Abstract
Tauopathies are a group of neurological disorders, including Alzheimer’s disease and frontotemporal dementia, which involve progressive neurodegeneration, cognitive deficits, and aberrant tau protein accumulation. The development of tauopathies cannot currently be stopped or slowed down by treatment measures. Given the significant contribution of tau burden in primary tauopathies and the strong association between pathogenic tau accumulation and cognitive deficits, there has been a lot of interest in creating therapies that can alleviate tau pathology and render neuroprotective effects. Recently, small molecules, immunotherapies, and gene therapy have been used to reduce the pathological tau burden and prevent neurodegeneration in animal models of tauopathies. However, the major pitfall of the current therapeutic approach is the difficulty of drugs and gene-targeting modalities to cross the blood-brain barrier and their unintended side effects. In this review, the current therapeutic strategies used for tauopathies including the use of oligonucleotide-based gene therapy approaches that have shown a promising result for the treatment of tauopathies and Alzheimer’s disease in preclinical animal models, have been discussed.
Keywords: dementia, gene therapies, immunotherapy, neurodegeneration, oligonucleotides, tau, tauopathies, therapies
Introduction
Tauopathy is a neurodegenerative disorder caused by aberrant tau protein aggregates in cells. A multipurpose, distinctive, and naturally disordered protein, tau protein is found in the brain and central nervous system. The tau protein forms oligomers or filaments and interacts with other molecules involved in various signaling pathways. The microtubule-associated protein tau (MAPT) gene contains 16 exons where alternative splicing of exons 2, 3, and 10 transcripts leads to the formation of six different molecular isoforms (Figure 1) ranging from 352–441 amino acids with a molecular weight of 45 kDa–65 kDa. These isoforms largely vary from each other by the number of repeats; three repeats (3R) or four repeats (4R), in their microtubule-binding domains (MTBR). Insertion or deletion of a 31 or 32 amino acid sequence coded by exon 10 results in 3R (R1, R3, R4) or 4R (R1, R2, R3, R4) tau protein isoforms (Wang and Mandelkow, 2016). The isoforms 3R and 4R form abnormal aggregates, thus tauopathies are categorized into three groups: 3R, 4R, and 3R/4R tauopathies (comprising 3R tau & 4R tau in equal proportions). Recent studies have demonstrated that paired helical filament (PHF)-6 motifs (275VQIINK280 or 306VQIVYK311) present within R2 and R3 MTBR are required for the pathological aggregation of tau protein and make them seed competent (Mirbaha et al., 2018). Exons 2 and 3 encode 29 or 58 amino acid sequences that, when inserted or deleted, result in the production of the tau protein isoforms 0N, 1N, and 2N, respectively. Interestingly, exon 3 is never expressed alone; it is always expressed in conjunction with exon 2. On the other hand, exon 2 seems to have no such anomaly. Moreover, the 2N tau protein isoforms are under expressed in the human brain (Wang and Mandelkow, 2016).
Figure 1.
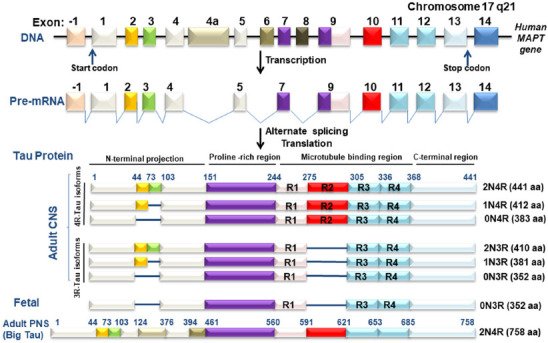
Schematic representation of the wild-type MAPT gene and its variants.
MAPT gene exons are numbered from -1 to 14. In the adult human brain, six isoforms are produced as a result of alternate splicing of exon 2 (yellow), exon 3 (green), and exon 10 (red) in the CNS. Each isoform is distinguished by either three (3R) or four (4R) microtubule-binding domains and 0N, 1N, and 2N amino-terminal end insertions or deletions. Exons 4a (brown) and 6 (dark brown) have been represented in isoforms that express in the PNS, whereas exon 8 (black) is not transcribed in the human brain. Fetal brain tau lacks exon 2 (yellow), exon 3 (green), and exon 10 (red). Created with Microsoft PowerPoint. aa: Amino acid; CNS: central nervous system; DNA: deoxyribonucleic acid; MAPT: microtubule-associated protein tau; mRNA: messenger RNA; PNS: peripheral nervous system.
The mature neurons of the human brain predominantly express tau protein, whereas oligodendrocytes and astrocytes express tau protein to a much smaller extent (Chung et al., 2021). Under normal circumstances, tau protein stabilizes the microtubules, a crucial component of the neuronal cytoskeleton, and regulates intracellular trafficking (Figure 2). Disruption in tau protein regulation and/or function have been linked to neuropathological conditions i.e., tauopathies including Alzheimer’s disease (AD), frontotemporal dementia (FTD), Pick disease, corticobasal syndrome (CBS), progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), and argyrophilic grain disease (Chung et al., 2021; Figure 2). It is evident that the neurons of patients with neurological disorders possess aggregates of various aberrant proteins, of which hyperphosphorylated tau is a key component. Glycogen synthase kinase-3 (GSK3) has been identified as the primary kinase involved in the hyperphosphorylation of tau protein (Sayas and Ávila, 2021). The GSK3-mediated phosphorylation of tau protein is attributed to the presence of amyloid beta (Aβ), which may inhibit insulin receptors to eventually promote GSK3 activation. The complexes that form as a result of the self-assembling of tau protein further complicate the disease condition through the degeneration of the glial and neuronal cells (You et al., 2022).
Figure 2.
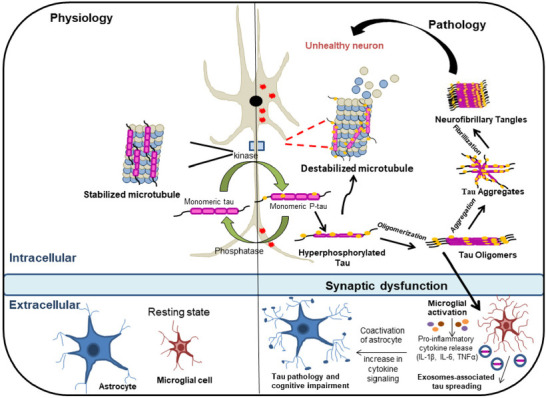
Schematic representation of the progression of tau pathology.
In a healthy state, tau protein controls microtubule stabilization. Hyperphosphorylation of tau protein causes a reduction of microtubule affinity in tauopathies. Eventually, pathological soluble tau oligomers are formed and further aggregate to form insoluble neurofibrillary tangles. Tau oligomers are released into the extracellular space, which helps in the propagation of pathological tau protein to nearby neurons. Pro-inflammatory mediators like IL-1β, IL-6, and TNF-α are produced by microglia in response to inflammatory stimuli, which increases the activity of kinases implicated in tau protein phosphorylation and further exacerbates the disease. Created with Microsoft PowerPoint. IL-1β: Interleukin-1 beta; IL-6: interleukin-6; TNF-α: tumor necrosis factor alpha.
The presence of hyperphosphorylated tau protein and its aberrant aggregates in neuronal cells induce apoptosis and cell death (Sayas and Ávila, 2021). There is a significant amount of evidence suggesting that the hyperphosphorylated tau protein could bind to normal microtubule-associated proteins (MAPs) such as MAP1B and MAP2, resulting in synaptic degeneration (Alonso et al., 1997). In AD, the intracellular tangles caused by pathological tau protein inversely affect the abundance of healthy neurons. These pathological conditions attributed directly or indirectly to the existence of abnormal tau protein aggregates uncover the urgency for the discovery of efficient therapeutics for treating tauopathies. Although there are no established and approved pharmacological treatments available to date, several small molecules have been developed to attenuate the causes that lead to tauopathy, such as hyperphosphorylation of tau protein. Immunotherapies and catalytic nucleic acid-based gene therapies are the new treatment strategies used against tauopathies (Figure 3). In this review, we will cover key features and findings of tauopathies, existing anti-tau RNA/protein therapies, their shortcomings, and therapeutic strategies that have been emerging as potential treatments in clinical settings.
Figure 3.
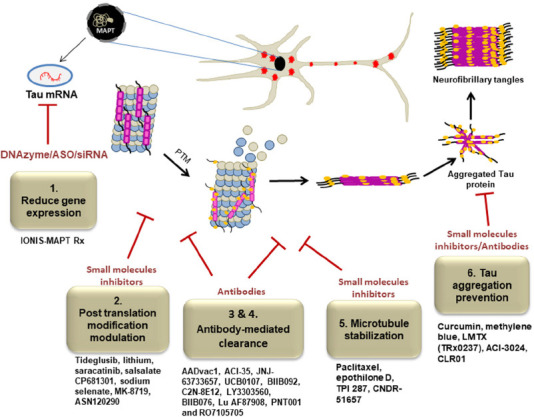
Schematic representation of potential therapeutic strategies for the treatment of tauopathies.
