The build-up of misfolded α-synuclein (α-syn) in the central nervous system is the pathological hallmark of a number of neurodegenerative diseases that are known as α-synucleinopathies. These include Parkinson’s disease (PD), Parkinson’s disease with dementia (PDD), dementia with Lewy body (LB), multiple system atrophy (MSA), and a subset of Alzheimer’s disease. Growing evidence underscores that the intercellular transmission and amplification of pathological α-syn are critical processes underlying the progression of α-synucleinopathies (Peng et al., 2020), and as such, the study of these processes could lead to the identification of promising therapeutics to mitigate disease progression. Most previous studies have focused solely on pathological seeds in relation to disease progression. However, successful amplification requires two components: the formation of pathological α-syn seeds and the transformation of soluble α-syn to the pathological conformation. The potential effects that soluble α-syn could have on pathological α-syn amplification have not been studied. Although many post-translational modifications (PTMs) have been identified on α-syn, most studies have focused on the effect of PTMs on pathological α-syn initiation, toxicity, or physiological function (He et al., 2021). However, the effects of soluble α-syn PTMs on pathological α-syn amplification remain unknown. In our recent study (Zhang et al., 2023), we focused on how PTMs on soluble α-syn regulate pathological α-syn spread in different diseases and discovered that PTMs on soluble α-syn drastically modulated pathological α-syn in different α-synucleinopathies.
The novelty of our study comes from the fact that our study represents the first systematic analysis of PTMs on soluble α-syn. A previous study has mainly focused on PTMs in pathological α-syn (He et al., 2021), whereas our study was able to identify a large number of new modifications on soluble α-syn from diseased brains. Our identification of these novel PTM sites on soluble α-syn could serve as a useful resource for future research on biological functions and possible pathogenic modulation of these PTMs. From our findings, we discovered that soluble α-syn PTMs could modulate the spread of pathological α-syn via two different mechanisms. In one mechanism, the PTMs on soluble α-syn could alter the ability of pathological seeds to recruit endogenous α-syn. In the other mechanism, the seeding characteristics of pathological α-syn could be modulated by soluble α-syn PTMs after they were incorporated.
Different pathological α-syn strains have been discovered in different α-synucleinopathies, which largely contribute to disease diversity. The diversity in α-synucleinopathies can be seen in baseline physiological differences. For example, in PD, PDD, dementia with LB, and Alzheimer’s disease, pathological α-syn accumulates as LB in neurons. In contrast, in MSA, pathological α-syn is found most frequently in glial cytoplasmic inclusions (GCI). In our study and several others, it was discovered that pathological α-syn in GCI (GCI-α-syn) and LB (LB-α-syn) were considered distinct strains of α-syn and consequently had unique seeding abilities and conformations (Peng et al., 2018). Interestingly, we found that LB-α-syn, GCI-α-syn, and α-syn preformed fibrils (artificially generated misfolded α-syn) showed very distinct responses to soluble α-syn PTMs, and the effect of soluble α-syn PTMs on pathological α-syn amplification was highly dependent on both sites of the PTM and strain of the pathological α-syn.
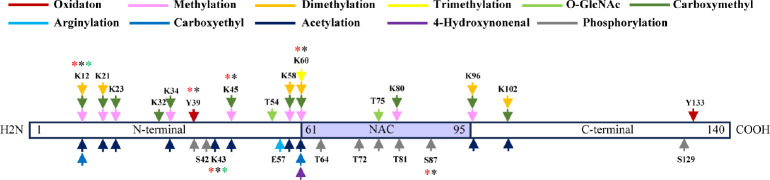
In our study, the majority of the PTMs that we identified (34 out of 51) were concentrated in the N-terminal domain. However, five out of the seven phosphorylation sites that we identified were located in the non-amyloid-component (NAC) domain (Figure 1). Out of these five phosphorylation sites found in the NAC domain, four of them were only identified in soluble α-syn prepared from MSA brains. We analyzed 11 different soluble α-syn PTM sites in relation to the amplification or seeding of pathological α-syn. Of these 11 PTM sites, eight were able to modulate the amplification of at least one of the pathological α-syn strains we tested (Additional Table 1), validating that the regulation of transmission of pathological α-syn through soluble α-syn is a common mechanism that is not limited to a specific type of modification or a specific modification site. Among the eight PTM sites that were able to modulate the amplification of pathological α-syn, only acetylation at K21 and K43 was able to modulate the amplification of all three α-syn strains studied (GCI-α-syn, LB-α-syn, and synthetic α-syn PPFs), indicating that effects on pathological transmission by soluble α-syn are sensitive to both conformation and site variation. This fact is further supported by the similarities in responses that primary neuron passaged GCI-α-syn (GCI-N), which have the mouse α-syn sequence and GCI-α-syn displayed when seeded with soluble α-syn PTMs (Additional Table 1), as their effects revealed to be more conformation-dependent rather than residue sequence dependent.
