using a 50:50 ratio randomly permuted block sizes constructed by the STATA package. Randomisation is stratified by centre and is performed centrally by the trial manager only when all necessary baseline data had been received at the trial co‐ordinating centre". |
| Allocation concealment (selection bias) | Low risk | Allocation was done only after all baseline data were recorded |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and operating surgeons not feasible in such a study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information not clear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis was intention‐to‐treat basis; all participants accounted for |
| Selective reporting (reporting bias) | Low risk | Reported on all predefined outcomes |
| Other bias | Low risk | None identified |
OVER.
| Methods |
Study design: Multicentre RCT Method of randomisation: permuted block design Exclusions post randomisation: EVAR = 17 (2 refused, 2 died, 1 repair aborted, 12 had OSR); OSR = 21 (4 refused, 1 died, 3 aborted, 13 had EVAR) Losses to follow up: 2 (both in EVAR group) Intention‐to‐treat analysis: Yes |
|
| Participants |
Country: USA Setting: Hospital (42 centres) Recruitment: 15 October 2002 to 15 October 2008 Numbers: 881 (EVAR = 444; OSR = 437) Mean age (SD): EVAR = 69.6 years (7.8); OSR = 70.5 years (7.8) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Treatment: EVAR Control: OSR |
|
| Outcomes |
Primary: Long‐term all‐cause mortality Secondary: 1) Procedure failure 2) Short‐term major morbidity (i.e. myocardial infarction, stroke, amputation or renal failure requiring dialysis) 3) Inhospital and intensive care unit stay 4) Other complications such as incisional hernia or claudication 5) HRQoL 6) Erectile dysfunction |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by 'permuted block design' |
| Allocation concealment (selection bias) | Low risk | Allocation was made only after baseline information was obtained and eligibility verified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and operating surgeons not feasible in such a study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were adjudicated by a blinded outcomes assessment committee |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis was intention‐to‐treat basis; all participants accounted for |
| Selective reporting (reporting bias) | Low risk | Reported on all predefined outcomes |
| Other bias | Low risk | None identified |
AAA: abdominal aortic aneurysmCT: computed tomography EVAR: endovascular aneurysm repair HRQoL: health‐related quality of life MRI: magnetic resonance imaging OSR: open surgical repair RCT: randomised controlled trial SD: standard deviation SVS/AAVS: Society for Vascular surgery/American Association of Vascular Surgery
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| CAESAR Trial | RCT comparing surveillance and selective surgical treatment for abdominal aortic aneurysm less than 5.5 cm in diameter versus early endovascular treatment. Excluded as EVAR was compared with surveillance but not open surgery but trial was not conducted in 'unfit' individuals. In addition the aneurysms were of small diameter. |
| Cuypers 2001 | A small RCT comparing cardiac response between EVAR and OSR. The randomisation method and allocation concealment were unclear. Mortality and long‐term outcomes were not the primary endpoints. Hence, the trial may not be powered to determine the outcomes considered for this review. |
| ECAR study 2010 | RCT comparing EVAR versus OSR in participants with ruptured aorto‐iliac aneurysms. |
| IMPROVE trial | RCT comparing EVAR versus OSR in participants with ruptured AAA. |
| Lottman 2004 | This study randomised participants in a 3:1 format, where 57 participants were randomised to EVAR and 19 to OSR. Further they present health‐related quality of life outcomes estimated at 3 months post surgery. The numbers of participants considered for study, especially in the OSR group, were small and the outcomes were estimated at 3 months only. Therefore, the results may not be a true representative of the effects of the interventions and hence the study was excluded from analysis. |
| PIVOTAL Trial | Study determined whether repair of small aneurysms is superior to surveillance with respect to the frequency of rupture or aneurysm‐related death. |
| Soulez 2005 | A small RCT (n = 40), comparing pain and quality of life in participants undergoing EVAR with those undergoing OSR. Randomisation method and allocation concealment were unclear. Mortality and long‐term outcomes were not the primary endpoints. |
EVAR: endovascular aneurysm repair OSR: open surgical repair RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NExT ERA.