Tau gene expression is decreased by targeting anti-sense oligonucleotides (DNAzyme, ASOs, siRNA) against the mRNA from the MAPT gene (targeted strategy 1). Kinase-mediated hyperphosphorylation, O-GlcNAc removal inhibition, and tau acetylation inhibition are blocked to sustain healthy PTM pathways (targeted strategy 2). Antibodies are utilized to remove or sequester pathologic tau species, inhibiting the spread of the condition from cell to cell (targeted strategies 3 & 4). Loss of function of tau is addressed by restoring microtubule stabilization (targeted strategy 5). The formation of pathological tau protein aggregates is prevented through antibodies mediated clearance and small molecules based inhibitors (targeted strategy 6). Each targeted strategy included examples of potential drug candidates for the treatment of tauopathies. Created with Microsoft PowerPoint. ASOs: Antisense oligonucleotides; DNAzymes: single-stranded catalytic DNA molecules; MAPT: microtubule-associated protein tau; PTM: post-translational modification; O-GlcNAc: O-linked -N-acetylglucosamine acetylation; siRNA: small interfering RNA.
Search Strategy
The literature search was performed for the period from 1994 to 2023 with the major emphasis on the last 5 years of publication using the PubMed database. Some information was also taken from the websites page of the World Health Organization and AlzForum (https://www.alzforum.org/). The search keywords were as follows: “Tauopathies” and “Neurodegenerative disorders” OR “COVID-19” OR “Dementia” OR “Small Molecules Inhibitors for tauopathies” OR “Vaccines for tauopathies” OR “Gene Therapy for tauopathies” OR “Oligonucleotides” OR Clinical Trials OR Animal Models OR Drug Discovery for tauopathies.
Periodic Prevalence of Tau Protein
Tauopathies are progressive and heterogeneous neurodegenerative disorders defined by the buildup of aberrant tau protein deposits in the brain. In line with the World Health Organization (WHO), more than 55 million individuals globally are estimated to be affected by dementia, and each year approximately 10 million new cases of dementia are emerging. The number is expected to rise as much as 138 million by 2050. FTD is considered one of the commonly occurred presenile dementia which contributes to 2.6% of all types of dementia (Leroy et al., 2021). The reported age-standardization incidence was 2.90 per 100,000 individuals per year, which is increasing with time and goes up between the age groups of 75–79 (Turcano et al., 2020; Leroy et al., 2021). In spite of having a similar progression rate and mental state examination, FTD patients are underdiagnosed due to a longer referral delay compared to AD patients. Reports demonstrated the shortest survival rate for FTD patients with primarily motor symptoms whereas the survival rate of those patients with behavioral variant FTD, and language variant FTD did not differ significantly (Leroy et al., 2021). For a better understanding of the perspective of FTD disease, a European observational research study is currently being conducted (Borroni et al., 2022). A handful of population-based studies have been conducted to understand the PSP and CBS, which are uncommon groups of tauopathies. It has been found that the PSP and CBS incidence are about 3–8 per 100,000 individuals per year which increase with the age of above 75–79 (Stang et al., 2020). The average survival rate of individuals diagnosed with PSP or CBS is about 6 years (Stang et al., 2020). Argyrophilic grain disease is a 4R, late-onset tauopathy associated with advanced age (> 80 years old). It affects both genders equally. Studies indicated that lower socioeconomic status and appetite disorders are also the contributing factor other than the older age (Wurm et al., 2020). CTE is another notable dementia that has received little attention due to a scarcity of cases. Several studies have linked CTE to sports, presenting evidence that the pathology of CTE is commonly observed in athletes, primarily men (Bieniek et al., 2020).
AD is the most prevalent type of dementia, contributing 60–70 percent of total cases worldwide. Global data show that low-income nations have a greater prevalence of AD (Leroy et al., 2021). The incidence of dementia is increasing at an accelerated rate among the aging population by all means, which increases the medical cost of AD, including the unpaid and unseen efforts of care providers (El-Hayek et al., 2019). Since its discovery, research has progressed to understand the pathological processes of AD over the past several decades, although the development of therapeutic tools to delay the progress of neurodegeneration is still unmet. The majority of the research attention in the search for AD therapies for managing disease has been focused on two pathological aspects of the disease: neurofibrillary tangles (NFTs), which are composed of hyperphosphorylated tau protein, and Aβ protein that aggregates into plaques. Unfortunately, efforts to either remove Aβ peptide or reduce its production in order to mitigate the disease course remained largely unsuccessful and showed negative results in several clinical trials over the decades. The failure of drugs targeting Aβ pathology in the brain has fueled increased interest in tau protein-targeting strategies. Aberrant tau protein is emerging as a promising therapeutic candidate for the discovery of effective treatments for AD and related tauopathies. Additionally, during the unprecedented COVID-19 era, the risk of cognitive decline has additionally been noted in both AD and non-AD patients with mild to severe COVID-19. The elevated expression of pathological tau protein as well as upregulation of deleterious pathways causing hyperphosphorylation of tau protein in COVID-19 patients (Frontera et al., 2022; Reiken et al., 2022) has alarmed the ongoing burden of disease by upticking future patients (Gordon et al., 2022). However, therapeutic intervention is needed to underpin the new wave of cognitive decline or dementia.
Tau Protein Expression and Regulation in Different Organs
The expression of the tau protein isoforms is tightly controlled. The 45 kDa 0N3R tau protein isoform is expressed only in the fetal human brain. While the mature human brain expresses 3R and 4R tau protein isoforms with the same proportion (1:1). The level of tau protein phosphorylation is highest during neuronal development, and the degree of tau protein phosphorylation contributes to cytoskeletal plasticity in developing neurons in the brain (Betters et al., 2023). In spite of having a highly phosphorylated state, the fetal tau protein isoform does not form PHF (Hefti et al., 2019). Tau protein is expressed not only in the adult central nervous system (CNS) but also in the muscle, liver, lung, kidney, skin, and other tissues. The adult peripheral nervous system specifically the sciatic nerve, optic nerves, spinal cord, and the cell lines derived from neuronal cells express a 110 kDa tau protein variant referred to as “Big tau” (Dugger et al., 2016). Big tau protein contains exon 4a and is developmentally regulated and expressed more in PNS neurons as they mature during adulthood (Fischer and Baas, 2020). The specific function of “Big tau” protein is still unknown; however, indirect evidence suggests that it may contribute to axonal stability and aid in microtubule stabilization (Fischer and Baas, 2020). The differential expression of tau protein in various tissues and the nervous system opens the door to further research into their relationship to the onset of AD and tauopathies.
The Role of Tau Protein in Neurodegeneration
Tau protein oligomers have become an impetus for tauopathies, especially in their abnormally hyperphosphorylated aggregate form. In these forms, tau protein loses its propensity for microtubules and begins to self-assemble to induce the development of PHF, straight filaments, or twisted ribbons, which together evolve as NFTs (Oakley et al., 2020; Figure 2). Along with its connection to synaptic dysfunction and neuronal death, NFTs density is highly linked with cognitive decline in AD (Hsieh et al., 2019). Several recent studies speculated that hyperphosphorylation of tau protein, particularly on serine and threonine residues, leads to their aggregation (Zhou et al., 2018). In addition, tau protein goes through additional post-translational modifications (PTMs), including sumoylation, acetylation, ubiquitination, cleavage, and glycation (Alquezar et al., 2021). Site-specific tau protein PTMs can alter tau protein solubility or disrupt the connection between tau protein and microtubules, which can affect tau protein pathophysiology. In comparison to the non-aggregated tau protein, the aggregated ones are more difficult to get dephosphorylated by the protein phosphatase 2A (PP2A) and result in tau pathology (Miao et al., 2019). There is evidence that a pathogenic tau protein similar to prion disease can directly infect nearby and synaptically linked cells (Duyckaerts et al., 2019). Tau protein is an intracellular protein, which upon axonal degeneration, neuronal death, or through direct translocation from cytoplasm to plasma membrane will release to extracellular space (Brunello et al., 2020). Tau protein translocation may also take place via presynaptic vesicle secretion, exosomes, and ectosomes or in its naked form (Polanco and Götz, 2022). Once released, it interacts with low-density lipoprotein receptor-related protein-1, heparan sulfate proteoglycans, or muscarinic receptors and will be internalized by other neurons via dynamin-mediated endocytosis and tunneling nanotubes connecting the cytoplasmic content of adjacent cells (Zhang et al., 2021). Once seeded, it stimulates the aggregation of natively folded tau protein inside the naive cells, causing cellular toxicity and the spread of disease, resembling a prion-like propagation hypothesis (Duyckaerts et al., 2019; Brunello et al., 2020). In a mouse model of tauopathy, the suppression of exosome production and the reduction in microglia activation stop the spread of abnormal tau protein (Asai et al., 2015). These results show that microglia play a crucial role in the spread of tauopathies through phagocytosis and the production of exosomes that carry tau protein. This suggests that microglia may be a new target in the progression of tauopathy therapies.
