Abstract
Spinal cord organoids are three-dimensional tissues derived from stem cells that recapitulate the primary morphological and functional characteristics of the spinal cord in vivo. As emerging bioengineering methods have led to the optimization of cell culture protocols, spinal cord organoids technology has made remarkable advancements in the past decade. Our literature search found that current spinal cord organoids do not only dynamically simulate neural tube formation but also exhibit diverse cytoarchitecture along the dorsal-ventral and rostral-caudal axes. Moreover, fused organoids that integrate motor neurons and other regionally specific organoids exhibit intricate neural circuits that allows for functional assessment. These qualities make spinal cord organoids valuable tools for disease modeling, drug screening, and tissue regeneration. By utilizing this emergent technology, researchers have made significant progress in investigating the pathogenesis and potential therapeutic targets of spinal cord diseases. However, at present, spinal cord organoid technology remains in its infancy and has not been widely applied in translational medicine. Establishment of the next generation of spinal cord organoids will depend on good manufacturing practice standards and needs to focus on diverse cell phenotypes and electrophysiological functionality evaluation.
Keywords: development, organoid-on-a-chip, pluripotent stem cells, progress, spinal cord diseases, spinal cord organoids, vascularization
Introduction
Organoids are three-dimensional (3D) constructs derived from stem cells in vitro that mimic the cytoarchitecture and physiological functionality of specific organs (Clevers, 2016). Unlike engineered tissues constructed by seeding stem cells on scaffolds, organoids are self-organized in terms of cell sorting and cytoarchitecture formation (Fedorchak et al., 2021). Lancaster et al. (2013) were the first to generate cerebral organoids using human induced pluripotent stem cells (hiPSCs) which possessed ability to develop into various distinct cerebral regions. This research represented a milestone in the construction of neural organoids that simulate the morphology and pathophysiology of the central nervous system (CNS). Since then, researchers have optimized cell culture protocols and manipulated caudalization signaling during CNS development to generate hindbrain and spinal cord organoids (Lippmann et al., 2015). Spinal cord organoids not only address the limitations of traditional two-dimensional cell culture, which cannot replicate the complex phenotype of the native spinal cord, but also overcome the challenges posed by phylogenetic differences between human and animal models (Li and Izpisua Belmonte, 2019). Therefore, spinal cord organoids provide excellent platforms for developmental studies, disease modeling, drug screening, and neural regeneration (Clevers, 2016; Olmsted and Paluh, 2021a). In this review, we comprehensively summarize research progress in the generation of spinal cord organoids over the past decade, focusing predominantly on state-of-the-art methodologies and significant advancements. We also introduce spinal cord organoids as versatile tools for investigating the pathogenesis of spinal cord conditions and identifying potential therapeutic targets, including neurodevelopmental diseases, neurodegenerative diseases, neuropathic pain, and spinal cord injury (SCI). Finally, we present the challenges and future perspectives of such in vitro modeling technologies and contemplate the next generation of spinal cord organoids.
Retrieval Strategy
The articles cited in this narrative review were retrieved from the PubMed database by use of the following search terms: spinal cord organoid, spinal organoid, and neural tube organoid. Most of the selected studies were published between 2013 and 2023.
Development Pattern of the Human Spinal Cord
The in vivo development pattern of the spinal cord is a highly orchestrated process. During the gastrulation stage, the ectoderm undergoes specialization into the neural plate, which subsequently invaginates to form the neural tube (Hawryluk et al., 2012). The specific pattern of migration and differentiation of neural progenitor cells within the neural tube are key mechanisms in forming a 3D structure of the spinal cord (Hawryluk et al., 2012). This process depends on the spatiotemporal gradient-dependent regulatory effects of morphogens, which induce distinct and spatially delimited gene expression patterns and lead to cell fate specification (Sagner and Briscoe, 2019). Inductive signaling molecules, such as bone morphogenetic proteins (BMPs), Sonic hedgehog (Shh), Wnt, retinoic acid (RA), Noggin, growth differentiation factor 11, and fibroblast growth factor (FGF), play crucial roles in the process of neural induction (Rogers and Schier, 2011; Leung and Shimeld, 2019). According to the classical viewpoint, the neural plate is divided into anterior and posterior aspects. The anterior neural plate is specified at the earliest stage and develops into the forebrain and midbrain. The posterior neural plate then undergoes a process of patterning that is similar to that of the anterior neural plate, resulting in the formation of the hindbrain and spinal cord via induced posteriorizing signaling (Stern, 2006). This process is formulated as the “activation-transformation” model (Stern, 2006). However, recent studies have confirmed that the spinal cord is primarily derived from neuromesodermal progenitors (NMPs) which are located in the node-streak border and adjacent caudal lateral epiblast (Henrique et al., 2015). NMPs co-express biomarkers of the early mesoderm and neural progenitors (Chalamalasetty et al., 2014; Garriock et al., 2015; Henrique et al., 2015). These cells migrate to the pre-neural tube to generate ventral neural tissue at the anterior spinal cord level, which then integrates with dorsal neural tissue derived from the anterior neural plate (Henrique et al., 2015). While in the more posterior spinal cord, NMPs generate both dorsal and ventral regions (Henrique et al., 2015). This discovery established a novel mechanism for caudalization that is independent of the “activation-transformation” model, thus providing a new concept for generating spinal cord organoids.
During spinal cord development, three axes are formed: the anterior-posterior (AP)/rostral-caudal axis, the dorsal-ventral (DV) axis, and the medial-lateral axis (Iyer and Ashton, 2022). The AP axis is patterned from the overlapping expression of homeobox (Hox) genes. The Hox gene family consists of four clusters: HoxA, HoxB, HoxC, and HoxD, which are located on chromosomes 7, 17, 12, and 2, respectively (Philippidou and Dasen, 2013). These genes can be further divided into thirteen homologous groups (Hox 1–13) based on sequence similarity, which are expressed in different regions along the AP axis. Cell identities are determined by Hox4–7 genes in cervical tissues, Hox8–9 genes in thoracic tissues, and Hox10–11 genes in lumbar tissues (Philippidou and Dasen, 2013). Two opposing mechanisms control the expression profiles of Hox genes. During development, RA induces the expression of Hox4–7 genes to promote cervical cell identities, whereas FGF induces the expression of thoracic Hox8–9 genes to promote posteriorization (Philippidou and Dasen, 2013). Both FGF and growth differentiation factor 11 are required to establish the most posterior cell identities (Sagner and Briscoe, 2019). The formation of the DV axis determines the identities of locomotor and somatosensory cells. During closure of the neural tube, ventral patterning is promoted by Shh, a factor secreted by the notochord and floor plate. On the other hand, dorsal patterning is promoted by proteins of the BMP and Wnt families which are secreted by the roof plate (Sagner and Briscoe, 2019). Opposing Shh and BMP/Wnt gradients generate cross-repressive transcriptional interactions and induce the formation of eleven discrete progenitor domains, including five ventral domains (p0–p3 and pMN domains), which differentiate into neuronal populations responsible for locomotor coordination, and six dorsal domains (dp1–dp6), which differentiate into proprioceptive and sensory neurons (Sagner and Briscoe, 2019). The neural progenitors in each domain express different transcription factors to form a cross-regulatory gene network which determines their response to morphogens (Kutejova et al., 2016). The medial-lateral axis is formed by the radial migration and differentiation of neural progenitor cells. During patterning of the DV axis, proliferating progenitors in the ependymal layer migrate to their final settling positions to form the mantle layer as they undergo cell differentiation (Andrews et al., 2019). The axons proceeding in and out of the mantle layer form the marginal layer. Subsequently, additional neuroblasts are deposited into the mantle layer, thus resulting in the formation of basal plate and alar plate (Hawryluk et al., 2012). As the mantle layer develops into the gray matter, the alar plate gives rise to the dorsal horn while the basal plate forms the ventral horn (Hawryluk et al., 2012). The marginal layer, which includes glial cells as well as ascending and descending nerve fibers, eventually develops into the white matter (Hawryluk et al., 2012). The developmental pattern of the human spinal cord is shown in Figure 1.
Figure 1.