| Trial name or title | NExT ERA: National Expertise Based Trial of Elective Repair of Abdominal Aortic Aneurysms: A Pilot Study |
| Methods | |
| Participants | All participants with an AAA considered to require non‐urgent repair after assessment by one of the participating surgeons will be considered. |
| Interventions | Open surgical repair or EVAR treatment of AAA |
| Outcomes | Mortality from the time of randomisation until hospital discharge or 30 days after surgery Non‐fatal myocardial infarction End‐organ ischaemic event rates (including renal failure, limb ischaemia, bowel ischaemia, non‐fatal stroke) Reintervention Quality of life Success of repair Mortality at 6 months |
| Starting date | September 2006 |
| Contact information | Tara M Mastracci, MD, FRCSC |
| Notes | NCT00358085: Not yet recruiting, no verification of information since 2006 |
AAA: abdominal aortic aneurysm EVAR: endovascular aneurysm repair
Differences between protocol and review
We investigated the risk of bias using the 'Risk of bias' tool developed by The Cochrane Collaboration (Higgins 2011), and not by the methods described by Jadad and Schulz, as originally planned in the protocol for this review (Thomas 2002). We have divided the types of outcomes measured into primary and secondary outcomes, in keeping with the most recent Cochrane recommendations. We have further defined and clarified outcomes to reflect current clinical relevance.
Contributions of authors
Sharath Paravastu (SP), Rubaraj Jayarajasingam (RJ) and Rachel Cottam (RC) identified all possible trials, considered them for inclusion, and assessed trial quality. SP, RJ and RC extracted the data. Simon Palfreyman contributed to the study protocol, data analysis and draft of the manuscript. Steve Thomas (ST) and Jonathan Michaels contributed to all stages of the review. ST takes overall responsibility for this work.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The PVD Group editorial base is supported by the Chief Scientist Office.
-
National Institute for Health Research (NIHR), UK.
The PVD Group editorial base is supported by a programme grant from the NIHR.
Declarations of interest
None known.
New
References
References to studies included in this review
ACE {published data only}
- Becquemin JP. The ACE trial: a randomized comparison of open versus endovascular repair in good risk patients with abdominal aortic aneurysm. Journal of Vascular Surgery 2009;50(1):222‐4. [DOI] [PubMed] [Google Scholar]
- Becquemin JP, Pillet JC, Lescalie F, Sapoval M, Goueffic Y, Lermusiaux P, et al. A randomized controlled trial of endovascular aneurysm repair versus open surgery for abdominal aortic aneurysms in low‐ to moderate‐risk patients. Journal of Vascular Surgery 2011;53(5):1167‐73. [DOI] [PubMed] [Google Scholar]
DREAM {published data only}
- Baas AF, Janssen KJ, Prinssen M, Buskens E, Blankensteijn JD. The Glasgow Aneurysm Score as a tool to predict 30‐day and 2‐year mortality in the patients from the Dutch Randomized Endovascular Aneurysm Management trial. Journal of Vascular Surgery 2008;47(2):277‐81. [DOI] [PubMed] [Google Scholar]
- Blankensteijn JD, Jong SE, Prinssen M, Ham AC, Buth J, Sterkenburg SM, et al. Two‐year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. New England Journal of Medicine 2005;352(23):2398‐405. [DOI] [PubMed] [Google Scholar]
- Bruin JL, Baas AF, Buth J, Prinssen M, Verhoeven EL, Cuypers PW, et al. Long‐term outcome of open or endovascular repair of abdominal aortic aneurysm. New England Journal of Medicine 2010;362(20):1881‐9. [DOI] [PubMed] [Google Scholar]
- Prinssen M, Buskens E, Blankensteijn J, DREAM‐Trial participants. Quality of life after endovascular and open AAA repair. Results of a randomised trial. European Society for Vascular Surgery, Programme and Abstract Book, XVII Annual Meeting and Course on Vascular Surgical Techniques. 2003:66‐7. [DOI] [PubMed]