Therapeutic Approaches for Targeting the Aberrant Tau Protein
Small molecule-based therapies
Tau protein undergoes several PTMs which disruptions lead to their pathological conditions, including tauopathy and AD. Increased activity of kinases and/or lack of activity of phosphatases can lead to the hyperphosphorylation of tau protein, one of the major causes of tauopathies. In the cytosol, hyperphosphorylated tau protein forms oligomers, or is self-assembled into PHFs or straight filaments, disrupting microtubule stability. Therefore, the majority of the initial treatments addressing tauopathies were based on small molecules inhibiting kinases, tau protein aggregations, PTMs, and/or stabilization of microtubules. However, these therapies have been turned down due to their toxicity, poor efficacy, or inability to cross the blood-brain barrier (BBB) (Soeda and Takashima, 2020; Wang et al., 2020). Considering the role of protein kinases in tau protein hyperphosphorylation, several kinase inhibitors targeting glycogen synthase kinase-3 beta (GSK-3β), cyclin-dependent kinase 5, extracellular signal-regulated kinase 2, and tyrosine kinase Fyn, are currently under investigation (Yadikar et al., 2020). In clinical trials, the GSK-3β inhibitor tideglusib has been shown to be well tolerated in AD patients, and lithium treatment was found to be beneficial in improving the cognitive performance of patients with mild cognitive impairment and AD (Snitow et al., 2021). Although the tideglusib has entered clinical trials, phase 2 didn’t yield any positive therapeutic outcomes, whereas for lithium, clinical trials are ongoing, and patients are being recruited for phase 3 studies. Saracatinib, a Fyn kinase inhibitor, did not have a significant effect on slowing down disease progression in AD patients (Nygaard, 2018). CP681301, a cyclin-dependent kinase 5 inhibitor, increased tau protein phosphorylation in transgenic mice overexpressing p25 and also diminished the activity of GSK-3β by indirect phosphorylation at S9 (Soeda and Takashima, 2020). Nonetheless, human clinical trials using kinase inhibitors have not produced encouraging outcomes in reducing the emergence of cognitive deficits.
In addition to the inhibition of kinases, many small-molecule therapies are deployed to improve the activities of phosphatases in order to combat the disease. Inactivation of various phosphatases is the most commonly observed pathological condition in AD and other tauopathies. The activity of PP2A is low in the AD brain (Wei et al., 2020). PP2A is responsible for around 70 percent of the overall tau protein phosphatase activity in the brain, suggesting that decreased PP2A activity in AD is responsible for the accumulation of hyperphosphorylated tau protein in the brain. Studies demonstrated that activation of PP2A by sodium selenate lowered tau protein phosphorylation, and reversed memory deficits in animal models of tauopathies (Ahmed et al., 2020). However, due to selenium buildup in the CNS, sodium selenate therapy failed in phase 2a clinical studies (Cardoso et al., 2019). Other compounds such as memantine, apolipoprotein E mimetic (COG112), and resveratrol are known to attenuate the phosphorylation of tau protein by interfering with the activity of PP2A (Taleski and Sontag. 2018). Moreover, tau protein is primarily acetylated in the fibril-forming core, which encourages stacking of the beta strand and decreases the solubility of tau protein. Salsalate, a nonsteroidal anti-inflammatory drug reduced the amount of tau protein acetylation at K174, preventing hippocampus atrophy and memory loss in PS19 transgenic (PS19Tg) mice, a model of tauopathy (Min et al., 2015). Salsalate, however, had no therapeutic impact in phase 1 open-label clinical trial with patients who had been diagnosed with Richardson’s syndrome (VandeVrede et al., 2020). The outcome has not been made available to the public. O-linked-N-acetylglucosamine (O-GlcNAc) acylation is a PTM in which O-GlcNAc transferase adds O-GlcNAc to serine and threonine residues of nucleus and cytoplasmic proteins. O-GlcNAcase is responsible for the removal of O-GlcNAc. It has been demonstrated that glycosylation of tau protein by O-GlcNAc prevents tauopathies, thus, inhibiting O-GlcNAcase activity in principle should prevent AD/tauopathies. As a result, reducing tau protein deglycosylation may be achieved by inhibiting the O-GlcNAcase enzyme. MK-8719, an O-GlcNAcase inhibitor, increased tau protein glycosylation levels in rTg4510 mouse models, reduced aberrant tau protein pathology, and slowed down brain atrophy (Wang et al., 2020). MK-8719 and ASN120290 were given orphan drug designations and have been used for the treatment of PSP; however, no clinical trials have been reported. A significant amount of research is also being carried out to target ubiquitination, sumoylation, and other PTMs (Alquezar et al., 2021).
The autophagy-lysosome pathway and the ubiquitin proteasomal system are the two main mechanistic pathways for the degradation of abnormal proteins to maintain cellular homeostasis, and aberrant tau protein accumulation in the brain impairs their ability to function. So, it stands to reason to stimulate these pathways in order to deal with tau protein accumulation. Protein breakdown by the lysosome or proteasome is accelerated by ubiquitination. A reduced level of phosphorylated tau protein and higher tau protein clearance were seen in the P301L mouse model after, a de-ubiquinating enzyme, USP13, was knocked down (Liu et al., 2019). Rapamycin, an mTOR kinase inhibitor is tested in phase 1 and phase 2 clinical trials for patients with mild cognitive impairment and early-onset AD (Silva et al., 2020). However, other mTOR kinase inhibitors, namely AZD2014, OSI-027, and AZD8055, showed greater efficacy compared to rapamycin in neuronal cell models from patient’s induced pluripotent stem cells (Silva et al., 2020).
Tau protein-aggregates and oligomers (Oakley et al., 2020) are one of the main components in AD and other tauopathies. Misfolded and oligomerized tau protein can gradually overload the nerve cells, resulting in neuronal cell death. Curcumin, a major component of turmeric has been shown to inhibit tau protein aggregation (Bijari et al., 2018). Nevertheless, due to its poor bioavailability and low stability in the body, curcumin failed to demonstrate significant clinical efficacy (Chen et al., 2018). However, clinical trial results of its analogs with improved bioavailability are still awaited (Okuda et al., 2017; Cascio et al., 2019). The anti-inflammatory compound, resveratrol has also been shown to reduce the level of hyperphosphorylated tau protein in mice (Yu et al., 2018). Though the molecule possesses additional benefits like antioxidant and anti-inflammatory properties, its low bioavailability and rapid metabolism (Chimento et al., 2019) are its major shortcomings. Interestingly, nickel administration, either in its metallic form or in the form of its conjugate with morpholine, inhibited tau protein aggregation in vitro (Gorantla et al., 2020). However, nickel deposition in the liver, kidney, and spleen is a matter of concern. An in vitro experiment revealed that folic acid prevents tau protein aggregation and reduces its phosphorylation by stabilizing its native conformation and regulating PP2A methylation (Ghasemzadeh and Riazi, 2020), respectively. Furthermore, methylene blue has been shown to prevent tau protein aggregation and improve memory impairment (deficit) in a mouse model of tauopathy (Soeda et al., 2019). However, a derivative of methylene blue, LMTX (TRx0237), failed to show any substantial improvement in phase 3 clinical trials (Gauthier et al., 2016). ACI-3024 is a small-molecule tau protein aggregation inhibitor. In cell-based experiments, ACI-3024 was shown to reduce intracellular pathogenic tau protein in a dose-dependent manner, as well as prevent microglial activation and neuronal death caused by tau PHF. Consistent with cell-based study, ACI-3024 treatment was claimed to lower aggregated and insoluble tau protein in rTg4510 mice. In these mice, treatment apparently decreased cerebrospinal fluid total tau protein and microglia activation in inverse relation to plasma drug levels. A phase 1 clinical study was carried out on healthy individuals, but there is no registry registration or published research to be discovered (Imbimbo et al., 2022). CLR01, a member of the broad-spectrum protein aggregation inhibitors called molecular tweezers was found to lessen tau protein aggregation, hyperphosphorylation, and oligomerization in P301S-tau mice (Di et al., 2021).