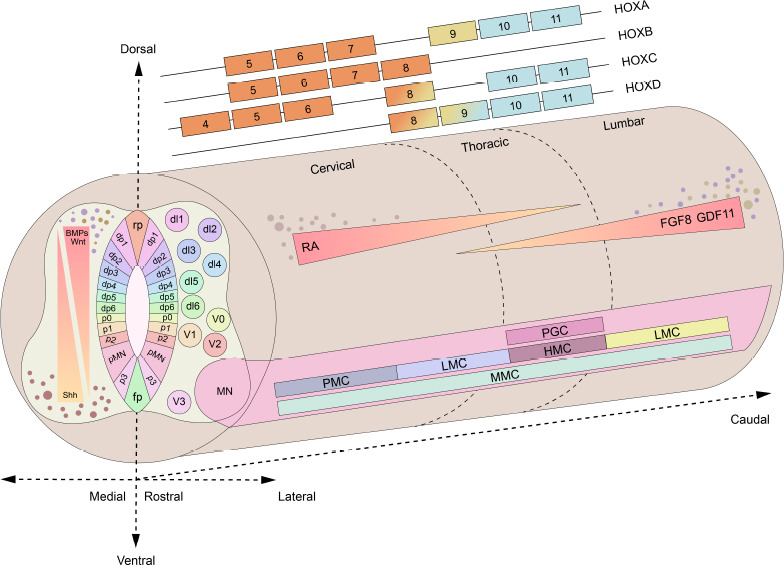
Schematics of the three axes during spinal cord development.
(A) The AP axis is defined by overlapping Hox gene expression in the cervical (Hox4–7), thoracic (Hox8–9), and lumbar (Hox10–11) regions. Delineation of progenitor domains along the AP axis is established in response to RA, FGFs, and GDF-11. These factors induce the expression of distinct Hox genes, which in turn generate unique columnar identities for neurons. For example, motor neurons (MNs) are specified along the AP axis into motor columns, including the median motor column (MMC) through the spinal cord, the phrenic motor column (PMC) in the cervical cord, the lateral motor column (LMC) in both cervical and lumbar cord, the hypaxial motor column (HMC) and the preganglionic column (PGC) in thoracic cord (Stifani, 2014). (B) Patterning of the DV axis is determined by Shh secreted by the floorplate and notochord, as well as BMP/Wnt secreted by the roofplate and ectoderm. Neural progenitor cells differentiate into 11 discrete progenitor domains, which can be identified by distinct homeodomain transcription factors. (C) The ML axis refers to the radial location of distinct domains and layers in the mature spinal cord from the nuclei to the margin. Created with Adobe Illustrator 2021. AP axis: Anterior-posterior axis; BMP: bone morphogenetic protein; DV axis: dorsal-ventral axis; FGFs: fibroblast growth factors; fp: floor plate; GDF-11: growth differentiation factor 11; Hox gene: homeobox gene; ML axis: medial-lateral axis; RA: retinoic acid; rp: roofplate; Shh: Sonic hedgehog.
Methodologies for the Generation of Spinal Cord Organoids
Protocols for cultivating spinal cord organoids typically involve three steps. First, embryoid bodies formation in which stem cells are cultured in chemically defined media to reach high confluency (Hor and Ng, 2020). Second, neural induction in which embryoid bodies are encapsulated in Matrigel or hydrogels for primary expansion and neural induction in neural patterning media (Hor and Ng, 2020). At this stage, protocols that provide minimal extrinsic cues induce the cell aggregates to self-organize and differentiate into multi-lineage organoids in which the cells acquire both neural fate and other mesodermal phenotypes (Hor and Ng, 2020). Alternatively, protocols with more specific morphogens, induce organoids to develop a precise neural fate while simultaneously reducing the diversity of cellular phenotypes (Fedorchak et al., 2021). Third, tissue differentiation and maturation in which cultures are transferred to bioreactors and regulatory cues are added to generate spinal cord organoids that mimic the intrinsic cytoarchitectures, cellular phenotypes, and neural circuits of their in vivo counterparts (Hor and Ng, 2020). Currently, spinal cord organoids are primarily derived from induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs) due to their inherent properties of stemness. Over recent years and along with elucidation of their pivotal role in spinal cord development, NMPs have demonstrated significant potential for generating organoids that incorporate the spinal cord and mesodermal tissues collectively (Faustino Martins et al., 2020; Olmsted and Paluh, 2021b). Unlike assembloids, which are assembled by region-specific organoids, organoids intiated from NMPs can simulate co-development of the human central and peripheral nervous system and allow for long-term functional assessments (Olmsted and Paluh, 2021b). However, there is a significant degree of variability in this type of organoid, which necessitates the precise regulation of extrinsic cues and growth factors.
Biomaterials and micropatterning
The biochemical and mechanical properties of the extracellular matrix have a profound impact on neuronal biological behavior, such as process projection and synapse formation. Therefore, biomaterials are essential when generating neural organoids (Kofman et al., 2022; Yang et al., 2023). Previous studies have revealed that the emergence of numerous neural rosettes inevitably interfere with the morphogenesis process, thus impeding tissue maturation and hindering organoid generation on the macroscale (Lancaster et al., 2013; Knight et al., 2018). Micropatterned biomaterials can provide geometric confinement to regulate both tissue size and geometry; this exerts significant influence on cell-cell signaling and cytoarchitecture (Abdel Fattah et al., 2021). By micropatterning the initial tissue morphology according to certain parameters, Knight et al. (2018) have induced a single neural rosette within hiPSCs that could be maintained throughout subsequent tissue morphogenesis. Currently, the biomaterials used to generate neural organoids mainly include Matrigel, synthetic hydrogels, and recombinant proteins. Derived from the secretion of mouse sarcoma cells, Matrigel is a widely used commercial matrix for the cultivation of organoids and is enriched with type IV collagen, laminin, proteoglycans, and growth factors. However, the undefined composition of Matrigel makes it difficult to identify the key signals necessary for establishing the structure and function of organoids (Kozlowski et al., 2021). The batch variation of Matrigel results in significant heterogeneity in different organoids. In addition, the animal origination may lead to immunogenicity (Kozlowski et al., 2021). Alternatively, synthetic hydrogels are better defined in composition and can be fabricated according to specific parameters. Synthetic hydrogels can guide the adhesion and migration of neural precursor cells, promote axonal regeneration, and stimulate angiogenesis (Wei et al., 2010; Zhang et al., 2016). In particular, hyaluronic acid hydrogels exhibit significant potential for human neural organoid technologies due to the high hyaluronic acid content in the human cerebral extracellular matrix (Xiang et al., 2017). Recently, researchers utilized a combination of porous chitosan microspheres and Matrigel to deliver a Shh agonist for spatial specification. This approach mimics the spinal cord organizing centers in vivo and generates distinct dorsoventral progenitor domains (Xue et al., 2023). Nevertheless, synthetic hydrogels must be incorporated with biochemical cues to ensure adequate cell attachment and prevent anoikis (Hagbard et al., 2018). In addition, the degradation of synthetic hydrogels may produce cytotoxic molecules, thus limiting the subtypes of polymers that can be used for cell culture (Vihola et al., 2005). Self-assembling peptides and recombinant proteins are fabricated with specific viscoelasticity, stiffness, and chemical functionality. The degradation rate of recombinant proteins can be programmed by incorporating protease recognition sites or by altering crosslinking chemistry (Kozlowski et al., 2021). The main disadvantages of self-assembling peptides and recombinant proteins are higher cost, potential endotoxin contamination and immunogenicity (Kozlowski et al., 2021). To date, the use of Matrigel-free matrix has been limited to only a narrow range of target tissues. The efficiency and biocompatibility of novel biomaterials for generating spinal cord organoids require further validation but they represent a promising area of research.
Bioreactors and vascularization
One of the primary limitations of organoid culture is the medullary necrosis caused by insufficient local oxygen and nutrients; this issue results in a limited tissue size and impaired functionality (Rambani et al., 2009). Spinner flasks and oscillatory bioreactors have been extensively used in expansion for large-scale tissues, as they can improve the homogeneous distribution of oxygen and nutrients by stirring the suspension medium (Fedorchak et al., 2021). However, these suspension bioreactors cannot replicate the signaling conditions in vivo or support long-term maintenance. The 3D-printed, miniaturized, multi-well and spinning bioreactor SpinΩ can improve throughput and successfully model region-specific brain organoids which can be maintained over 200 days (Qian et al., 2018). Another promising strategy to prevent medullary necrosis in a hypoxic environment is vascularization (Cakir et al., 2019; Ham et al., 2020). Currently, there are five approaches that can be used to vascularize neural organoids. First, multidirectional differentiation in which VEGF and basic FGF are added to the culture system during embryoid body formation and neural induction to induce the co-differentiation of endothelial-like cells without inhibiting the process of neurogenesis (Ham et al., 2020). Second, cocultivation of hiPSCs and endothelial cells or assembly of neural and vascular aggregates (Pham et al., 2018). Third, genetic engineering in which ESCs are engineered to overexpress reprogramming factors, such as ETV2 and NEUROD1; these ESCs are mixed with normal iPSCs in a specific proportion to build vasculized neural organoids (Cakir et al., 2019). Fourth, ectopic transplantation in which 40–50 day-old organoids are directly implanted into the retrosplenial cortices of mice to yield functional vascular networks (Mansour et al., 2018). Finally, bioengineering approaches such as microfluidics chips, 3D bioprinting, and photopolymerization (Grebenyuk and Ranga, 2019). Seeding microvascular endothelial cells onto a spinal cord organoid-on-a-chip has been shown to enhance vascular-neural interaction and tissue maturation (Sances et al., 2018). Vascularization facilitates the delivery of oxygen and nutrients to the medulla of the organoids and impacts their metabolic networks; this effect is rarely observed in organoids derived from normal iPSCs (Li et al., 2023b).