Figure 1.
Summary of PTMs identified on soluble α-syn purified from different α-synucleinopathy and control brains.
If the PTM (phosphorylation or acetylation) significantly affected LB-α-syn amplification, the PTM site is flagged with red *, if it significantly affected GCI-α-syn amplification, the site is flagged with black *, if it significantly affected PFF-α-syn amplification, the site is flagged with green *. Created with Microsoft PowerPoint. GCI: Glial cytoplasmic inclusion; LB: Lewy body; PFF: preformed fibrils; PTM: post-translational modification; α-syn: α-synuclein.
Additional Table 1.
Summary of sites and effects of alpha-synuclein PTMs.
| PTM Site | Modification | Previously Reported | LB-α-syn amplification | GCI-α-syn amplification | PFF amplification | GCI-N amplification | GCI seeding property | PFF seeding property | Effects of majorly studied α-Syn PTMs and Reference | |
|---|---|---|---|---|---|---|---|---|---|---|
| K12 | Acetylation | No | N/A | N/A | N/A | N/A | N/A | N/A | N terminus acetylation inhibits aggregation | Bing at al., 2017 |
| K21 | Acetylation | No | Down | Down | Down | Down | N/A | N/A | N terminus acetylation inhibits aggregation | Bing at al., 2017 |
| K23 | Acetylation | No | N/A | N/A | N/A | N/A | N/A | N/A | N terminus acetylation inhibits aggregation | Bing at al., 2017 |
| K34 | Acetylation | No | n.s. | n.s. | n.s. | n.s. | N/A | N/A | N terminus acetylation inhibits aggregation | Bing at al., 2017 |
| K43 | Acetylation | No | Down | Down | Down | Down | N/A | N/A | N terminus acetylation inhibits aggregation | Bing at al., 2017 |
| K45 | Acetylation | No | Down | Down | n.s. | Down | N/A | N/A | N terminus acetylation inhibits aggregation | Bing at al., 2017 |
| K58 | Acetylation | No | N/A | N/A | N/A | N/A | N/A | N/A | N terminus acetylation inhibits aggregation | Bing at al., 2017 |
| K60 | Acetylation | No | Down | Up | n.s. | n.s. | N/A | N/A | N terminus acetylation inhibits aggregation | Bing at al., 2017 |
| K96 | Acetylation | No | n.s. | n.s. | n.s. | n.s. | N/A | N/A | N/A | |
| K102 | Acetylation | No | n.s. | n.s. | n.s. | n.s. | N/A | N/A | N/A | |
| Y39 | Phosphorylation | Yes | Down | Down | n.s. | Down | P1: seeding down P2: seeding down | P1: n.s. P2: n.s. | Promotes aggregation | Brahmachari et al., 2016 |
| S42 | Phosphorylation | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| T64 | Phosphorylation | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| T72 | Phosphorylation | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| T75 | Phosphorylation | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| T81 | Phosphorylation | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| S87 | Phosphorylation | Yes | Up | Down | n.s. | Down | P1: seeding down P2: seeding down | P1: n.s. P2: seeding down | Inhibits aggregation | Paleologou et al., 2010 |
| Y125 | Phosphorylation | Yes | Down | n.s. | n.s. | n.s. | P1: n.s. P2: n.s. | P1: n.s. P2: n.s. | Inhibits aggregation | Negro wt al., 2002 |
| S129 | Phosphorylation | Yes | N/A | N/A | N/A | N/A | N/A | N/A | Promotes or inhibits aggregation | Fujiwara et al., 2002; Oueslati et al., 2013 |
| Y133 | Phosphorylation | Yes | Down | Down | n.s. | Down | P1: n.s. P2: n.s. | P1: n.s. P2: n.s. | N/A | |
| K12 | Methylation | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K21 | Methylation | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K23 | Methylation | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K34 | Methylation | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K45 | Methylation | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K58 | Methylation | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K60 | Methylation | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K80 | Methylation | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K96 | Methylation | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K12 | Dimethylation | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K21 | Dimethylation | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K58 | Dimethylation | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K60 | Dimethylation | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K96 | Dimethylation | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K102 | Dimethylation | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K60 | Trimethylation | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| E57 | Arginylation | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Y39 | Oxidation | Yes | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Y133 | Oxidation | Yes | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K12 | Carboxyethyl | Yes | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K60 | Carboxyethyl | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K12 | Carboxymethyl | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K21 | Carboxymethyl | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K23 | Carboxymethyl | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K32 | Carboxymethyl | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K34 | Carboxymethyl | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K45 | Carboxymethyl | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K58 | Carboxymethyl | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K60 | Carboxymethyl | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K80 | Carboxymethyl | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K96 | Carboxymethyl | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| K102 | Carboxymethyl | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| T54 | O-GlcNAc | Yes | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| T75 | O-GlcNAc | Yes | N/A | N/A | N/A | N/A | N/A | N/A | Inhibits aggregation | Levine et al., 2019 |
| K60 | 4-Hydroxynonenal | Yes | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
Up represents increased amplification; Down represents reduced amplification; Seeding down represents reduced seeding ability; n.s. represents no significant difference; N/A represents not tested.