Reduced binding affinity caused by hyperphosphorylation of tau protein in tauopathies destabilizes the microtubules, which in turn causes axonal deficiency and tau pathology. Paclitaxel was the first microtubule stabilizer to be researched, and the focus of that study was on its anti-tumor capabilities. Its further study was put on hold due to the low quality of crossover BBB. Several microtubule-stabilizing compounds, such as epothilone D and TPI 287 have been tested in animal models of tauopathy (Miller and Das, 2020) and showed promising results in clinical trials. However, the clinical trials of epithilone D and TPI 287 were discontinued due to the adverse side effects and lack of tolerance in patients with AD (Tsai et al., 2020), respectively. Triazolo-pyrimidines CNDR-51657, another potential anti-cancer agent, when given orally to 9-month-old female PS19Tg mice, attenuated tau pathology, axonal dystrophy, and microtubule abnormalities (Zhang et al., 2018). No negative consequences were noted. Triazolo-pyrimidines interact with tubulin and their strong oral bioavailability and brain penetration, making them strong candidates for microtubule stabilizers (Oukoloff et al., 2021).
Acetylcholinesterase inhibitors like donepezil, rivastigmine, galantamine, and N-methyl D-aspartate receptor-like memantine have been shown to improve cognitive decline in AD patients. Few studies also reported the beneficial effect of acetylcholinesterase inhibitors in preclinical models of tauopathies (Yoshiyama et al., 2010; Riedel et al., 2020). For instance, a study by Yoshiyama and coworkers (Yoshiyama et al., 2010) reported that donepezil treatment ameliorated neuroinflammation, tau protein pathology, and neurodegeneration in PS19Tg mice. Acetylcholinesterase inhibitors alleviate the levels of synaptic acetylcholine, improve the interaction within cholinergic neurons, and delay the progression of diseases. In spite of having a positive impact on AD patients, these molecules are often associated with significant side effects (Sharma, 2019). Neurotrophic factor supplementation, including brain-derived neurotrophic factor (Giuffrida et al., 2018), ciliary neurotrophic factor (Wei et al., 2021), and nerve growth factor (Cuello et al., 2019), have been found to ameliorate deficits in neurogenesis, synaptic plasticity, and cognition. These approaches, however, have been slowed by concerns about the specificity, limited BBB permeability, and potential side effects of the available drugs in animal studies (Thoenen and Sendtner, 2002). Although histone deacetylase inhibitors have shown neuroprotective roles (Lee et al., 2018), successful treatments in the animal model are rarely translated into clinical trials.
Several small molecules inhibitors are developed to target the PTMs of tau protein, but very few have entered clinical trials, notably, sodium selenate, BPN14770, rapamycin, LMTX, curcumin, lithium, tideglusib, and saracatinib. These small molecules prevent phosphorylation of tau protein isoforms at tyrosine, serine, and threonine residues, acetylation at lysine 174, and aggregation by inhibiting the disulfide bond formation in oligomers. However, low stability, poor bioavailability, CNS toxicity, off-target effect, or lack of human efficacy constitutes the bottlenecks of small-molecule mode of therapeutic approaches. Multi-target drug candidates or combination therapy can be viable alternatives to combat tauopathies but can be hampered by the risk of drug-drug interactions and poor BBB permeability. Yet, it cannot be averted while developing treatments for challenging disorders, especially like AD. Novel formulation or drug delivery methods need to be adopted for the small-molecule therapeutics of tauopathies that lack brain exposure despite having efficacy. Growing research in nanodrug delivery methods can bring remarkable improvement in bioavailability and brain exposure of drug candidates against tauopathies.
Tau protein immunotherapies
Immunization is a desirable treatment strategy since it can boost the host immune system in response to disease or induce the generation of autoantibodies through active immunization. The same effect can also be produced by the administration of laboratory-made antibodies through passive immunization. Full-length human tau protein, shorter N-terminal region of tau protein, and phosphorylated tau protein, are available as immunogens. The effectiveness of an anti-tau protein vaccine called AV1980R/A with an adjuvant called AdvaxCpG has been evaluated in wild-type and transgenic mice. Interestingly, elevated levels of IgG in the brains of animals were observed upon immunization and showed significant improvement in memory function. Additionally, AV1980R/A showed a decrease in the level of hyperphosphorylated tau protein at Ser 396 with no effect on other phosphorylated tau protein forms in the vaccinated mouse brain (Joly-Amado et al., 2020). Several tau protein vaccines and antibodies are being tested in mouse models of tauopathy (Albert et al., 2019). AADvac1, a synthetic peptide that targets the MTBR of tau protein, was the first anti-tau protein vaccine to enter clinical trials. AADVac1 was proven to be risk-free in a phase 2 clinical study on patients with moderate AD dementia, but there was no clear evidence that it had any effect on cognitive tests (Novak et al., 2021). ACI-35, a liposomal vaccine, constituting tau protein fragments with several phosphorylated serine residues decreased soluble tau protein phosphorylation at S396 and induced antibody formation in P301L mice (Theunis et al., 2013). The phase 1b/2a clinical trial has shown that ACI-35 treatment is both safe and immunogenic in patients with mild to moderate AD dementia (No authors listed, 2022). Considering the pathogenicity of tau protein and the seeding of tau protein aggregation in naive cells, passive immunotherapies are emerging to target both extra and intracellular tau protein. JNJ-63733657 and UCB0107 antibodies binding to the N-terminus or C-terminus region and mid-domain region of phosphorylated tau protein inhibited cellular uptake of tau protein and efficiently prevented the seeding and propagation of the aberrant protein in naive or healthy cells (Ji and Sigurdsson, 2021). While JNJ-63733657 targets MTBR of tau protein, UCB0107 targets central region (amino acids 235-246) of tau protein. Studies demonstrated that targeting intracellular tau protein is more effective in reducing tau protein pathology in rTg4510 mouse model of tauopathy. The intrabodies, CP13i and PHF1i targeting intracellular tau protein, reduced tau protein pathology, whereas single-chain variable fragments, which target extracellular tau, had no therapeutic effect in the rTg4510 mice (Goodwin et al., 2021). However, the high molecular weight of antibodies is hindering their penetration into the cells (Soeda and Takashima, 2020). To address this limitation, anti-tau protein monoclonal antibodies that target intracellular tau protein have been developed. As of now, a significant number of antibodies have been developed and are being investigated at various stages of clinical trials. Monoclonal antibodies such as BIIB092, C2N-8E12, UCB0107, LY3303560, BIIB076, JNJ-63733657, Lu AF87908, PNT001, and RO7105705 are found to be well tolerated and safe as per phase 1 or 2 study data, and for a few, the trials are ongoing, and results are awaited (Soeda and Takashima, 2020). However, in two phase 2 clinical trials, the antibodies that bind to the N-terminal domain of tau protein (C2N-8E12, tilavonemab, and BIIB092; gosuranemab) were found to be ineffective for PSP patients (Höglinger et al., 2021; Jabbari and Duff, 2021). The binding occurs as expected, as evidenced by a decrease in cerebrospinal fluid-free tau protein and an increase in tau protein in the lysosomes of newly developing perivascular vesicular astrocytes (Kim et al., 2021). Factors affecting the outcome of clinical trials are challenges associated with the earlier PSP diagnosis, particularly in preclinical stages, ineffective epitopes, and extracellular targeting [204]. A phase 2 clinical trial is being conducted to examine C2N-8E12 in AD patients.
The progression of AD is dependent on tau protein phosphorylation at S396/S404 and S422 residues; thus, antibodies such as Lu AF87908 and ACI-35 that can bind to the phosphorylation sites in a sequence-specific manner might be effective in preventing disease progression. However, RG7345, which targets phosphorylated tau protein in tauopathies, was discontinued from clinical development due to the unfavorable pharmacokinetics of the antibody (Sandusky-Beltran and Sigurdsson, 2020). Various tau protein immunotherapies with a wide array of advantages are currently under development. Although most of them succeed to some extent in addressing the advancement of AD and related tauopathies, tau immunotherapies do have a few roadblocks. Expensive production, a short half-life, systemic administration, and the accumulation and formation of anti-antibodies causing undesirable immunological side effects together contributed to the failure of immunotherapies as effective therapeutic tools and hence a parallel need for alternative therapies.
Immune-related adverse anomalies are one of the considerable side effects of tau immunotherapies. To circumvent this issue, prior establishment of a safety profile needs to be done to achieve prophylactic immunotherapy for tauopathy. While developing antibodies for inducing passive immunization, specificity of antibodies and deliverability to the brain should be considered as highly preferable features. Efficient new techniques that can deliver the designed antibodies into the target brain region in sufficient quantities should be developed which can render immunotherapy a successful therapeutic tool to treat tauopathies.