Organoids-on-a-chip
Culturing spinal cord organoids requires the precise regulation of morphogenetic signaling to induce physical segregation and phenotype specification. In traditional protocols, these events are accomplished by adding exogenous morphogens at specific time points. The spontaneous dispersion of morphogens and cell-secreted soluble components generate biochemical gradients in the culture environment. However, simulating the gradient necessary for in vivo organogenesis is challenging (Rogers and Schier, 2011). Over recent years, organoid-on-a-chip has emerged as a powerful tool to bridge the gap between organoid technologies and developmental biology (Habibey et al., 2022). Organoid-on-a-chip technology integrates organoids and microfluidic chips, which possess microchannels for establishing stable gradients via the precise control of fluid flow; this recreates an intricate microenvironment for tissue patterning and expansion in vitro (Park et al., 2019). Each chip features a circulatory system that continuously provides nutrients for tissue survival and maturation while also removing spent medium (Kofman et al., 2022). By utilizing this microfluidic system, researchers have replicated the spatiotemporal chemical environments present in vivo and promoted axial patterning in spinal cord organoids (Demers et al., 2016a, b). The microHIVE (microhexagon interlace for generation of versatile and fine gradients) platform has been shown to optimize the profile of morphogens and induce the differentiation of iPSCs into a spatial continuum of distinct motor neuron subtypes, even from cervical to lumbar spinal cord (Lim et al., 2019). The combination of microfluidic chambers and micropumps enable fresh medium to be irrigated into the microenvironment in a manner that closely resembles the human vascular system (Zheng et al., 2021a). Organoid-on-a-chip technology also allows us to manipulate parameters associated with the specific cultivation environment, including geometry and constraints, thus providing an excellent platform for studying the impact of mechanical forces on spinal cord development and function (Kofman et al., 2022). A previous study utilized a brain organoids-on-a-chip system to investigate the impact of compressive forces on cortical folding (Karzbrun et al., 2018). Similar techniques could also be applied to the study of neural tube formation in the future. Moreover, the incorporation of biochemical sensors and actuators in organoids-on-a-chip allows us to monitor the physiological state of cells and adjust the culture conditions in real time (Park et al., 2019). Ultimately, the utilization of spinal cord organoid-on-a-chip technology provides us with novel opportunities for investigating the environmental factors that contribute to both normal and pathological neural developmental processes (Habibey et al., 2022; Kofman et al., 2022).
Genetical engineering
Genetically modified neural organoids exhibit specific morphogenic patterns and functionality. Novel CRISPR-Cas9-based technologies enable researchers to generate organoids with specific neurodevelopmental disorders. CRISPR-Cas9-mediated homology-independent transgenesis (CRISPR-HOT) was previously applied to target and insert in-frame protein tags in human organoids with minimal insertions, deletions, and out-of-frame mutations (Artegiani et al., 2020). CRISPR-lineage tracing at a cellular resolution (CRISPR-LICHT) has also been utilized in heterogeneous tissue for loss-of-function screening and knock-out investigations in neural organoids (Esk et al., 2020). For example, TBX6-deleted ESCs derived spinal cord organoids developed additional neural tubes that mirrored the in vivo mutant phenotype during embryonic development (Veenvliet et al., 2020). There are other applications of genetic engineering that should be considered, including the ectopic expression of specific genes that promote vascularization, myelination, and electrophysiological maturation; these processes cannot be observed in simple organoids from a neural lineage (Porciúncula et al., 2021). As mentioned previously, mixing iPSCs with engineered ESCs that express ETV2 ectopically could generate brain organoids with vascular-like networks (Cakir et al., 2019). Finally, gene engineering can also be utilized to photoactivate transcription factors and allow for optogenetic patterning in neural organoids (Guy et al., 2021). It is also worth noting that when using genome editing technologies to construct organoids, it is necessary to compare them to isogenic controls to validate the causal relationship between phenotype discrepancies and genetic alterations (Guy et al., 2021).
Research Progress in the Generation of Spinal Cord Organoids
Since the generation of cerebral organoids in 2013, researchers have focused on cultivating region-specific organoids to decipher the functions and developmental rules associated with different parts of the CNS. Following established protocols for generating neural organoids, region-specific organoids such as the retina, hippocampal, thalamus, midbrain, and cerebellum have been successfully constructed (Guy et al., 2021). However, the spinal cord is a complex tubular structure that consists of over 20 neuronal subtypes and 30 segments along the AP axis. This complexity presents a significant challenge when attempting to reconstitute the spinal cord in vitro. Initially, researchers focused on generating tissues with the characteristics of the hindbrain and spinal cord by manipulating caudalization signaling of the embryonic neural tube (Lippmann et al., 2015). In 2014, single mouse ESCs was induced to clonally form 3D neuroepithelial cysts with clear apical/basolateral polarity; furthermore, these cysts could be caudalized to cervical levels by RA (Meinhardt et al., 2014). Similar techniques were subsequently employed to investigate how chemically specified microenvironments affect neural tube patterning (Ranga et al., 2016). However, these studies were unable to generate patterned 3D human spinal cord tissues that included multiple subtypes of neurons. In 2018, dorsal and ventral spinal cord-like tissues were generated by the induction of hiPSCs with BMP4 and SAG (an agonist of the Shh signaling pathway), respectively. These tissues formed a roof plate or floor plate signaling centers and generated several subtypes of spinal neurons that evolved into distinct progenitor domains (Ogura et al., 2018). In addition to endogenous signaling centers, exogenous additives incorporated into the matrix can also effectively stimulate the neural patterning of spinal organoids. Spatiotemporal patterning can be altered by modifying the concentration, timing, and duration of exposure (Duval et al., 2019). Although these studies represented a significant advancement, one limitation is that they are unable to generate both ventral and dorsal structures simultaneously within a single organoid (Winanto et al., 2019). This challenge has been addressed by the establishment of Shh-BMP4 cross-gradients along the DV axis (Andersen et al., 2020). By applying microfluidic chips that can maintain different gradients of morphogens over time, it is now possible to generate spinal cord organoids that consist of both motor and sensory neurons. Another significant development was the creation of oriented differentiation protocols that combine rostrocaudal and dorsoventral patterning (Mouilleau et al., 2021; Park et al., 2022). However, establishing in vitro models with mature cell diversity to replicate morphological development of the spinal cord is still a challenging task, as this requires both differentiation efficiency and phenotypic diversity. Lee et al. (2022) established a robust method for generating spinal cord organoids through neurulation-like morphological processes. These organoids mimic in vivo neural tube formation and produce both neurons and glial cells. When co-cultured with skeletal muscle tubules and dorsal root ganglion (DRG), these organoids can establish synaptic connections (Lee et al., 2022).
The spinal cord contains numerous ascending and descending fibers that connect to the brain and the peripheral motor-sensory units. In-depth investigations of functional assessment and disease modeling require comprehensive models that incorporate intricate cell-to-cell interactions and neural circuits. The technology underlying fused organoids involves integrating regionally specific organoids, thus increasing the complexity of neural organoids which exhibit the entire neural network connecting the brain, spinal cord, and target tissue. Fused organoids can be generated through two approaches. The first is to assemble spinal cord organoids with other regional organoids that have distinct cellular identities; these are termed as assembloids. For instance, spinal cord organoids were assembled by forebrain cortical organoids to form cortico-spinal assembloids, which were subsequently integrated with skeletal myoblasts to form cortico-spinal-muscle organoids. These cortico-motor assembloids maintained long-range functional connections and induced muscle contraction by glutamate release or photogenetic stimulation of the cortex, thus indicating the morphological and functional integrity of the fused organoids (Andersen et al., 2020). Another approach is to induce iPSCs to NMPs and utilize their multi-lineage characteristics to generate spinal, mesodermal, and neural crest tissues in a simultaneous manner. Organoids derived from NMPs can synchronously recapitulate the development of the spinal cord and mesodermal lineages. For example, the neuromuscular organoids contain neurons, Schwann cells, and skeletal muscle. The two lineages were shown to interact and self-organize to form functional neuromuscular junctions during the maturation process and acquire electrophysiological characteristics that drove contractile activity (Faustino Martins et al., 2020). By modifying the initial induction protocols, Olmsted and Paluh (2021b) developed elongated multi-lineage organized gastruloids using human iPSCs that achieved increased morphogenetic complexity and multi-system physiology. Elongated multi-lineage organoids simulate the co-development of the human CNS-peripheral nervous system with their targets, including skeletal muscles and the heart.