Previous studies have demonstrated that different pathological α-syn strains have distinct spreading patterns in the central nervous system (Peng et al., 2018). Although, the mechanisms for these processes are still unknown. However, the findings from our study indicate that the highly diverse responses from the different pathological α-syn strains to the same PTM may contribute to their distinct spreading patterns in the central nervous system and consequently play a role in determining the clinical diversity of different α-synucleinopathies. This further highlights the importance of studying these PTM sites and their diversity in transmissive effects.
For many of the highly studied PTM sites, there have been a variety of different effects reported in regard to modulation of pathological α-syn amplification, fibrillization, and toxicity. Acetylation is a potential PTM for mitigating the amplification of pathological α-syn. Acetylation on the N-terminal domain was shown to block α-syn aggregation (de Oliveira et al., 2017). Within our study, we found that the effects of acetylation on the amplification of LB-α-syn and GCI-α-syn can vary depending on the specific acetylation site on α-syn. However, overall, we found that acetylation was able to attenuate the amplification of different pathological α-syn strains, indicating that heightened acetylation levels on α-syn could be a possible defense in preventing the spread of pathogenic α-syn and may be beneficial for different α-synucleinopathy patients.
In addition to acetylation, phosphorylation is another potential PTM for mitigating the amplification of pathological α-syn. Previous research revealed that phosphorylation of α-syn monomers at S87 inhibited α-syn oligomerization in vitro using a phosphomimetic, and also attenuated α-syn aggregation and toxicity in vivo in rat models with α-synucleinopathies (Paleologou et al., 2010; Oueslati et al., 2012). However, in our study, we found that phosphorylation at S87 had varying effects on pathological α-syn, depending on the conformation of the protein. Phosphorylation at S87 slightly increased the amplification of LB-α-syn, indicating that PTMs can take on different roles during the different stages of pathogenesis. In contrast, phosphorylation at S87 blocked the amplification of GCI-α-syn and reduced its seeding capacity as well. These findings provide promising evidence of a new possible drug target site for MSA patients. In addition, as there are multiple phosphorylation sites in the NAC domain (including S87), hyperphosphorylation of the NAC domain could function as a potential target for defense against transmission of pathological α-syn in MSA patients.
Another phosphorylation site, Y39, was shown to reduce α-syn fibrillization in vitro (Dikiy et al., 2016) but increase α-syn aggregation in vivo (Brahmachari et al., 2016). In our study, we found that Y39 displayed promising results in attenuating the amplification of pathological α-syn. Phosphorylation at Y39 decreased the amplification of LB-α-syn and GCI-α-syn, indicating that this PTM site could be a target of interest for drug therapies for PD, dementia with LB, or MSA patients. Additionally, Y39 phosphorylation was able to dramatically reduce the seeding potential of amplified GCI-α-syn, highlighting that a single PTM site may play several roles in modulating the different stages of disease progression. However, phosphorylation at Y39 may also promote aggregation (Brahmachari et al., 2016), indicating that the specificity of pathogenic developments or attenuations may also be enzyme-dependent.