Oligonucleotide based therapies
Theoretically, gene therapy delivers a transgene that either substitutes a defective gene or generally supports cells to combat the disease environment in order to treat a disease. But the selection of the vector, delivery system, dose optimization, host immune response, and vector interaction makes it a complex process. With the recent advancement in technology, oligonucleotide-based therapies like antisense oligonucleotide (ASO) technology, RNA interference (RNAi), and single-stranded catalytic DNA molecules (DNAzyme) are emerging as gene silencing approaches. These methods make use of short, single-stranded (15–20 nucleotide) oligonucleotides that bind to the corresponding RNA sequences based on Watson-Crick base pairing, leading to the eradication of the target RNA. As a major breakthrough, these have been used to address neurodegenerative diseases, and researchers are now targeting tau mRNA or RNA that interferes with the expression of tau mRNA (RNAi) to reduce the production of tau protein to stop their aggregation (Figure 4; DeVos et al. 2013; Xu et al. 2014). Although adeno-associated virus containing RNAi and ASOs therapies effectively reduce mRNA and protein expression and mitigate disease progression, they require direct injection into the brain and have been shown to have off-target effects. Given safety concerns with repeated injections, the risks may limit their use during AD and other tauopathies, which would then limit their therapeutic value.
Figure 4.
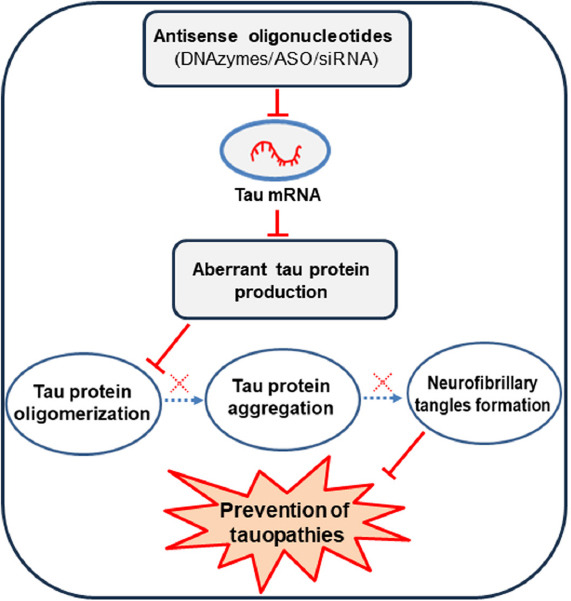
Overview of oligonucleotide-based tau therapies for the treatment of tauopathies.
The accumulation of aberrant tau protein may be minimized by inhibiting the tau gene expression using anti-sense oligonucleotides (DNAzyme, ASOs, siRNA) against the mRNA from the MAPT gene. Created with Microsoft PowerPoint. ASOs: Antisense oligonucleotides; DNAzymes: single-stranded catalytic DNA molecules; MAPT: microtubule-associated protein tau; siRNA: Small interfering RNA.
ASOs
ASOs are short single-stranded DNA sequence ranging from 18–30 nucleotides in length (Scoles et al., 2019). They bind to the complementary mRNA target in a Watson-Crick- based hybridization manner. Upon binding, they modify the expression of target mRNA in an RNase H dependent and independent manner. The RNase H enzyme detects DNA-RNA complexes and eventually cleaves and degrades the target mRNA from the complexes. However, the activation of RNase H upon its hybridization with ASO is highly dependent on the sequence specificity of ASO towards target RNA. On the other hand, the RNase H independent ASOs act as steric blockers and modulate the processing events of mRNA such as splicing, 5’ cap addition, or 3’ end polyadenylation (Roberts et al., 2020). The ASOs are subject to nuclease degradation in biological systems in unmodified form. Several modifications have been made to enhance the biological effectiveness of target therapy. Alterations in the backbone of ASOs using phosphorothioate have proven to improve the susceptibility to degradation by nuclease while retaining the cleavage activity (Jadhav et al., 2019). Further modification of ASOs through the incorporation of a sugar moiety at the 2’ position not only enhanced their potency and nuclease resistance but also remarkably improved their toxicity profile, which makes ASOs feasible for therapeutic purposes (Anderson et al., 2021). In SH-SY5Y cells, ASO targeting the exon 4 of the MAPT gene reduced tau RNA expression and protein levels by 96% and 74% respectively (Chakravarthy et al., 2020). ASO has also been shown to decrease tau mRNA and protein in PS19Tg mice with a reversal in tau protein pathology (DeVos et al., 2017). The ASO, IONIS-MAPT Rx, is currently being tested against tauopathy and showed competitive binding to pre-mRNA of MAPT gene through intron 9. In the phase 1 clinical trials IONIS-MAPT Rx delivered intrathecally in patients with mild AD appeared to be safe (NCT03186989), and it did reduce both total tau protein and phospho tau-181 levels in the cerebrospinal fluid by more than 50 percent from baseline (Mummery et al., 2023). This finding endorses the ASO translatability to humans and suggested that tau-lowering therapy is well-tolerated and safe. However, further studies are required to evaluate the therapeutic effect of ASOs in AD and other tauopathies.
Small interfering RNA
Small interfering RNAs (siRNAs) are short non-coding double-stranded RNA (dsRNA) sequences ranging from 19–21 nucleotides in length, with a 2 nucleotide (TT or UU) overhang on the 3’ end of either strand (Alshaer et al., 2021). They show high specificity towards target RNA and degrade the RNA in RNase H dependent manner. They emerge as a drug-targeting tool by modulating the expression of targeted genes through transcriptional gene silencing. When it enters the cell, the dsRNA is recognized by either the Dicer-2 or Argonaute-2 enzymes to form the RNA-inducing silencing complex and result in the degradation of the passenger strand. The remaining siRNA then binds to the complementary RNA and stimulates its degradation (Alshaer et al., 2021). Hoglinger and coworkers have shown that siRNA against MAPT (AccellTM SMART pool), in the absence of carrier molecule, was able to inhibit the expression of human tau protein in primary cortical neurons of PS19 transgenic mice expressing P301S mutant form of human tau protein. Moreover, neither neurotoxicity, neuroinflammation, nor apoptosis was observed during the presence of MAPT siRNA in the mouse hippocampus (Xu et al., 2014). However, siRNA shortcomings are its limited BBB permeability, cellular uptake, and susceptibility to nuclease-mediated degradation (Dana et al., 2017). Several studies have shown that direct delivery of adeno-associated virus containing RNAi molecules to the CNS can effectively reduce tau protein production and slow or prevent AD progression in mouse models of AD (Ising et al., 2017). Given safety concerns with repeated CNS injections, the risks may limit their use in the course of tauopathy, which would then limit their therapeutic value.
DNAzymes
Breaker and Joyce in 1994, using in-vitro selection methods, discovered a population of single-stranded catalytic DNA (DNAzyme) that could cleave target RNA in a cations-dependent manner (Breaker and Joyce, 1994). Since then, DNAzyme has emerged as a molecular tool in gene therapy, and many investigators globally have explored the pertinent growing scope of DNA catalytic activity, including its applications (Wang et al., 2021). DNAzyme, also known as a single-stranded catalytic DNA molecule, is an RNA-cleaving DNA enzyme. The structure of a DNAzyme comprises three key components, two variable flanking domains or binding arms that are bound to a central catalytic domain with RNA endonuclease activity composed of a specific sequence of 15 or 13 deoxynucleotides. Through Watson-Crick base hybridization, the binding arms help bind the DNAzyme to a specific target mRNA. DNAzyme is a metal-dependent enzyme. It uses a metal ion such as Pb2+, Mg2+, Zn2+, or Cu2+ to polarize a water molecule, catalyzing the hydrolysis of the phosphodiester bond in an RNA molecule. The mRNA cleavage products are further degraded by intracellular RNases, whereas the DNAzyme is then available for a next cycle of catalytic action. With this high turnover cycle, a relatively low concentration of DNAzyme is sufficient to considerably reduce levels of specific disease-causing proteins, thus constituting an effective gene therapy approach. Presently, 10–23 and 8–17 designs of DNAzymes are known to catalyze the efficient cleavage of RNA into degraded products. DNAzymes can be engineered to function in an allele-specific manner to selectively cleave an RNA molecule having a point mutation. Thus, the cleavage of diseased or abnormal mRNAs diminishes the level of disease-causing proteins without interfering with the expression of the wild-type protein (Ma and Liu, 2020). The backbone modification or the addition of a sugar moiety to DNAzyme molecule has been shown to substantially improve its stability and potency in serum (Wang et al., 2021). Structural fine tuning of DNAzyme has improved its half-life, resistance against endogenous nucleases, and most importantly, affinity towards the target complementary sequences (Wang et al., 2021). The distribution, clearance, and safety of DNAzyme have recently been described. (Hallett et al., 2013). In this study, Hallett et al. (2013) have shown that DNAzymes are stable, can cross the BBB, and can be delivered repeatedly by systemic injections, thus avoiding the need for direct brain injections. The therapeutic utility of DNAzyme is further supported by clinical trials of DNAzyme targeting c-jun (Grassi and Grassi, 2013), EBV LMP1 protein (Cao et al., 2014), and GATA-3 (Cho et al., 2013) that have shown that it is safe and well-tolerated.