- Prinssen M, Buskens E, Blankensteijn JD. The Dutch Randomised Endovascular Aneurysm Management (DREAM) trial. Background, design and methods. Journal of Cardiovascular Surgery 2002;43(3):379‐84. [PubMed] [Google Scholar]
- Prinssen M, Buskens E, Blankensteijn JD, DREAM trial participants. Quality of life endovascular and open AAA repair; Results of a randomised trial. European Journal of Vascular and Endovascular Surgery 2004;27(2):121‐7. [DOI] [PubMed] [Google Scholar]
- Prinssen M, Buskens E, Nolthenius RP, Sterkenburg SM, Teijink JA, Blankensteijn JD. Sexual dysfunction after conventional and endovascular AAA repair: results of the DREAM trial. Journal of Endovascular Therapy 2004;11(6):613‐20. [DOI] [PubMed] [Google Scholar]
- Prinssen M, Buskens E, Jong SE, Buth J, Mackaay AJ, Sambeek MR, et al. Cost‐effectiveness of conventional and endovascular repair of abdominal aortic aneurysms: Results of a randomized trial. Journal of Vascular Surgery 2007;46(5):883‐90. [DOI] [PubMed] [Google Scholar]
- Prinssen M, Verhoeven EL, Buth J, Cuypers PW, Sambeek MR, Balm R, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. New England Journal of Medicine 2004;351(16):1607‐18. [DOI] [PubMed] [Google Scholar]
EVAR1 {published data only}
- Brown L, Greenhalgh R, Powell J, Thompson S, on behalf of the EVAR Trial Participants. Cardiovascular events and the convergence of mid‐term mortality after endovascular aneurysm repair (EVAR) or open repair of abdominal aortic aneurysm. The Vascular Society of Great Britain & Ireland Yearbook 44th Annual Meeting; 18‐20 November 2009, Liverpool, UK. 2009:63.
- Brown LC, Brown EA, Greenhalgh RM, Powell JT, Thompson SG, UK EVAR Trial Participants. Renal function and abdominal aortic aneurysm (AAA): the impact of different management strategies on long‐term renal function in the UK EndoVascular Aneurysm Repair (EVAR) trials. Annals of Surgery 2010;251(5):966‐75. [DOI] [PubMed] [Google Scholar]
- Brown LC, Epstein D, Manca A, Beard JD, Powell JT, Greenhalgh RM. The UK Endovascular Aneurysm Repair (EVAR) trials: design, methodology and progress. European Journal of Vascular and Endovascular Surgery 2004;27(4):372‐81. [DOI] [PubMed] [Google Scholar]
- Brown LC, Greenhalgh RM, Howell S, Powell JT, Thompson SG. Patient fitness and survival after abdominal aortic aneurysm repair in patients from the UK EVAR trials. British Journal of Surgery 2007;94(6):709‐16. [DOI] [PubMed] [Google Scholar]
- Brown LC, Greenhalgh RM, Kwong GP, Powell JT, Thompson SG, Wyatt MG. Secondary interventions and mortality following endovascular aortic aneurysm repair: device‐specific results from the UK EVAR Trials. European Journal of Vascular and Endovascular Surgery 2007;34(3):281‐90. [DOI] [PubMed] [Google Scholar]
- Brown LC, Thompson SG, Greenhalgh RM, Powell JT, on behalf of the Endovascular Aneurysm Repair trial participants. Incidence of cardiovascular events and death after open or endovascular repair of abdominal aortic aneurysm in the randomized EVAR trial 1. British Journal of Surgery 2011;98(7):935‐42. [DOI] [PubMed] [Google Scholar]
- EVAR trial participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet 2005;365(9478):2179‐86. [DOI] [PubMed] [Google Scholar]
- Greenhalgh RM, Brown LC, Kwong GP, Powell JT, Thompson SG, EVAR trial participants. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30‐day operative mortality results: randomised controlled trial. Lancet 2004;364(9437):843‐8. [DOI] [PubMed] [Google Scholar]
- ISRCTN55703451. EndoVascular Aneurysm Repair (EVAR) trials 1 and 2. http://www.controlled‐trials.com/ISRCTN55703451 (accessed 6 December 2013).