In addition to the above-mentioned PTMs, previous studies have found that O-GlcNAc on α-syn was able to prevent α-syn aggregation (Levine et al., 2019). In our study, we identified two O-GlcNAc sites on α-syn (T54 and T75). Previous research has indicated that these PTM sites may be efficient at blocking the pathogenesis of α-syn, and the study of these sites may also be of interest for future research, as they could prove to be potential therapeutic targets to protect against the development and spread of α-synucleinopathies. Similar to O-GlcNAc, the arginylation of α-syn has also been shown to block aggregation of α-syn (Zhao et al., 2022). In our research, we were able to identify one arginylation site in the N-terminal domain (E57), but were unable to test this specific PTM in relation to the modulation of pathogenic α-syn. However, this PTM could be an interesting potential topic for future study, as the effects of the arginylation on α-syn are unstudied and unknown. Moreover, other studies have found that another type of PTM, namely glycation, promoted the aggregation of α-syn (Vicente Miranda et al., 2017). In our study, we were able to identify several glycation sites on soluble α-syn purified from diseased brains. Studying these glycation sites and how they regulate the transmission and amplification of pathological α-syn would also be an interesting topic for future research. Lastly, there has not been any research on methylation and dimethylation of α-syn. In our research, we identified several new methylation and dimethylation sites in soluble α-syn, which could also prove to be important PTMs for future study. There have been many PTMs identified within our study and that of others, but very little research has been performed on these specific sites, prompting the need for further research on these PTMs and their possible effects on aggregation, transmission, seeding, and toxicity of α-syn.
In addition to considering single PTMs as putative therapeutic target sites, it is important to point out that the effects of individual PTMs may be modulated upon the occurrence of other simultaneous PTMs. As previous studies indicate, even normal physiological roles of α-syn prompted by PTMs (such as interactions with membranes) may be regulated by multiple PTMs, including N-terminal acetylation, phosphorylation, and oxidation. While multiple PTMs on α-syn may have competing or additive effects that can alter pathogenic α-syn characteristics and behaviors, studying the PTMs in an isolated manner initially is important to fully determine what each PTM may contribute to the pathogenesis or pathological preventative nature of α-syn. Further research into the effects of multiple PTMs on α-syn may allow for a more accurate physio-pathological study, and possibly unveil potential therapeutic targets that can be precisely mitigated due to an enhanced understanding of PTM interactions.
In conclusion, our findings revealed the previously unrecognized regulatory role of soluble α-syn in the transmission of pathological α-syn. As there have been many unknowns surrounding the factors that determine the diversity in spreading patterns and transmission of pathological α-syn, it has been hard to discern possible mechanisms underlying these processes. In addition, the immense diversity and complexity of PTMs on soluble α-syn and the complicated interactions between soluble α-syn PTMs and pathological α-syn have made it difficult to truly understand disease progression. However, the regulatory roles of PTMs on soluble α-syn could play a significant role in understanding the clinical and pathological landscape of diverse α-synucleinopathies. Given the varying and dramatic effects of soluble α-syn PTMs on pathological α-syn amplification, aggregation, and seeding, future studies in this area could focus on further verifying these findings in animal models and patients. The regulatory role of soluble α-syn PTMs can have further applications to other neurodegenerative diseases such as tauopathies. For example, several PTMs have also been identified on soluble tau, and various tau strains have been reported, indicating that PTMs on soluble tau could produce similar results regarding transmission and amplification and their dependence on strain and site specificity as PTMs on soluble α-syn. Additionally, many possible new drug targets could be explored within the context of the PTMs identified and tested in our study, which could mitigate the transmission of pathological α-syn and ameliorate disease progression.
Additional files:
Additional file 1: Open peer review reports 1 (90.3KB, pdf) and 2 (88.9KB, pdf) .
Additional Table 1: Summary of sites and effects of alpha-synuclein PTMs.
Footnotes
Open peer reviewers: Francisco Cayabyab, University of Saskatchewan, Canada; Sebastian Kügler, University Medical Center Göttingen, Germany.