The molecular events contributing to neuronal death or degeneration are not yet clear and are under investigation. Evidence suggests that there is a close relationship between AD and the level of β-secretase (also known as BACE1 or β-site APP cleaving enzyme 1). BACE1 is an aspartyl protease that contributes to the development of Aβ plaque, the pathophysiological hallmark of AD. DNAzyme generated against BACE mRNA has been shown to reduce BACE gene expression by 50–70% in the HEK293 cells (Nawrot, 2004). In SH-SY5Y cells, DNAzyme (RNV563) targeting MAPT exon 13 reduced MAPT mRNA expression by more than 50% (Chakravarthy et al., 2020). The finding from these preclinical studies encourages more comprehensive and rigorous studies to examine the distribution, safety, and therapeutic benefit of DNAzyme, which is essential for clinical translation and the expansion of therapeutic applications for tauopathies and several other neurodegenerative diseases.
Oligonucleotide-based therapies have made astonishing advances over the last few years with the approval of ASO and with promising developments of several potential therapeutic agents for neurodegenerative diseases. However, off-target effect and effective delivery of oligonucleotides to its site of action remains a major issue. Efficient oligonucleotides with improved nuclease resistance, susceptibility to degradation, and toxicity profile need to be developed to make the treatment method more feasible and safer. Several strategies including structural modification of the oligonucleotide, utilization of various lipid or polymeric nanocarriers, tagging of oligonucleotides to target receptors, and use of small molecules can be adopted to enhance oligonucleotide effectiveness.
Conclusion and Future Perspectives
Tauopathies including AD are a group of neurodegenerative diseases that are characterized by an abnormal accumulation of hyperphosphorylated tau protein in the brain cells and progressive cognitive decline. Despite gaining considerable knowledge about the pathological mechanisms of tauopathies over the last several decades, we know little about how to stop or slow down the ongoing neurodegenerative process. In the search for effective disease-modifying intervention for tauopathies, several biological targets have been and continue to be determined. Considering the strong association between pathogenic tau protein accumulation and cognitive deficits along with genetic evidence that tau mutations cause progressive neurodegeneration, there has been a lot of interest in developing anti-tau RNA/protein therapies as a means to treat AD and related tauopathies. The majority of the tau-based therapies are targeting either the gain of toxic function of tau protein by aiming PTMs and tau protein aggregation, or the loss of function of tau protein by aiming microtubule instability. Each approach has its advantages and shortcomings. A common limitation that can be seen in these approaches is either poor BBB permeability and/or off-target effect. Thus, new therapeutic strategies to downregulate the production of pathological tau aggregates, and deliver the therapeutic agent to CNS, with tolerable toxicity will signify promising interventions in treating tauopathies. Given that tau pathology may differ across tauopathies, a realistic therapeutic approach may be to regulate the tau transcripts instead of tau protein, which would rule out the possibility of tau protein toxicity. The current advancements in gene therapy, genome editing techniques, and oligonucleotide-based therapies are opening the doors to a better understanding of the cellular mechanisms of tauopathy and the development of new therapies for these complex disorders. To this end, ASO, RNAi, and DNAzymes represent potential therapeutic approaches that target tau mRNA to regulate its accumulation and toxicity during pathological conditions (Figure 4). While ASOs and DNAzymes have been investigated for several neural and extraneural diseases, there has been considerable interest in testing the therapeutic benefits of ASOs and DNAzyme in tauopathies. Considering the positive outcome of tau-targeting ASO MAPTRx in AD, more preclinical studies are required to determine the therapeutic benefits of these tau-lowering gene therapies for other primary tauopathies. Moreover, attempts to improve the BBB penetrability of ASOs or DNAzyme are required to enhance the effectiveness of these therapies which may possibly persuade the approach to be a much more competent option to treat neurodegenerative disorders in the future. Although gene therapies including ASOs and DNAzyme are safe and effective in reducing mRNA expression of target genes, these are still in the initial stage of development and further comprehensive studies will be needed for evaluation of these approaches in preclinical models of tauopathies for clinical translation.
Funding Statement
Funding: This work was supported by National Institute of Health grant number R03AG075597 (to MMK and TP) and Department of Defense Award Number HT9425-23-1-0043 (to MMK).
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Data availability statement: Not applicable.
C-Editors: Liu WJ, Zhao M, Qiu Y; T-Editor: Jia Y
References
- 1.Ahmed T, Van der Jeugd A, Caillierez R, Buée L, Blum D, D'hooge R, Balschun D. Chronic sodium selenate treatment restores deficits in cognition and synaptic plasticity in a murine model of tauopathy. Front Mol Neurosci. (2020);13:570223. doi: 10.3389/fnmol.2020.570223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert M, Mairet-Coello G, Danis C, Lieger S, Caillierez R, Carrier S, Skrobala E, Landrieu I, Michel A, Schmitt M. Prevention of tau seeding and propagation by immunotherapy with a central tau epitope antibody. Brain. (2019);142:1736–1750. doi: 10.1093/brain/awz100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso AdC, Grundke-Iqbal I, Barra HS, Iqbal K. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration:sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc Natl Acad Sci U S A. (1997);94:298–303. doi: 10.1073/pnas.94.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alquezar C, Arya S, Kao AW. Tau post-translational modifications:dynamic transformers of tau function degradation and aggregation. Front Neurol. (2021);11:595532. doi: 10.3389/fneur.2020.595532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alshaer W, Zureigat H, Al Karaki A, Al-Kadash A, Gharaibeh L, Ma'mon MH, Aljabali AA, Awidi A. siRNA:mechanism of action challenges and therapeutic approaches. Eur J Pharmacol. (2021);905:174178. doi: 10.1016/j.ejphar.2021.174178. [DOI] [PubMed] [Google Scholar]
- 6.Anderson BA, Freestone GC, Low A, De-Hoyos CL, Iii WJD, Østergaard ME, Migawa MT, Fazio M, Wan WB, Berdeja A. Towards next generation antisense oligonucleotides:mesylphosphoramidate modification improves therapeutic index and duration of effect of gapmer antisense oligonucleotides. Nucleic Acids Res. (2021);49:9026–9041. doi: 10.1093/nar/gkab718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T, Wolozin B, Butovsky O, Kügler S, Ikezu T. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci. (2015);18:1584–1593. doi: 10.1038/nn.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Betters RK, Luhmann E, Gottschalk AC, Xu Z, Shin MR, Ptak CP, Fiock KL, Radoshevich LC, Hefti MM. Characterization of the tau interactome in human brain reveals isoform-dependent interaction with 14-3-3 family proteins. eNeuro 10. (2023) doi: 10.1523/ENEURO.0503-22.2023. doi:10.1523/ENEURO.0503-22.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bieniek KF, Blessing MM, Heckman MG, Diehl NN, Serie AM, Paolini MA, Boeve BF, Savica R, Reichard RR, Dickson DW. Association between contact sports participation and chronic traumatic encephalopathy:a retrospective cohort study. Brain Pathol. (2020);30:63–74. doi: 10.1111/bpa.12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bijari N, Balalaie S, Akbari V, Golmohammadi F, Moradi S, Adibi H, Khodarahmi R. Effective suppression of the modified PHF6 peptide/1N4R Tau amyloid aggregation by intact curcumin not its degradation products:another evidence for the pigment as preventive/therapeutic “functional food”. Int J Biol Macromol. (2018);120:1009–1022. doi: 10.1016/j.ijbiomac.2018.08.175. [DOI] [PubMed] [Google Scholar]