- United Kingdom EVAR Trial Investigators. Endovascular versus open repair of abdominal aortic aneurysm. New England Journal of Medicine 2010;362(20):1863‐71. [DOI] [PubMed] [Google Scholar]
EVAR2 {published data only}
- Brown LC, Epstein D, Manca A, Beard JD, Powell JT, Greenhalgh RM. The UK Endovascular Aneurysm Repair (EVAR) trials: design, methodology and progress. European Journal of Vascular and Endovascular Surgery 2004;27(4):372‐81. [DOI] [PubMed] [Google Scholar]
- Brown LC, Greenhalgh RM, Howell S, Powell JT, Thompson SG. Patient fitness and survival after abdominal aortic aneurysm repair in patients from the UK EVAR trials. British Journal of Surgery 2007;94(6):709‐16. [DOI] [PubMed] [Google Scholar]
- Brown LC, Greenhalgh RM, Thompson SG, Powell JT, EVAR Trial Participants. Does EVAR alter the rate of cardiovascular events in patients with abdominal aortic aneurysm considered unfit for open repair? Results from the randomised EVAR trial 2. European Journal of Vascular and Endovascular Surgery 2010;39(4):396‐402. [DOI] [PubMed] [Google Scholar]
- EVAR 2 Trial Participants. Does aneurysm rupture risk decrease in patients who are anatomically suitable for endovascular repair?. The Vascular Society of Great Britain & Ireland 42nd Annual Meeting; 28‐30 November 2007; Manchester, UK. 2007:89.
- EVAR trial participants. Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial. Lancet 2005;365(9478):2187‐92. [DOI] [PubMed] [Google Scholar]
- EVAR trial participants. Secondary interventions and mortality following endovascular aortic aneurysm repair: Device‐specific results from the UK EVAR Trials. European Journal of Vascular and Endovascular Surgery 2007;34(3):281‐90. [DOI] [PubMed] [Google Scholar]
- ISRCTN55703451. EndoVascular Aneurysm Repair (EVAR) trials 1 and 2. http://www.controlled‐trials.com/ISRCTN55703451 (accessed 6 December 2013).
- United Kingdom EVAR Trial Investigators, Greenhalgh RM, Brown LC, Powell JT, Thompson SG, Epstein D. Endovascular repair of aortic aneurysm in patients physically ineligible for open repair. New England Journal of Medicine 2010;362(20):1872‐80. [DOI] [PubMed] [Google Scholar]
OVER {published data only}
- Kyriakides TC, Lederle F, Freischlag J. Open versus endovascular repair of abdominal aortic aneurysms: the OVER trial. Clinical Trials 2004; Vol. 1, issue Suppl 1:212.
- Lederle FA, Freischlag JA, Kyriakides TC, Matsumura JS, Padberg FT Jr, Kohler TR, et al. Long‐term comparison of endovascular and open repair of abdominal aortic aneurysm. New England Journal of Medicine 2012;367(21):1988‐97. [DOI] [PubMed] [Google Scholar]
- Lederle FA, Freischlag JA, Kyriakides TC, Padberg FT Jr, Matsumura JS, Kohler TR, et al. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial. JAMA 2009;302(14):1535‐42. [DOI] [PubMed] [Google Scholar]
- Lederle FA, Freischlag JA, Kyriakides TC, Padberg FTJ, Matsumura JS Kohler TR. Two‐year comparison of endovascular and open repair of abdominal aortic aneurysms. Journal of Vascular Surgery 2009;50(2):447. [Google Scholar]
- Lederle FA, Stroupe KT, Open versus Endovascular Repair (OVER) Veterans Affairs Cooperative Study Group. Cost‐effectiveness at two years in the VA Open versus Endovascular Repair trial. European Journal of Vascular and Endovascular Surgery 2012;44(6):543‐8. [DOI] [PubMed] [Google Scholar]
- NCT00094575. Standard open surgery versus endovascular repair of abdominal aortic aneurysm (AAA). http://clinicaltrials.gov/ct/show/NCT00094575?order=1 (accessed 6 December 2013).