P-Reviewers: Cayabyab F, Kügler S; C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- 1.Brahmachari S, Ge P, Lee SH, Kim D, Karuppagounder SS, Kumar M, Mao X, Shin JH, Lee Y, Pletnikova O, Troncoso JC, Dawson VL, Dawson TM, Ko HS. Activation of tyrosine kinase c-Abl contributes to alpha-synuclein-induced neurodegeneration. J Clin Invest. (2016);126:2970–2988. doi: 10.1172/JCI85456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Oliveira RM, Vicente Miranda HA-O, Francelle L, Pinho R, SzegöÉ M, Martinho R, Munari F, Lázaro DF, Moniot S, Guerreiro P, Fonseca-Ornelas L, Marijanovic Z, Antas P, Gerhardt E, Enguita FJ, Fauvet B, Penque D, Pais TF, Tong Q, Becker S, et al. The mechanism of sirtuin 2-mediated exacerbation of alpha-synuclein toxicity in models of Parkinson disease. PLoS Biol. (2017);15:e1002601. doi: 10.1371/journal.pbio.2000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dikiy I, Fauvet B, Jovičić A, Mahul-Mellier AL, Desobry C, El-Turk F, Gitler AD, Lashuel HA, Eliezer D. Semisynthetic and in vitro phosphorylation of alpha-synuclein at Y39 promotes functional partly helical membrane-bound states resembling those induced by PD mutations. ACS Chem Biol. (2016);11:2428–2437. doi: 10.1021/acschembio.6b00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He S, Wang F, Yung KKL, Zhang S, Qu S. Effects of α-synuclein-associated post-translational modifications in Parkinson's disease. ACS Chem Neurosci. (2021);12:1061–1071. doi: 10.1021/acschemneuro.1c00028. [DOI] [PubMed] [Google Scholar]
- 5.Levine PM, Galesic A, Balana AT, Mahul-Mellier AL, Navarro MX, De Leon CA, Lashuel HA, Pratt MR. alpha-Synuclein O-GlcNAcylation alters aggregation and toxicity revealing certain residues as potential inhibitors of Parkinson's disease. Proc Natl Acad Sci U S A. (2019);116:1511–1519. doi: 10.1073/pnas.1808845116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oueslati A, Paleologou KE, Schneider BL, Aebischer P, Lashuel HA. Mimicking phosphorylation at serine 87 inhibits the aggregation of human alpha-synuclein and protects against its toxicity in a rat model of Parkinson's disease. J Neurosci. (2012);32:1536–1544. doi: 10.1523/JNEUROSCI.3784-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paleologou KE, Oueslati A, Shakked G, Rospigliosi CC, Kim HY, Lamberto GR, Fernandez CO, Schmid A, Chegini F, Gai WP, Chiappe D, Moniatte M, Schneider BL, Aebischer P, Eliezer D, Zweckstetter M, Masliah E, Lashuel HA. Phosphorylation at S87 is enhanced in synucleinopathies inhibits alpha-synuclein oligomerization and influences synuclein-membrane interactions. J Neurosci. (2010);30:3184–3198. doi: 10.1523/JNEUROSCI.5922-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng C, Trojanowski JQ, Lee VM. Protein transmission in neurodegenerative disease. Nat Rev Neurol. (2020);16:199–212. doi: 10.1038/s41582-020-0333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng C, Gathagan RJ, Covell DJ, Medellin C, Stieber A, Robinson JL, Zhang B, Pitkin RM, Olufemi MF, Luk KC, Trojanowski JQ, Lee VM. Cellular milieu imparts distinct pathological alpha-synuclein strains in alpha-synucleinopathies. Nature. (2018);557:558–563. doi: 10.1038/s41586-018-0104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vicente Miranda H, Szego ÉM, Oliveira LMA, Breda C, Darendelioglu E, de Oliveira RM, Ferreira DG, Gomes MA, Rott R, Oliveira M, Munari F, Enguita FJ, Simões T, Rodrigues EF, Heinrich M, Martins IC, Zamolo I, Riess O, Cordeiro C, Ponces-Freire A, et al. Glycation potentiates α-synuclein-associated neurodegeneration in synucleinopathies. Brain. (2017);140:1399–1419. doi: 10.1093/brain/awx056. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S, Zhu R, Pan B, Xu H, Olufemi MF, Gathagan RJ, Li Y, Zhang L, Zhang J, Xiang W, Kagan EM, Cao X, Yuan C, Kim SJ, Williams CK, Magaki S, Vinters HV, Lashuel HA, Garcia BA, James Petersson E, et al. Post-translational modifications of soluble α-synuclein regulate the amplification of pathological α-synuclein. Nat Neurosci. (2023);26:213–225. doi: 10.1038/s41593-022-01239-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao J, Pan B, Fina M, Huang Y, Shimogawa M, Luk KC, Rhoades E, Petersson EJ, Dong DW, Kashina A. α-Synuclein arginylation in the human brain. Transl Neurodegener. (2022);11:20. doi: 10.1186/s40035-022-00295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.