Over recent years, in vitro models have been established to recapitulate the early stages of spinal cord development. Gastruloids, which are stem cell-derived embryonic organoids, can mimic differentiation of the three germ layers and form a string of single somites (van den Brink et al., 2020). Mouse ESC-derived trunk-like structures were shown to consist of both neural tube and bilateral somites with embryo-like polarity (Veenvliet et al., 2020). Somitoids can be used to model the later stages of axial specification and recapitulate the segmentation clock of the somites; this involves NMPs and populations of somite cells with appropriate polarities (Budjan et al., 2022; Sanaki-Matsumiya et al., 2022). The comprehensive and tractable nature of gastruloids and somitoids renders these structures complementary platforms to better decipher the dynamics of early embryonic spinal cord development. Progress in the development of spinal cord organoids is summarized in Figure 2.
Figure 2.
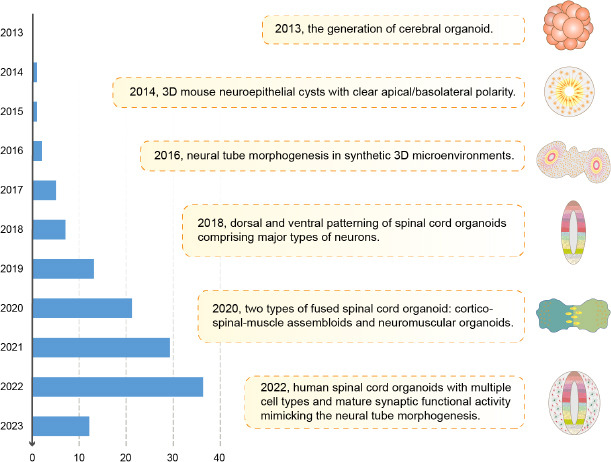
Timeline of major progress in spinal cord organoid research.
The left of this figure shows the number of papers identified by searching PubMed with the following keywords on the 12th of March 2023: “((spinal cord organoid) OR (spinal organoid)) OR (neural tube organoid).” The middle and right of the figure shows the main advancements in generating spinal cord organoids. Created with Adobe Illustrator 2021. 3D: Three-dimensional.
Spinal Cord Organoids for Investigating Disease
Organoids are cutting-edge innovations that recapitulate the physiological processes of the in vivo organs and offer numerous advantages over traditional two-dimensional cultures and animal models. These organoids exhibit almost physiological cytoarchitecture and functionality, allow for extended cultivation, maintain genome stability, and reduce experimental complexity. These qualities make organoids valuable tools for disease modeling, drug screening, and tissue regeneration (Li and Izpisua Belmonte, 2019; Zhou et al., 2023; Figure 3). In combination with multi-omics and bioengineering technologies, organoids can also serve as excellent models for investigating pathogenic conditions of the spinal cord.
Figure 3.
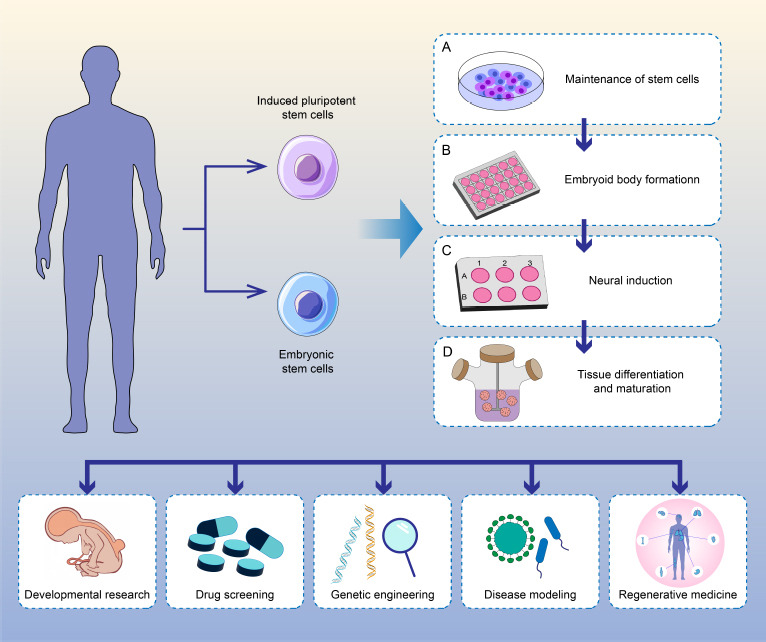
Schematic showing the cultivation protocols and applications of spinal cord organoids.
Protocols for generating spinal cord organoids typically involve the following steps: acquisition of seed cells, formation of embryoid bodies, neural induction, tissue differentiation and maturation. After successful construction, spinal cord organoids can be used for developmental research, disease modeling, drug screening, and tissue regeneration. Created with Adobe Illustrator 2021.
Neurodevelopmental diseases
Compared with traditional animal models, spinal cord organoids are more versatile for studying early neuropathogenesis by virtue of the intuitive assessment of planar cell polarity and morphological changes during neural tube closure. Moreover, the generation of neural tube defect organoids by gene editing has enabled researchers to directly investigate the progression of diseases and simultaneously conduct drug screening (Li and Chen, 2022). Winanto et al. (2020) developed a patient-derived spinal cord organoid to study mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS), a maternally inherited mitochondrial DNA disease. By comparing this organoid with its isogenically corrected counterpart, they discovered that elevated levels of Notch signaling contribute to restricted neurogenesis and neurite outgrowth; these changes are associated with the pathogenesis of MELAS. The inhibition of Notch signaling by DAPT has been shown to reverse these neurodevelopmental defects in MELAS organoids (Winanto et al., 2020). Organoids can also be utilized to study the impact of mechanical forces on neural tube development. Previously, researchers used an equibiaxial stretching device to exert active mechanical forces on neural tube organoids and adjust neural patterning and morphogenesis. This biomechanical system could facilitate the decoding of regulatory networks responsible for mechanical forces in the developmental process (Abdel Fattah et al., 2021). Furthermore, organoids can serve as tools to investigate the neurotoxic effects of chemicals that induce developmental defects in the fetus (Zheng et al., 2021b). By utilizing spinal cord organoids to investigate the impact of anti-epileptic drugs on the neural tube at different concentrations, Lee et al. (2022) demonstrated that valproic acid and carbamazepine had a dose-dependent effect on the failure of neural tube closure.
Motor neuron and neurodegenerative diseases
Fused neuromuscular organoids can self-organize into functional neuromuscular junctions and exhibit central pattern generator-like circuits, thus providing a valuable platform for modeling a range of motor neuron diseases including amyotrophic lateral sclerosis, spinal muscular atrophy (SMA), and myasthenia gravis (Ichida and Ko, 2020). The construction of patient-specific neuromuscular organoids might provide novel insights into the underlying mechanisms of these diseases and facilitate the exploration of novel therapeutics. Notably, with the optimization of cultivation protocols over recent years, timely exposure to specific morphogens in the culture system could drive the expansion of native oligodendrocyte precursor cell populations and induce oligodendrocyte differentiation in neural organoids (Madhavan et al., 2018; Marton et al., 2019). Previous studies have confirmed that organoids that model the motor neuron axonal fascicle can be subjected to morphological, electrical, and physical analysis (Kawada et al., 2017). Establishing motor nerve organoids containing oligodendrocytes holds significant potential for studying the myelination and remyelination process in neural degenerative diseases. Hor et al. (2018) developed a ventral spinal organoid model of SMA that does not exhibit any obvious developmental neurogenetic defects but showed extensive motor neuron degeneration during the cultivation process, thus indicating that SMA is more likely a neurodegenerative disorder than a neurodevelopmental disorder. The motor neurons of the SMA express high levels of cyclin-dependent kinases and the inhibition of cyclin-dependent kinases has been shown to rescue degeneration, thus suggesting that cyclin-dependent kinase inhibitors may be potential therapeutic drugs for SMA (Hor et al., 2018). By employing gene editing technologies, it is possible to model different disease variants and investigate heterogeneity in an in-depth manner. For instance, sensorimotor organoids modeling different clinical variants of amyotrophic lateral sclerosis exhibited different degrees of damage in neuromuscular junctions (Pereira et al., 2021). Daviaud et al. (2023) constructed cerebral organoids using iPSCs from patients with multiple sclerosis (MS) to investigate the pathological mechanisms of different clinical subtypes. These authors identified dysregulation in the proliferation and differentiation of NPCs in MS organoids, with the most significant effect observed in primary progressive MS. This form of dysregulation resulted in an increased number of neurons but a decrease in the number of oligodendrocytes. This imbalance of proliferation and differentiation was associated with the down-regulated expression of the cell cycle inhibitor p21, thus yielding a novel therapeutic target for MS (Daviaud et al., 2023).