- 11.Borroni B, Graff C, Hardiman O, Ludolph AC, Moreno F, Otto M, Piccininni M, Remes AM, Rowe JB, Seelaar H. FRONTotemporal dementia Incidence European Research Study—FRONTIERS:rationale and design. Alzheimers Dement. (2022);18:498–506. doi: 10.1002/alz.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breaker RR, Joyce GF. A DNA enzyme that cleaves RNA. Chem Biol. (1994);1:223–229. doi: 10.1016/1074-5521(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 13.Brunello CA, Merezhko M, Uronen R-L, Huttunen HJ. Mechanisms of secretion and spreading of pathological tau protein. Cell Mol Life Sci. (2020);77:1721–1744. doi: 10.1007/s00018-019-03349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao Y, Yang L, Jiang W, Wang X, Liao W, Tan G, Liao Y, Qiu Y, Feng D, Tang F. Therapeutic evaluation of Epstein-Barr virus-encoded latent membrane protein-1 targeted DNAzyme for treating of nasopharyngeal carcinomas. Mol Ther. (2014);22:371–377. doi: 10.1038/mt.2013.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardoso BR, Roberts BR, Malpas CB, Vivash L, Genc S, Saling MM, Desmond P, Steward C, Hicks RJ, Callahan J. Supranutritional sodium selenate supplementation delivers selenium to the central nervous system:results from a randomized controlled pilot trial in Alzheimer's disease. Neurotherapeutics. (2019);16:192–202. doi: 10.1007/s13311-018-0662-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cascio FL, Puangmalai N, Ellsworth A, Bucchieri F, Pace A, Piccionello AP, Kayed R. Toxic tau oligomers modulated by novel curcumin derivatives. Sci Rep. (2019);9:1–16. doi: 10.1038/s41598-019-55419-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakravarthy M, Chen S, Wang T, Veedu RN. Development of novel chemically-modified nucleic acid molecules for efficient inhibition of human MAPT gene expression. Genes (Basel) (2020);11:667. doi: 10.3390/genes11060667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen M, Du ZY, Zheng X, Li DL, Zhou RP, Zhang K. Use of curcumin in diagnosi prevention and treatment of Alzheimer's disease. Neural Regen Res. (2018);13:742. doi: 10.4103/1673-5374.230303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chimento A, De Amicis F, Sirianni R, Sinicropi MS, Puoci F, Casaburi I, Saturnino C, Pezzi V. Progress to improve oral bioavailability and beneficial effects of resveratrol. Int J Mol Sci. (2019);20:1381. doi: 10.3390/ijms20061381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho EA, Moloney FJ, Cai H, Au-Yeung A, China C, Scolyer RA, Yosufi B, Raftery MJ, Deng JZ, Morton SW. Safety and tolerability of an intratumorally injected DNAzyme Dz13 in patients with nodular basal-cell carcinoma:a phase 1 first-in-human trial (DISCOVER) Lancet. (2013);381:1835–1843. doi: 10.1016/S0140-6736(12)62166-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung DC, Roemer S, Petrucelli L, Dickson DW. Cellular and pathological heterogeneity of primary tauopathies. Mol Neurodegener. (2021);16:57. doi: 10.1186/s13024-021-00476-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuello AC, Pentz R, Hall H. The brain NGF metabolic pathway in health and in Alzheimer's pathology. Front Neurosci. (2019);13:62. doi: 10.3389/fnins.2019.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dana H, Chalbatani GM, Mahmoodzadeh H, Karimloo R, Rezaiean O, Moradzadeh A, Mehmandoost N, Moazzen F, Mazraeh A, Marmari V. Molecular mechanisms and biological functions of siRNA. Int J Biomed Sci. (2017);13:48. [PMC free article] [PubMed] [Google Scholar]
- 24.DeVos SL, Goncharoff DK, Chen G, Kebodeaux CS, Yamada K, Stewart FR, Schuler DR, Maloney SE, Wozniak DF, Rigo F. Antisense reduction of tau in adult mice protects against seizures. J Neurosci. (2013);33:12887–12897. doi: 10.1523/JNEUROSCI.2107-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeVos SL, Miller RL, Schoch KM, Holmes BB, Kebodeaux CS, Wegener AJ, Chen G, Shen T, Tran H, Nichols B. Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Sci Transl Med. (2017);9:eaag0481. doi: 10.1126/scitranslmed.aag0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di J, Siddique I, Li Z, Malki G, Hornung S, Dutta S, Hurst I, Ishaaya E, Wang A, Tu S, Boghos A, Ericsson I, Klärner FG, Schrader T, Bitan G. The molecular tweezer CLR01 improves behavioral deficits and reduces tau pathology in P301S-tau transgenic mice. Alzheimers Res Ther. (2021);13:6. doi: 10.1186/s13195-020-00743-x. Erratum in:Alzheimers Res Ther 13:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dugger BN, Whiteside CM, Maarouf CL, Walker DG, Beach TG, Sue LI, Garcia A, Dunckley T, Meechoovet B, Reiman EM. The presence of select tau species in human peripheral tissues and their relation to Alzheimer's disease. J Alzheimers Dis. (2016);51:345–356. doi: 10.3233/JAD-150859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duyckaerts C, Clavaguera F, Potier MC. The prion-like propagation hypothesis in Alzheimer's and Parkinson's disease. Curr Opin Neurol. (2019);32:266–271. doi: 10.1097/WCO.0000000000000672. [DOI] [PubMed] [Google Scholar]
- 29.El-Hayek YH, Wiley RE, Khoury CP, Daya RP, Ballard C, Evans AR, Karran M, Molinuevo JL, Norton M, Atri A. Tip of the iceberg:assessing the global socioeconomic costs of Alzheimer's disease and related dementias and strategic implications for stakeholders. J Alzheimers Dis. (2019);70:323–341. doi: 10.3233/JAD-190426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischer I, Baas PW. Resurrecting the mysteries of big tau. Trends Neurosci. (2020);43:493–504. doi: 10.1016/j.tins.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frontera JA, Boutajangout A, Masurkar AV, Betensky RA, Ge Y, Vedvyas A, Debure L, Moreira A, Lewis A, Huang J. Comparison of serum neurodegenerative biomarkers among hospitalized COVID-19 patients versus non-COVID subjects with normal cognition mild cognitive impairment or Alzheimer's dementia. Alzheimers Dement. (2022);18:899–910. doi: 10.1002/alz.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gauthier S, Feldman HH, Schneider LS, Wilcock GK, Frisoni GB, Hardlund JH, Moebius HJ, Bentham P, Kook KA, Wischik DJ. Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer's disease:a randomised controlled double-blind parallel-arm phase 3 trial. Lancet. (2016);388:2873–2884. doi: 10.1016/S0140-6736(16)31275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghasemzadeh S, Riazi GH. Inhibition of Tau amyloid fibril formation by folic acid:in-vitro and theoretical studies. Int J Biol Macromol. (2020);154:1505–1516. doi: 10.1016/j.ijbiomac.2019.11.032. [DOI] [PubMed] [Google Scholar]
- 34.Giuffrida ML, Copani A, Rizzarelli E. A promising connection between BDNF and Alzheimer's disease. Aging (Albany NY) (2018);10:1791. doi: 10.18632/aging.101518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodwin MS, Sinyavskaya O, Burg F, O'Neal V, Ceballos-Diaz C, Cruz PE, Lewis J, Giasson BI, Davies P, Golde TE. Anti-tau scFvs targeted to the cytoplasm or secretory pathway variably modify pathology and neurodegenerative phenotypes. Mol Ther. (2021);29:859–872. doi: 10.1016/j.ymthe.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorantla NV, Das R, Balaraman E, Chinnathambi S. Transition metal nickel prevents Tau aggregation in Alzheimer's disease. Int J Biol Macromol. (2020);156:1359–1365. doi: 10.1016/j.ijbiomac.2019.11.176. [DOI] [PubMed] [Google Scholar]
- 37.Gordon MN, Heneka MT, Le Page LM, Limberger C, Morgan D, Tenner AJ, Terrando N, Willette AA, Willette SA. Impact of COVID-19 on the onset and progression of Alzheimer's disease and related dementias:a roadmap for future research. Alzheimers Dement. (2022);18:1038–1046. doi: 10.1002/alz.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grassi G, Grassi M. First-in-human trial of Dz13 for nodular basal-cell carcinoma. Lancet. (2013);381:1797–1798. doi: 10.1016/S0140-6736(13)60633-9. [DOI] [PubMed] [Google Scholar]
- 39.Hallett MA, Dalal P, Sweatman TW, Pourmotabbed T. The distribution clearance and safety of an anti-MMP-9 DNAzyme in normal and MMTV-PyMT transgenic mice. Nucleic Acid Ther. (2013);23:379–388. doi: 10.1089/nat.2012.0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hefti MM, Kim S, Bell AJ, Betters RK, Fiock KL, Iida MA, Smalley ME, Farrell K, Fowkes ME, Crary JF. Tau phosphorylation and aggregation in the developing human brain. J Neuropathol Exp Neurol. (2019);78:930–938. doi: 10.1093/jnen/nlz073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Höglinger GU, Litvan I, Mendonca N, Wang D, Zheng H, Rendenbach-Mueller B, Lon H-K, Jin Z, Fisseha N, Budur K. Safety and efficacy of tilavonemab in progressive supranuclear palsy:a phase 2 randomised placebo-controlled trial. Lancet Neurol. (2021);20:182–192. doi: 10.1016/S1474-4422(20)30489-0. [DOI] [PubMed] [Google Scholar]