- Stroupe KT, Lederle FA, Matsumura JS, Kyriakides TC, Jonk YC, Ge L, et al. Cost‐effectiveness of open versus endovascular repair of abdominal aortic aneurysm in the OVER trial. Journal of Vascular Surgery 2012;56(4):901‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
CAESAR Trial {published data only}
- Cao P, CAESAR Trial Collaborators. Comparison of surveillance vs Aortic Endografting for Small Aneurysm Repair (CAESAR) trial: study design and progress. European Journal of Vascular and Endovascular Surgery 2005;30(3):245‐51. [DOI] [PubMed] [Google Scholar]
- Cao P, Rango P, Verzini F, Parlani G, Romano L, Cieri E. Comparison of Surveillance Versus Aortic Endografting for Small Aneurysm Repair (CAESAR): results from a randomised trial. European Journal of Vascular and Endovascular Surgery 2011;41(1):13‐25. [DOI] [PubMed] [Google Scholar]
- NCT00118573. Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR). http://clinicaltrials.gov/ct/show/NCT00118573?order=1 (accessed 6 December 2013).
Cuypers 2001 {published data only}
- Cuypers PW, Gardien M, Buth J, Peels CH, Charbon JA, Hop WC. Randomized study comparing cardiac response in endovascular and open abdominal aortic aneurysm repair. British Journal of Surgery 2001;88(8):1059‐65. [DOI] [PubMed] [Google Scholar]
ECAR study 2010 {published data only}
- Desgranges P, Kobeiter H, Castier Y, Senechal M, Majewski M, Krimi A. The Endovasculaire vs Chirurgie dans les Anevrysmes Rompus PROTOCOL trial update. Journal of Vascular Surgery 2010;51(1):267‐70. [DOI] [PubMed] [Google Scholar]
IMPROVE trial {unpublished data only}
- IMPROVE Trial, Powell JT, Thompson SG, Thompson MM, Grieve R, Nicholson AA, Ashleigh R, et al. The Immediate Management of the Patient with Rupture: Open Versus Endovascular repair (IMPROVE) aneurysm trial‐‐ISRCTN 48334791 IMPROVE trialists. Acta Chirurgica Belgica 2009;109(6):678‐80. [DOI] [PubMed] [Google Scholar]
Lottman 2004 {published data only}
- Lottman PE, Laheij RJ, Cuypers PW, Bender M, Buth J. Health‐related quality of life outcomes following elective open or endovascular AAA repair: a randomized controlled trial. Journal of Endovascular Therapy 2004;11(3):323‐9. [DOI] [PubMed] [Google Scholar]
PIVOTAL Trial {published data only}
- NCT00444821. The (PIVOTAL) study. http://clinicaltrials.gov/ct/show/NCT00444821?order=1 (accessed 6 December 2013).
- Ouriel K. The PIVOTAL study: A randomized comparison of endovascular repair versus surveillance in patients with smaller abdominal aortic aneurysms. Journal of Vascular Surgery 2009;49(1):266‐9. [DOI] [PubMed] [Google Scholar]
- Ouriel K, Clair DG, Kent KC, Zarins CK, PIVOTAL Investigators. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. Journal of Vascular Surgery 2009;50(2):449. [DOI] [PubMed] [Google Scholar]
Soulez 2005 {published data only}
- Soulez G, Thérasse E, Monfared AA, Blair JF, Choiniére M, Elkouri S, et al. Pain and quality of life assessment after endovascular versus open repair of abdominal aortic aneurysms in patients at low risk. Journal of Vascular Interventional Radiology 2005;16(8):1093‐100. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NExT ERA {unpublished data only}
- Mastracci TM, Clase CM, Devereaux PJ, Cinà CS. Open versus endovascular repair of abdominal aortic aneurysm: a survey of Canadian vascular surgeons. Canadian Journal of Surgery 2008;51(2):142‐8, quiz 149. [PMC free article] [PubMed] [Google Scholar]
Additional references
AAAQIP 2011
- The Abdominal Aortic Aneurysm Quality Improvement Programme Team. National abdominal aortic aneurysm quality improvement programme. Vascular Society of Great Britain and Ireland, http://www.vascularsociety.org.uk/vascular/wp‐content/uploads/2012/11/National‐AAA‐QIP‐Interim‐Report.pdf (accessed 4 July 2013).