Pain
DRGs contain a large number of small nerve fiber nuclei and multiple pro-nociceptive molecules, making them crucial relay stations for transmitting and generating pain signals (Martínez-Lavín, 2021; Deng et al., 2023; Li et al., 2023a). Targeting DRGs to alleviate neuropathic pain has been a hot topic for several decades. However, the limited availability of human DRG tissues has hindered many pre-clinical studies, especially those involving drug screening. DRG organoids derived from fibroblasts exhibit cellular and molecular characteristics comparable to those of native DRGs (Xiao et al., 2020). By integrating organoid and co-culture techniques, it is possible to create fused organoids that feature fully developed sensory neurons and receptors. A model of human tissue-engineered skin was created by coculturing human keratinocytes and fibroblasts with sensory neurons and Schwann cells derived from iPSCs in a 3D collagen sponge model. This model exhibited higher levels of neuronal cell maturity and the extension of neurites to reach epidermal-like layers, thus yielding significant potential for investigating skin-related pain pathologies (Muller et al., 2018). Recently, researchers have engineered spinal cord organoids-on-a-chip to recapitulate the intricate nociceptive circuitry. These organoids can be effectively integrated with multiple-electrode array system, thus allowing for plug-and-play measurement of neural activity (Ao et al., 2022; Cai et al., 2023). Pain-evoking substances and drugs that modulate nociception have been used to validate these devices, which demonstrating a promising direction for the etiological investigation of pain (Ao et al., 2022).
SCI
SCI is a devastating neural trauma that affects millions of people worldwide. The limited intrinsic regenerative capacity and complex inhibitory microenvironment associated with SCI leads to permanent motor-sensory dysfunction. Stem cell transplantation was once regarded as a promising strategy; however, the therapeutic outcome of this technology was restricted by poor survival rates, uncontrollable differentiation, inefficient integration, and tumorigenicity. To tackle these issues, combination of bioscaffolds and stem cell transplantation presents a promising strategy to create conducive microenvironments for cell survival. Bioscaffolds can reduce stem cell dispersion, alleviate local inflammation, enhance stem cell aggregation, and promote favorable interactions between cells and extracellular matrices (Chen et al., 2021). However, mechanical mismatches, improper biodegradation rates, and immune reactions have limited their clinical application (Liu et al., 2018). Stem cell-derived spinal cord-like tissues (SCLTs) resemble native spinal cords in both geometrical structure and mature cytoarchitecture. SCLTs can interact with their surrounding environment and exhibit robust neurite outgrowth, making them a promising candidate for reconstructing the neural network of the injured spinal cord. To avoid immunogenicity, the biomaterials used in the culture of SCLTs can be extracted from the tissues of individuals; this can also provide the somatic cells to generate iPSCs (Wertheim et al., 2022). Cells and hydrogels derived from the same individual have been shown to exhibit a synergistic effect in mimicking the process of embryonic spinal cord formation. The transplantation of engineered SCLTs has been shown to significantly alleviate the motor dysfunction of chronic SCI (Wertheim et al., 2022). Lai et al. (2018) constructed white matter-like tissues and gray matter-like tissues separately, and then assembled the two modules into SCLTs to recapitulate the cytoarchitecture and functionality of adult spinal cord tissue. Transplanting SCLTs to transected spinal cord was shown to rebuild the neural circuits and improve functional recovery. The therapeutic efficacy of this technique was further reinforced when combined with tail nerve electrical stimulation (Lai et al., 2023). Recently, a spinal cord organoid with functional neurons specific to the dorsal and ventral domains has been generated by reprogramming human astrocytes into early neuroectodermal cells. When grafted into complete models of SCI, these organoids formed synaptic connectivity with host neurons to bridge the injured tissues (Xu et al., 2023). By utilizing a biomaterial delivery system, it may be possible to achieve the in situ reprogramming of human astrocytes at the injury site, thereby avoiding invasive procedures. In conclusion, spinal cord organoids and SCLTs have a promising future and could shed light on new avenues for the treatment of SCI. Although a previous study found no significant advantage of transplanting neural organoids over immature stem cell-loaded Matrigel beads in SCI treatment, the potential superiority of organoids needs to be reconfirmed by controlling key variables in well-designed experiments (Wang et al., 2022).
Challenges and Future Perspectives
Although tremendous progress has been achieved over the past decade, spinal cord organoid technology remains in its infancy and has not been broadly applied in basic and translational medicine due to several limitations. Firstly, the lack of standardized protocols and evaluation systems for spinal cord organoids cultivation results in significant heterogeneity and limited reproducibility. Differentiation protocols adopted by different research teams vary in terms of stem cell lines, morphogenic cues, cultivation period, and biomaterials. These discrepancies cause variations in morphology and cytoarchitecture. Even the same differentiation protocol may generate heterogeneous tissues (Cowan et al., 2020). Generating mass-scale organoids for translational applications requires protocols and reagents that meet good manufacturing practice standards. Secondly, current spinal cord organoids are unable to fully replicate the characteristics of the spinal cord in vivo. Most of these organoids only possess the main types of neurons along the DV axis and lack both glial cells and nerve fibers. Therefore, it is challenging to establish a complete neural circuit and achieve integrated functionality (Iyer and Ashton, 2022). Furthermore, because of the relatively short culture period and our limited comprehension of developmental signaling, spinal cord organoids can only replicate the early developmental stage, thus making it difficult to reproduce the fully developed native spinal cord (Guy et al., 2021). This limitation presents significant obstacles for studying age-related pathogenesis (Castillo Bautista and Sterneckert, 2022). Optimized protocols and advancements in the field of developmental biology of the spinal cord will facilitate long-term culture in the future. Finally, the assessment of spinal cord organoids has primarily focused on their morphology and has not considered their electrophysiological functionality and bioelectrical environment. As bioelectric fields have been shown to regulate neurodevelopment, the application of exogenous electric fields may enhance cell survival, proliferation, and specification while reducing death and necrosis in the culture of neural organoids (O’Hara-Wright et al., 2022). The optimization of bioelectric stimulation and electrophysiological functionality evaluation is vital if we are to develop the next generation of spinal cord organoids.
Conclusions
Spinal cord organoids provide experimentally tractable systems for developmental studies, disease modeling, drug screening, and regenerative medicine. Compared with conventional animal models and two-dimensional cell culturing, spinal cord organoids provide a similar physiological microenvironment and present fewer ethical restrictions for studying spinal cord diseases. Organoids generated from patient-derived iPSCs can reflect specific genetic backgrounds and serve as a valuable tool for investigating the molecular basis of certain pathologies. However, the widespread use of spinal cord organoids is hindered by tissue heterogeneity, variability in differentiation, central necrosis, and immature functionality. Despite these obstacles, current bioengineering techniques hold great promise for overcoming these limitations and unraveling the intricate mechanisms of human spinal cord development. The next generation of spinal cord organoids should be optimized for good manufacturing practice standard, vascularization, phenotypic diversity, and electrophysiological maturation. Because of the rapid advancements in spinal cord organoid technology and the limitations of our retrieval strategies, some cutting-edge knowledge and the very latest articles may not be included in this review. However, we believe our review provides a state-of-the-art outline of studies relating to spinal cord organoids. We eagerly look forward to the day when spinal cord organoids will spark a revolution in the field of neuroscience.
Funding Statement
Funding: This work was supported by the sup-project of National Key R&D Program of China, No. 2018YFA0108602; CAMS Innovation Fund for Medical Sciences, No. CIFMS, 2021-I2M-C&T-B-016; and National High Level Hospital Clinical Research Funding, No. 2022-PUMCH-B-112 (all to JG).
Footnotes
Conflicts of interest: The authors have no conflicts of interest to declare.
Data availability statement: Not applicable.
C-Editors: Wang J, Zhao M; S-Editors: Yu J, Li CH; L-Editors: Yu J, Song LP; T-Editor: Jia Y
References
- 1.Abdel Fattah AR, Daza B, Rustandi G, Berrocal-Rubio M, Gorissen B, Poovathingal S, Davie K, Barrasa-Fano J, Cóndor M, Cao X, Rosenzweig DH, Lei Y, Finnell R, Verfaillie C, Sampaolesi M, Dedecker P, Van Oosterwyck H, Aerts S, Ranga A. Actuation enhances patterning in human neural tube organoids. Nat Commun. (2021);12:3192. doi: 10.1038/s41467-021-22952-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen J, Revah O, Miura Y, Thom N, Amin ND, Kelley KW, Singh M, Chen X, Thete MV, Walczak EM, Vogel H, Fan HC, Paşca SP. Generation of functional human 3D cortico-motor assembloids. Cell. (2020);183:1913–1929.e26. doi: 10.1016/j.cell.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews MG, Kong J, Novitch BG, Butler SJ. New perspectives on the mechanisms establishing the dorsal-ventral axis of the spinal cord. Curr Top Dev Biol. (2019);132:417–450. doi: 10.1016/bs.ctdb.2018.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ao Z, Cai H, Wu Z, Krzesniak J, Tian C, Lai YY, Mackie K, Guo F. Human spinal organoid-on-a-chip to model nociceptive circuitry for pain therapeutics discovery. Anal Chem. (2022);94:1365–1372. doi: 10.1021/acs.analchem.1c04641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Artegiani B, Hendriks D, Beumer J, Kok R, Zheng X, Joore I, Chuva de Sousa Lopes S, van Zon J, Tans S, Clevers H. Fast and efficient generation of knock-in human organoids using homology-independent CRISPR-Cas9 precision genome editing. Nat Cell Biol. (2020);22:321–331. doi: 10.1038/s41556-020-0472-5. [DOI] [PubMed] [Google Scholar]
- 6.Budjan C, Liu S, Ranga A, Gayen S, Pourquié O, Hormoz S. Paraxial mesoderm organoids model development of human somites. Elife. (2022);11:e68925. doi: 10.7554/eLife.68925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai H, Ao Z, Tian C, Wu Z, Kaurich C, Chen Z, Gu M, Hohmann AG, Mackie K, Guo F. Engineering human spinal microphysiological systems to model opioid-induced tolerance. Bioact Mater. (2023);22:482–490. doi: 10.1016/j.bioactmat.2022.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cakir B, Xiang Y, Tanaka Y, Kural MH, Parent M, Kang YJ, Chapeton K, Patterson B, Yuan Y, He CS, Raredon MSB, Dengelegi J, Kim KY, Sun P, Zhong M, Lee S, Patra P, Hyder F, Niklason LE, Lee SH, et al. Engineering of human brain organoids with a functional vascular-like system. Nat Methods. (2019);16:1169–1175. doi: 10.1038/s41592-019-0586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castillo Bautista CM, Sterneckert J. Progress and challenges in directing the differentiation of human iPSCs into spinal motor neurons. Front Cell Dev Biol. (2022);10:1089970. doi: 10.3389/fcell.2022.1089970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalamalasetty RB, Garriock RJ, Dunty WC, Jr, Kennedy MW, Jailwala P, Si H, Yamaguchi TP. Mesogenin 1 is a master regulator of paraxial presomitic mesoderm differentiation. Development. (2014);141:4285–4297. doi: 10.1242/dev.110908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Wang Y, Zhou G, Hu X, Han S, Gao J. The combination of nanoscaffolds and stem cell transplantation:Paving a promising road for spinal cord injury regeneration. Biomed Pharmacother. (2021);143:112233. doi: 10.1016/j.biopha.2021.112233. [DOI] [PubMed] [Google Scholar]
- 12.Clevers H. Modeling development and disease with organoids. Cell. (2016);165:1586–1597. doi: 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 13.Cowan CS, Renner M, De Gennaro M, Gross-Scherf B, Goldblum D, Hou Y, Munz M, Rodrigues TM, Krol J, Szikra T, Cuttat R, Waldt A, Papasaikas P, Diggelmann R, Patino-Alvarez CP, Galliker P, Spirig SE, Pavlinic D, Gerber-Hollbach N, Schuierer S, et al. Cell types of the human retina and its organoids at single-cell resolution. Cell. (2020);182:1623–1640.e34. doi: 10.1016/j.cell.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daviaud N, Chen E, Edwards T, Sadiq SA. Cerebral organoids in primary progressive multiple sclerosis reveal stem cell and oligodendrocyte differentiation defect. Biol Open. (2023);12:bio059845. doi: 10.1242/bio.059845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demers CJ, Cox G, Collins SD, Smith RL. Directing the spatial patterning of motor neuron differentiation in engineered microenvironments. Annu Int Conf IEEE Eng Med Biol Soc. (2016a);2016:477–480. doi: 10.1109/EMBC.2016.7590743. [DOI] [PubMed] [Google Scholar]
- 16.Demers CJ, Soundararajan P, Chennampally P, Cox GA, Briscoe J, Collins SD, Smith RL. Development-on-chip:in vitro neural tube patterning with a microfluidic device. Development. (2016b);143:1884–1892. doi: 10.1242/dev.126847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng YF, Xiang P, Du JY, Liang JF, Li X. Intrathecal liproxstatin-1 delivery inhibits ferroptosis and attenuates mechanical and thermal hypersensitivities in rats with complete Freund's adjuvant-induced inflammatory pain. Neural Regen Res. (2023);18:456–462. doi: 10.4103/1673-5374.346547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duval N, Vaslin C, Barata TC, Frarma Y, Contremoulins V, Baudin X, Nedelec S, Ribes VC. BMP4 patterns Smad activity and generates stereotyped cell fate organization in spinal organoids. Development. (2019);146:dev175430. doi: 10.1242/dev.175430. [DOI] [PubMed] [Google Scholar]
- 19.Esk C, Lindenhofer D, Haendeler S, Wester RA, Pflug F, Schroeder B, Bagley JA, Elling U, Zuber J, von Haeseler A, Knoblich JA. A human tissue screen identifies a regulator of ER secretion as a brain-size determinant. Science. (2020);370:935–941. doi: 10.1126/science.abb5390. [DOI] [PubMed] [Google Scholar]
- 20.Faustino Martins JM, Fischer C, Urzi A, Vidal R, Kunz S, Ruffault PL, Kabuss L, Hube I, Gazzerro E, Birchmeier C, Spuler S, Sauer S, Gouti M. Self-organizing 3D human trunk neuromuscular organoids. Cell Stem Cell. (2020);27:498. doi: 10.1016/j.stem.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Fedorchak NJ, Iyer N, Ashton RS. Bioengineering tissue morphogenesis and function in human neural organoids. Semin Cell Dev Biol. (2021);111:52–59. doi: 10.1016/j.semcdb.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garriock RJ, Chalamalasetty RB, Kennedy MW, Canizales LC, Lewandoski M, Yamaguchi TP. Lineage tracing of neuromesodermal progenitors reveals novel Wnt-dependent roles in trunk progenitor cell maintenance and differentiation. Development. (2015);142:1628–1638. doi: 10.1242/dev.111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grebenyuk S, Ranga A. Engineering organoid vascularization. Front Bioeng Biotechnol. (2019);7:39. doi: 10.3389/fbioe.2019.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guy B, Zhang JS, Duncan LH, Johnston RJ., Jr Human neural organoids:Models for developmental neurobiology and disease. Dev Biol. (2021);478:102–121. doi: 10.1016/j.ydbio.2021.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Habibey R, Rojo Arias JE, Striebel J, Busskamp V. Microfluidics for neuronal cell and circuit engineering. Chem Rev. (2022);122:14842–14880. doi: 10.1021/acs.chemrev.2c00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagbard L, Cameron K, August P, Penton C, Parmar M, Hay DC, Kallur T. Developing defined substrates for stem cell culture and differentiation. Philos Trans R Soc Lond B Biol Sci. (2018);373:20170230. doi: 10.1098/rstb.2017.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ham O, Jin YB, Kim J, Lee MO. Blood vessel formation in cerebral organoids formed from human embryonic stem cells. Biochem Biophys Res Commun. (2020);521:84–90. doi: 10.1016/j.bbrc.2019.10.079. [DOI] [PubMed] [Google Scholar]
- 28.Hawryluk GW, Ruff CA, Fehlings MG. Development and maturation of the spinal cord:implications of molecular and genetic defects. Handb Clin Neurol. (2012);109:3–30. doi: 10.1016/B978-0-444-52137-8.00001-2. [DOI] [PubMed] [Google Scholar]