Armon 1998
- Armon MP, Hopkinson BR. How much does an aorto‐uni‐iliac stent‐graft increase the endovascular options for abdominal aortic aneurysm repair?. In: Greenhalgh RM editor(s). Indications in Vascular and Endovascular Surgery. London: Saunders, 1998:221‐7. [Google Scholar]
Bengtsson 1993
- Bengtsson H, Bergqvist D. Ruptured abdominal aortic aneurysm: a population‐based study. Journal of Vascular Surgery 1993;18(1):74‐80. [DOI] [PubMed] [Google Scholar]
Budd 1989
- Budd JS, Finch DR, Carter PG. A study of the mortality from ruptured abdominal aortic aneurysms in a district community. European Journal of Vascular Surgery 1989;3(4):351‐4. [DOI] [PubMed] [Google Scholar]
Campbell 1986
- Campbell WB, Collin J, Morris PJ. The mortality of abdominal aortic aneurysm. Annals of the Royal College of Surgeons of England 1986;68(5):275‐8. [PMC free article] [PubMed] [Google Scholar]
Dangas 2012
- Dangas G, O'Connor D, Firwana B, Brar S, Ellozy S, Vouyouka A, et al. Open versus endovascular stent graft repair of abdominal aortic aneurysms: a meta‐analysis of randomized trials. JACC: Cardiovascular Interventions 2012;5(10):1071‐80. [DOI] [PubMed] [Google Scholar]
Eskandari 1998
- Eskandari MK, Bowles SA, Webster MW, Steed DL, Makaroun MS, Muluk SC, et al. Ruptured abdominal aortic aneurysms in the 1990s: resource utilization, long‐term survival and quality of life after repair. Vascular and Endovascular Surgery 1998;32(5):415‐24. [Google Scholar]
EVAR1 2005
- EVAR trial participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet 2005;365(9478):2179‐86. [DOI] [PubMed] [Google Scholar]
Feinglass 1995
- Feinglass J, Cowper D, Dunlop D, Slavensky R, Martin GJ, Pearce WH. Late survival risk factors for abdominal aortic aneurysm repair: experience from fourteen Department of Veterans Affairs hospitals. Surgery 1995;118(1):16‐24. [DOI] [PubMed] [Google Scholar]
Gadowski 1994
- Gadowski GR, Pilcher DB, Ricci MA. Abdominal aortic aneurysm expansion rate: effect of size and beta‐adrenergic blockade. Journal of Vascular Surgery 1994;19(4):727‐31. [DOI] [PubMed] [Google Scholar]
Giles 2011
- Giles KA, Landon BE, Cotterill P, O'Malley AJ, Pomposelli FB, Schermerhorn ML. Thirty‐day mortality and late survival with reinterventions and readmissions after open and endovascular aortic aneurysm repair in Medicare beneficiaries. Journal of Vascular Surgery 2011;53(1):6‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG. Chapter 8: Assessing Risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011. Available from www.cochrane‐handbook.org.
Holt 2009
- Holt PJ, Poloniecki JD, Khalid U, Hinchliffe RJ, Loftus IM, Thompson MM. Effect of endovascular aneurysm repair on the volume‐outcome relationship in aneurysm repair. Circulation. Cardiovascular quality and outcomes 2009;2(6):624‐32. [DOI] [PubMed] [Google Scholar]
Ingoldby 1986
- Ingoldby CJ, Wujanto R, Mitchell JE. Impact of vascular surgery on community mortality from ruptured aortic aneurysms. British Journal of Surgery 1986;73(7):551‐3. [DOI] [PubMed] [Google Scholar]
Johansson 1986
- Johansson G, Swedenborg J. Ruptured abdominal aortic aneurysms: a study of incidence and mortality. British Journal of Surgery 1986;73(2):101‐3. [DOI] [PubMed] [Google Scholar]
Kantonen 1999
- Kantonen I, Lepantalo M, Brommels M, Luther M, Salenius JP, Ylonen K. Mortality in ruptured abdominal aortic aneurysms. The Finnvasc Study Group. European Journal of Vascular and Endovascular Surgery 1999;17(3):208‐12. [DOI] [PubMed] [Google Scholar]
Karthikesalingam 2013
- Kathikesalingam A, Thompson MM. Repair of infrarenal aortic aneurysm ‐ the debate is OVER. Nature Review in Cardiology 2013;10(3):122‐4. [DOI] [PubMed] [Google Scholar]
Khoo 1994
- Khoo DE, Ashton H, Scott RA. Is screening once at age 65 an effective method for detection of abdominal aortic aneurysms?. Journal of Medical Screening 1994;1(4):223‐5. [DOI] [PubMed] [Google Scholar]
Leach 1988
- Leach SD, Toole AL, Stern H, DeNatale RW, Tilson MD. Effect of beta‐adrenergic blockade on the growth rate of abdominal aortic aneurysms. Archives of Surgery 1988;123(5):606‐9. [DOI] [PubMed] [Google Scholar]
Mutirangura 1989
- Mutirangura P, Stonebridge PA, Clason AE, McClure JH, Wildsmith JA, Nolan B, et al. Ten‐year review of non‐ruptured aortic aneurysms. British Journal of Surgery 1989;76(12):1251‐4. [DOI] [PubMed] [Google Scholar]
NICE EVAR 2012
- National Institute for Health and Clinical Excellence. Endovascular stent–grafts for the treatment of abdominal aortic aneurysms. http://www.nice.org.uk/ (accessed 4 July 2013).