- 29.Henrique D, Abranches E, Verrier L, Storey KG. Neuromesodermal progenitors and the making of the spinal cord. Development. (2015);142:2864–2875. doi: 10.1242/dev.119768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hor JH, Ng SY. Generating ventral spinal organoids from human induced pluripotent stem cells. Methods Cell Biol. (2020);159:257–277. doi: 10.1016/bs.mcb.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Hor JH, Soh ES, Tan LY, Lim VJW, Santosa MM, Winanto, Ho BX, Fan Y, Soh BS, Ng SY. Cell cycle inhibitors protect motor neurons in an organoid model of Spinal Muscular Atrophy. Cell Death Dis. (2018);(1100);9 doi: 10.1038/s41419-018-1081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ichida JK, Ko CP. Organoids develop motor skills:3D human neuromuscular junctions. Cell Stem Cell. (2020);26:131–133. doi: 10.1016/j.stem.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Iyer NR, Ashton RS. Bioengineering the human spinal cord. Front Cell Dev Biol. (2022);10:942742. doi: 10.3389/fcell.2022.942742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karzbrun E, Kshirsagar A, Cohen SR, Hanna JH, Reiner O. Human brain organoids on a chip reveal the physics of folding. Nat Phys. (2018);14:515–522. doi: 10.1038/s41567-018-0046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawada J, Kaneda S, Kirihara T, Maroof A, Levi T, Eggan K, Fujii T, Ikeuchi Y. Generation of a motor nerve organoid with human stem cell-derived neurons. Stem Cell Reports. (2017);9:1441–1449. doi: 10.1016/j.stemcr.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knight GT, Lundin BF, Iyer N, Ashton LM, Sethares WA, Willett RM, Ashton RS. Engineering induction of singular neural rosette emergence within hPSC-derived tissues. Elife. (2018);7:e37549. doi: 10.7554/eLife.37549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kofman S, Mohan N, Sun X, Ibric L, Piermarini E, Qiang L. Human mini brains and spinal cords in a dish:Modeling strategies current challenges and prospective advances. J Tissue Eng. (2022);13:20417314221113391. doi: 10.1177/20417314221113391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kozlowski MT, Crook CJ, Ku HT. Towards organoid culture without Matrigel. Commun Biol. (2021);4:1387. doi: 10.1038/s42003-021-02910-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kutejova E, Sasai N, Shah A, Gouti M, Briscoe J. Neural progenitors adopt specific identities by directly repressing all alternative progenitor transcriptional programs. Dev Cell. (2016);36:639–653. doi: 10.1016/j.devcel.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lai BQ, Feng B, Che MT, Wang LJ, Cai S, Huang MY, Gu HY, Jiang B, Ling EA, Li M, Zeng X, Zeng YS. A modular assembly of spinal cord-like tissue allows targeted tissue repair in the transected spinal cord. Adv Sci (Weinh) (2018);5:1800261. doi: 10.1002/advs.201800261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai BQ, Wu RJ, Han WT, Bai YR, Liu JL, Yu HY, Yang SB, Wang LJ, Ren JL, Ding Y, Li G, Zeng X, Ma YH, Quan Q, Xing LY, Jiang B, Wang YQ, Zhang L, Chen ZH, Zhang HB, et al. Tail nerve electrical stimulation promoted the efficiency of transplanted spinal cord-like tissue as a neuronal relay to repair the motor function of rats with transected spinal cord injury. Biomaterials. (2023);297:122103. doi: 10.1016/j.biomaterials.2023.122103. [DOI] [PubMed] [Google Scholar]
- 42.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. (2013);501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JH, Shin H, Shaker MR, Kim HJ, Park SH, Kim JH, Lee N, Kang M, Cho S, Kwak TH, Kim JW, Song MR, Kwon SH, Han DW, Lee S, Choi SY, Rhyu IJ, Kim H, Geum D, Cho IJ, et al. Production of human spinal-cord organoids recapitulating neural-tube morphogenesis. Nat Biomed Eng. (2022);6:435–448. doi: 10.1038/s41551-022-00868-4. [DOI] [PubMed] [Google Scholar]
- 44.Leung B, Shimeld SM. Evolution of vertebrate spinal cord patterning. Dev Dyn. (2019);248:1028–1043. doi: 10.1002/dvdy.77. [DOI] [PubMed] [Google Scholar]
- 45.Li DY, Gao SJ, Sun J, Zhang LQ, Wu JY, Song FH, Liu DQ, Zhou YQ, Mei W. Targeting the nitric oxide/cGMP signaling pathway to treat chronic pain. Neural Regen Res. (2023a);18:996–1003. doi: 10.4103/1673-5374.355748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li M, Izpisua Belmonte JC. Organoids - preclinical models of human disease. N Engl J Med. (2019);380:569–579. doi: 10.1056/NEJMra1806175. [DOI] [PubMed] [Google Scholar]
- 47.Li M, Gao L, Zhao L, Zou T, Xu H. Toward the next generation of vascularized human neural organoids. Med Res Rev. (2023b);43:31–54. doi: 10.1002/med.21922. [DOI] [PubMed] [Google Scholar]
- 48.Li P, Chen Y. Progress in modeling neural tube development and defects by organoid reconstruction. Neurosci Bull. (2022);38:1409–1419. doi: 10.1007/s12264-022-00896-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lim GS, Hor JH, Ho NRY, Wong CY, Ng SY, Soh BS, Shao H. Microhexagon gradient array directs spatial diversification of spinal motor neurons. Theranostics. (2019);9:311–323. doi: 10.7150/thno.29755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lippmann ES, Williams CE, Ruhl DA, Estevez-Silva MC, Chapman ER, Coon JJ, Ashton RS. Deterministic HOX patterning in human pluripotent stem cell-derived neuroectoderm. Stem Cell Reports. (2015);4:632–644. doi: 10.1016/j.stemcr.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Z, Tang M, Zhao J, Chai R, Kang J. Looking into the future:toward advanced 3D biomaterials for stem-cell-based regenerative medicine. Adv Mater. (2018);30:e1705388. doi: 10.1002/adma.201705388. [DOI] [PubMed] [Google Scholar]
- 52.Madhavan M, Nevin ZS, Shick HE, Garrison E, Clarkson-Paredes C, Karl M, Clayton BLL, Factor DC, Allan KC, Barbar L, Jain T, Douvaras P, Fossati V, Miller RH, Tesar PJ. Induction of myelinating oligodendrocytes in human cortical spheroids. Nat Methods. (2018);15:700–706. doi: 10.1038/s41592-018-0081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mansour AA, Goncalves JT, Bloyd CW, Li H, Fernandes S, Quang D, Johnston S, Parylak SL, Jin X, Gage FH. An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol. (2018);36:432–441. doi: 10.1038/nbt.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martínez-Lavín M. Dorsal root ganglia:fibromyalgia pain factory? Clin Rheumatol. (2021);40:783–787. doi: 10.1007/s10067-020-05528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marton RM, Miura Y, Sloan SA, Li Q, Revah O, Levy RJ, Huguenard JR, Paşca SP. Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat Neurosci. (2019);22:484–491. doi: 10.1038/s41593-018-0316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meinhardt A, Eberle D, Tazaki A, Ranga A, Niesche M, Wilsch-Bräuninger M, Stec A, Schackert G, Lutolf M, Tanaka EM. 3D reconstitution of the patterned neural tube from embryonic stem cells. Stem Cell Reports. (2014);3:987–999. doi: 10.1016/j.stemcr.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mouilleau V, Vaslin C, Robert R, Gribaudo S, Nicolas N, Jarrige M, Terray A, Lesueur L, Mathis MW, Croft G, Daynac M, Rouiller-Fabre V, Wichterle H, Ribes V, Martinat C, Nedelec S. Dynamic extrinsic pacing of the HOX clock in human axial progenitors controls motor neuron subtype specification. Development. (2021);148:dev.194514. doi: 10.1242/dev.194514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muller Q, Beaudet MJ, De Serres-Bérard T, Bellenfant S, Flacher V, Berthod F. Development of an innervated tissue-engineered skin with human sensory neurons and Schwann cells differentiated from iPS cells. Acta Biomater. (2018);82:93–101. doi: 10.1016/j.actbio.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 59.O'Hara-Wright M, Mobini S, Gonzalez-Cordero A. Bioelectric potential in next-generation organoids:electrical stimulation to enhance 3D structures of the central nervous system. Front Cell Dev Biol. (2022);10:901652. doi: 10.3389/fcell.2022.901652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ogura T, Sakaguchi H, Miyamoto S, Takahashi J. Three-dimensional induction of dorsal and ventral spinal cord tissues from human pluripotent stem cells. Development. (2018);145:dev162214. doi: 10.1242/dev.162214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olmsted ZT, Paluh JL. Stem cell neurodevelopmental solutions for restorative treatments of the human trunk and spine. Front Cell Neurosci. (2021a);15:667590. doi: 10.3389/fncel.2021.667590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olmsted ZT, Paluh JL. Co-development of central and peripheral neurons with trunk mesendoderm in human elongating multi-lineage organized gastruloids. Nat Commun. (2021b);12:3020. doi: 10.1038/s41467-021-23294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park J, Hsiung HA, Khven I, La Manno G, Lutolf MP. Self-organizing in vitro mouse neural tube organoids mimic embryonic development. Development. (2022);149:dev201052. doi: 10.1242/dev.201052. [DOI] [PubMed] [Google Scholar]