Olsen 1991
- Olsen PS, Schroeder T, Agerskov K, Roder O, Sorensen S, Perko M, et al. Surgery for abdominal aortic aneurysms. A survey of 656 patients. Journal of Cardiovascular Surgery 1991;32(5):636‐42. [PubMed] [Google Scholar]
Parodi 1991
- Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Annals of Vascular Surgery 1991;5(6):491‐9. [DOI] [PubMed] [Google Scholar]
Scott 1995
- Scott RA, Wilson NM, Ashton HA, Kay DN. Influence of screening on the incidence of ruptured abdominal aortic aneurysm: 5‐year results of a randomized controlled study. British Journal of Surgery 1995;82(8):1066‐70. [DOI] [PubMed] [Google Scholar]
Steyerberg 1995
- Steyerberg EW, Kievit J, Mol Van Otterloo JC, Bockel JH, Eijkemans MJ, Habbema JD. Perioperative mortality of elective abdominal aortic aneurysm surgery. A clinical prediction rule based on literature and individual patient data. Archives of Internal Medicine 1995;155(18):1998‐2004. [PubMed] [Google Scholar]
Takolander 1998
- Takolander R. Conservative treatment, stent grafting or open repair of abdominal aortic aneurysms. Annales Chirurgiae et Gynaecologiae 1998;87(2):167‐70. [PubMed] [Google Scholar]
Thomas 1988
- Thomas PR, Stewart RD. Abdominal aortic aneurysm. British Journal of Surgery 1988;75(8):733‐6. [DOI] [PubMed] [Google Scholar]
Thomas 2002
- Thomas SM, Palfreyman SSJ, Michaels JA, Cleveland TJ, Gaines PA. Endovascular repair of abdominal aortic aneurysm. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD004178] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2005
- Thomas SM, Beard JD, Ireland M, Ayers S, Vascular Society of Great Britain and Ireland, British Society of Interventional Radiology. Results from the prospective registry of endovascular treatment of abdominal aortic aneurysms (RETA): mid term results to five years. European Journal of Vascular and Endovascular Surgery 2005;29(6):563‐70. [DOI] [PubMed] [Google Scholar]
UKSAT 1998
- The UK Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet 1998;352(9141):1649‐55. [PubMed] [Google Scholar]
Walton 1999
- Walton LJ, Franklin IJ, Bayston T, Brown LC, Greenhalgh RM, Taylor GW, et al. Inhibition of prostaglandin E2 synthesis in abdominal aortic aneurysms: implications for smooth muscle cell viability, inflammatory processes, and the expansion of abdominal aortic aneurysms. Circulation 1999;100(1):48‐54. [DOI] [PubMed] [Google Scholar]
Zarins 1997
- Zarins CK, Harris EJ Jr. Operative repair for aortic aneurysms: the gold standard [review]. Journal of Endovascular Surgery 1997;4(3):232‐41. [DOI] [PubMed] [Google Scholar]