- 64.Park SE, Georgescu A, Huh D. Organoids-on-a-chip. Science. (2019);364:960–965. doi: 10.1126/science.aaw7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pereira JD, DuBreuil DM, Devlin AC, Held A, Sapir Y, Berezovski E, Hawrot J, Dorfman K, Chander V, Wainger BJ. Human sensorimotor organoids derived from healthy and amyotrophic lateral sclerosis stem cells form neuromuscular junctions. Nat Commun. (2021);12:4744. doi: 10.1038/s41467-021-24776-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pham MT, Pollock KM, Rose MD, Cary WA, Stewart HR, Zhou P, Nolta JA, Waldau B. Generation of human vascularized brain organoids. Neuroreport. (2018);29:588–593. doi: 10.1097/WNR.0000000000001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Philippidou P, Dasen JS. Hox genes:choreographers in neural development, architects of circuit organization. Neuron. (2013);80:12–34. doi: 10.1016/j.neuron.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Porciúncula LO, Goto-Silva L, Ledur PF, Rehen SK. The age of brain organoids:tailoring cell identity and functionality for normal brain development and disease modeling. Front Neurosci. (2021);15:674563. doi: 10.3389/fnins.2021.674563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qian X, Jacob F, Song MM, Nguyen HN, Song H, Ming GL. Generation of human brain region-specific organoids using a miniaturized spinning bioreactor. Nat Protoc. (2018);13:565–580. doi: 10.1038/nprot.2017.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rambani K, Vukasinovic J, Glezer A, Potter SM. Culturing thick brain slices:an interstitial 3D microperfusion system for enhanced viability. J Neurosci Methods. (2009);180:243–254. doi: 10.1016/j.jneumeth.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ranga A, Girgin M, Meinhardt A, Eberle D, Caiazzo M, Tanaka EM, Lutolf MP. Neural tube morphogenesis in synthetic 3D microenvironments. Proc Natl Acad Sci U S A. (2016);113:E6831–E6839. doi: 10.1073/pnas.1603529113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rogers KW, Schier AF. Morphogen gradients:from generation to interpretation. Annu Rev Cell Dev Biol. (2011);27:377–407. doi: 10.1146/annurev-cellbio-092910-154148. [DOI] [PubMed] [Google Scholar]
- 73.Sagner A, Briscoe J. Establishing neuronal diversity in the spinal cord:a time and a place. Development. (2019);146:dev182154. doi: 10.1242/dev.182154. [DOI] [PubMed] [Google Scholar]
- 74.Sanaki-Matsumiya M, Matsuda M, Gritti N, Nakaki F, Sharpe J, Trivedi V, Ebisuya M. Periodic formation of epithelial somites from human pluripotent stem cells. Nat Commun. (2022);13:2325. doi: 10.1038/s41467-022-29967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sances S, Ho R, Vatine G, West D, Laperle A, Meyer A, Godoy M, Kay PS, Mandefro B, Hatata S, Hinojosa C, Wen N, Sareen D, Hamilton GA, Svendsen CN. Human iPSC-derived endothelial cells and microengineered organ-chip enhance neuronal development. Stem Cell Reports. (2018);10:1222–1236. doi: 10.1016/j.stemcr.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stern CD. Neural induction:10 years on since the 'default model'. Curr Opin Cell Biol. (2006);18:692–697. doi: 10.1016/j.ceb.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 77.Stifani N. Motor neurons and the generation of spinal motor neuron diversity. Front Cell Neurosci. (2014);8:293. doi: 10.3389/fncel.2014.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van den Brink SC, Alemany A, van Batenburg V, Moris N, Blotenburg M, Vivié J, Baillie-Johnson P, Nichols J, Sonnen KF, Martinez Arias A, van Oudenaarden A. Single-cell and spatial transcriptomics reveal somitogenesis in gastruloids. Nature. (2020);582:405–409. doi: 10.1038/s41586-020-2024-3. [DOI] [PubMed] [Google Scholar]
- 79.Veenvliet JV, Bolondi A, Kretzmer H, Haut L, Scholze-Wittler M, Schifferl D, Koch F, Guignard L, Kumar AS, Pustet M, Heimann S, Buschow R, Wittler L, Timmermann B, Meissner A, Herrmann BG. Mouse embryonic stem cells self-organize into trunk-like structures with neural tube and somites. Science. (2020);370:eaba4937. doi: 10.1126/science.aba4937. [DOI] [PubMed] [Google Scholar]
- 80.Vihola H, Laukkanen A, Valtola L, Tenhu H, Hirvonen J. Cytotoxicity of thermosensitive polymers poly(N-isopropylacrylamide) poly(N-vinylcaprolactam) and amphiphilically modified poly(N-vinylcaprolactam) Biomaterials. (2005);26:3055–3064. doi: 10.1016/j.biomaterials.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 81.Wang Z, Zhao H, Tang X, Meng T, Khutsishvili D, Xu B, Ma S. CNS organoid surpasses cell-laden microgel assembly to promote spinal cord injury repair. Research (Wash D C) (2022);2022:9832128. doi: 10.34133/2022/9832128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wei YT, He Y, Xu CL, Wang Y, Liu BF, Wang XM, Sun XD, Cui FZ, Xu QY. Hyaluronic acid hydrogel modified with nogo-66 receptor antibody and poly-L-lysine to promote axon regrowth after spinal cord injury. J Biomed Mater Res B Appl Biomater. (2010);95:110–117. doi: 10.1002/jbm.b.31689. [DOI] [PubMed] [Google Scholar]
- 83.Wertheim L, Edri R, Goldshmit Y, Kagan T, Noor N, Ruban A, Shapira A, Gat-Viks I, Assaf Y, Dvir T. Regenerating the injured spinal cord at the chronic phase by engineered iPSCs-derived 3D neuronal networks. Adv Sci (Weinh) (2022);9:e2105694. doi: 10.1002/advs.202105694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Winanto, Khong ZJ, Hor JH, Ng SY. Spinal cord organoids add an extra dimension to traditional motor neuron cultures. Neural Regen Res. (2019);14:1515–1516. doi: 10.4103/1673-5374.255966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Winanto, Khong ZJ, Soh BS, Fan Y, Ng SY. Organoid cultures of MELAS neural cells reveal hyperactive Notch signaling that impacts neurodevelopment. Cell Death Dis. (2020);11:182. doi: 10.1038/s41419-020-2383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xiang Y, Tanaka Y, Patterson B, Kang YJ, Govindaiah G, Roselaar N, Cakir B, Kim KY, Lombroso AP, Hwang SM, Zhong M, Stanley EG, Elefanty AG, Naegele JR, Lee SH, Weissman SM, Park IH. Fusion of regionally specified hPSC-derived organoids models human brain development and interneuron migration. Cell Stem Cell. (2017);21:383–398.e7. doi: 10.1016/j.stem.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xiao D, Deng Q, Guo Y, Huang X, Zou M, Zhong J, Rao P, Xu Z, Liu Y, Hu Y, Shen Y, Jin K, Xiang M. Generation of self-organized sensory ganglion organoids and retinal ganglion cells from fibroblasts. Sci Adv. (2020);6:eaaz5858. doi: 10.1126/sciadv.aaz5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu J, Fang S, Deng S, Li H, Lin X, Huang Y, Chung S, Shu Y, Shao Z. Generation of neural organoids for spinal-cord regeneration via the direct reprogramming of human astrocytes. Nat Biomed Eng. (2023);7:253–269. doi: 10.1038/s41551-022-00963-6. [DOI] [PubMed] [Google Scholar]
- 89.Xue W, Li B, Liu H, Xiao Y, Li B, Ren L, Li H, Shao Z. Generation of dorsoventral human spinal cord organoids via functionalizing composite scaffold for drug testing. Science. (2023);26:105898. doi: 10.1016/j.isci.2022.105898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang L, Feng HY, Xu YJ. Current status of 3D printing medical applications and center construction. Zhongguo Zuzhi Gongcheng Yanjiu. (2023);27:2110–2115. [Google Scholar]
- 91.Zhang ZN, Freitas BC, Qian H, Lux J, Acab A, Trujillo CA, Herai RH, Nguyen Huu VA, Wen JH, Joshi-Barr S, Karpiak JV, Engler AJ, Fu XD, Muotri AR, Almutairi A. Layered hydrogels accelerate iPSC-derived neuronal maturation and reveal migration defects caused by MeCP2 dysfunction. Proc Natl Acad Sci U S A. (2016);113:3185–3190. doi: 10.1073/pnas.1521255113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zheng F, Xiao Y, Liu H, Fan Y, Dao M. Patient-specific organoid and organ-on-a-chip:3D cell-culture meets 3D printing and numerical simulation. Adv Biol (Weinh) (2021a);5:e2000024. doi: 10.1002/adbi.202000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zheng Y, Zhang F, Xu S, Wu L. Advances in neural organoid systems and their application in neurotoxicity testing of environmental chemicals. Genes Environ. (2021b);43:39. doi: 10.1186/s41021-021-00214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou JQ, Zeng LH, Li CT, He DH, Zhao HD, Xu YN, Jin ZT, Gao C. Brain organoids are new tool for drug screening of neurological diseases. Neural Regen Res. (2023);18:1884–1889. doi: 10.4103/1673-5374.367983. [DOI] [PMC free article] [PubMed] [Google Scholar]