- 42.Hsieh YC, Guo C, Yalamanchili HK, Abreha M, Al-Ouran R, Li Y, Dammer EB, Lah JJ, Levey AI, Bennett DA. Tau-mediated disruption of the spliceosome triggers cryptic RNA splicing and neurodegeneration in Alzheimer's disease. Cell Rep. (2019);29:301–316. doi: 10.1016/j.celrep.2019.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imbimbo BP, Ippati S, Watling M, Balducci C. A critical appraisal of tau-targeting therapies for primary and secondary tauopathies. Alzheimers Dement. (2022);18:1008–1037. doi: 10.1002/alz.12453. [DOI] [PubMed] [Google Scholar]
- 44.Ising C, Gallardo G, Leyns CE, Wong CH, Jiang H, Stewart F, Koscal LJ, Roh J, Robinson GO, Remolina Serrano J. AAV-mediated expression of anti-tau scFvs decreases tau accumulation in a mouse model of tauopathy. J Exp Med. (2017);214:1227–1238. doi: 10.1084/jem.20162125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jabbari E, Duff KE. Tau-targeting antibody therapies:too late wrong epitope or wrong target? Nat Med. (2021);27:1341–1342. doi: 10.1038/s41591-021-01465-9. [DOI] [PubMed] [Google Scholar]
- 46.Jadhav S, Avila J, Schöll M, Kovacs GG, Kövari E, Skrabana R, Evans LD, Kontsekova E, Malawska B, de Silva R. A walk through tau therapeutic strategies. Acta Neuropathol Commun. (2019);7:22. doi: 10.1186/s40478-019-0664-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ji C, Sigurdsson EM. Current status of clinical trials on tau immunotherapies. Drugs. (2021);81:1135–1152. doi: 10.1007/s40265-021-01546-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joly-Amado A, Davtyan H, Serraneau K, Jules P, Zitnyar A, Pressman E, Zagorski K, Antonyan T, Hovakimyan A, Paek H. Active immunization with tau epitope in a mouse model of tauopathy induced strong antibody response together with improvement in short memory and pSer396-tau pathology. Neurobiol Dis. (2020);134:104636. doi: 10.1016/j.nbd.2019.104636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim B, Mikytuck B, Suh E, Gibbons GS, Van Deerlin VM, Vaishnavi SN, Spindler MA, Massimo L, Grossman M, Trojanowski JQ. Tau immunotherapy is associated with glial responses in FTLD-tau. Acta Neuropathol. (2021);142:243–257. doi: 10.1007/s00401-021-02318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee HY, Fan SJ, Huang FI, Chao HY, Hsu KC, Lin TE, Yeh TK, Lai MJ, Li YH, Huang HL. 5-Aroylindoles act as selective histone deacetylase 6 inhibitors ameliorating Alzheimer's disease phenotypes. J Med Chem. (2018);61:7087–7102. doi: 10.1021/acs.jmedchem.8b00151. [DOI] [PubMed] [Google Scholar]
- 51.Leroy M, Bertoux M, Skrobala E, Mode E, Adnet-Bonte C, Le Ber I, Bombois S, Cassagnaud P, Chen Y, Deramecourt V, Lebert F, Mackowiak MA, Sillaire AR, Wathelet M, Pasquier F, Lebouvier T, Abied R, Adnet C, Barois A, Baude S, et al. Characteristics and progression of patients with frontotemporal dementia in a regional memory clinic network. Alzheimers Res Ther. (2021);13:19. doi: 10.1186/s13195-020-00753-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu X, Hebron ML, Mulki S, Wang C, Lekah E, Ferrante D, Shi W, Kurd-Misto B, Moussa C. Ubiquitin specific protease 13 regulates tau accumulation and clearance in models of Alzheimer's disease. J Alzheimers Dis. (2019);72:425–441. doi: 10.3233/JAD-190635. [DOI] [PubMed] [Google Scholar]
- 53.Ma L, Liu J. Catalytic nucleic acids:biochemistry chemical biology biosensors and nanotechnology. Science. (2020);23:100815. doi: 10.1016/j.isci.2019.100815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miao J, Shi R, Li L, Chen F, Zhou Y, Tung YC, Hu W, Gong C-X, Iqbal K, Liu F. Pathological tau from Alzheimer's brain induces site-specific hyperphosphorylation and SDS-and reducing agent-resistant aggregation of tau in vivo. Front Aging Neurosci. (2019);11:34. doi: 10.3389/fnagi.2019.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller JH, Das V. Potential for treatment of neurodegenerative diseases with natural products or synthetic compounds that stabilize microtubules. Curr Pharm Des. (2020);26:4362–4372. doi: 10.2174/1381612826666200621171302. [DOI] [PubMed] [Google Scholar]
- 56.Min SW, Chen X, Tracy TE, Li Y, Zhou Y, Wang C, Shirakawa K, Minami SS, Defensor E, Mok SA. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat Med. (2015);21:1154–1162. doi: 10.1038/nm.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mirbaha H, Chen D, Morazova OA, Ruff KM, Sharma AM, Liu X, Goodarzi M, Pappu RV, Colby DW, Mirzaei H. Inert and seed-competent tau monomers suggest structural origins of aggregation. Elife. (2018);7:e36584. doi: 10.7554/eLife.36584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mummery CJ, Borjesson-Hanson A, Blackburn DJ, Vijverberg EGB, De Deyn PP, Ducharme S, Jonsson M, Schneider A, Rinne JO, Ludolph AC, Bodenschatz R, Kordasiewicz H, Swayze EE, Fitzsimmons B, Mignon L, Moore KM, Yun C, Baumann T, Li D, Norris DA, et al. Tau-targeting antisense oligonucleotide MAPT(Rx) in mild Alzheimer's disease:a phase 1b, randomized, placebo-controlled trial. Nat Med. (2023);29:1437–1447. doi: 10.1038/s41591-023-02326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nawrot B. Targeting BACE with small inhibitory nucleic acids-a future for Alzheimer's disease therapy? Acta Biochim Pol. (2004);51:431–444. [PubMed] [Google Scholar]
- 60.No authors listed. Two new stabs at vaccinating people against pathologic tau. (2022). [Accessed July 13, 2023]. Available at: https://www.alzforum.org/news/conference-coverage/two-new-stabs-vaccinating-people-against-pathologic-tau .
- 61.Novak P, Kovacech B, Katina S, Schmidt R, Scheltens P, Kontsekova E, Ropele S, Fialova L, Kramberger M, Paulenka-Ivanovova N. ADAMANT:a placebo-controlled randomized phase 2 study of AADvac1 an active immunotherapy against pathological tau in Alzheimer's disease. Nat Aging. (2021);1:521–534. doi: 10.1038/s43587-021-00070-2. [DOI] [PubMed] [Google Scholar]
- 62.Nygaard HB. Targeting Fyn kinase in Alzheimer's disease. Biol Psychiatry. (2018);83:369–376. doi: 10.1016/j.biopsych.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oakley SS, Maina MB, Marshall KE, Al-Hilaly YK, Harrington CR, Wischik CM, Serpell LC. Tau filament self-assembly and structure:tau as a therapeutic target. Front Neurol. (2020);11:590754. doi: 10.3389/fneur.2020.590754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okuda M, Fujita Y, Hijikuro I, Wada M, Uemura T, Kobayashi Y, Waku T, Tanaka N, Nishimoto T, Izumi Y. PE859 a novel curcumin derivativ inhibits >amyloid-βand tau aggregation and ameliorates cognitive dysfunction in senescence-accelerated mouse prone 8. J Alzheimers Dis. (2017);59:313–328. doi: 10.3233/JAD-161017. [DOI] [PubMed] [Google Scholar]
- 65.Oukoloff K, Nzou G, Varricchio C, Lucero B, Alle T, Kovalevich J, Monti L, Cornec A-S, Yao Y, James MJ. Evaluation of the structure–activity relationship of microtubule-targeting 1 2 4-triazolo [15-a] pyrimidines identifies new candidates for neurodegenerative tauopathies. J Med Chem. (2021);64:1073–1102. doi: 10.1021/acs.jmedchem.0c01605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Polanco JC, Götz J. Exosomal and vesicle-free tau seeds—propagation and convergence in endolysosomal permeabilization. FEBS J. (2022);289:6891–6907. doi: 10.1111/febs.16055. [DOI] [PubMed] [Google Scholar]
- 67.Reiken S, Sittenfeld L, Dridi H, Liu Y, Liu X, Marks AR. Alzheimer's-like signaling in brains of COVID-19 patients. Alzheimers Dement. (2022);18:955–965. doi: 10.1002/alz.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Riedel G, Klein J, Niewiadomska G, Kondak C, Schwab K, Lauer D, Magbagbeolu M, Steczkowska M, Zadrozny M, Wydrych M, Cranston A, Melis V, Santos RX, Theuring F, Harrington CR, Wischik CM. Mechanisms of anticholinesterase interference with tau aggregation inhibitor activity in a tau-transgenic mouse model. Curr Alzheimer Res. (2020);17:285–296. doi: 10.2174/1567205017666200224120926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roberts TC, Langer R, Wood MJ. Advances in oligonucleotide drug delivery. Nat Rev Drug Discov. (2020);19:673–694. doi: 10.1038/s41573-020-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sandusky-Beltran L, Sigurdsson E. Tau immunotherapies:lessons learned current status and future considerations. Neuropharmacology. (2020);175:108104. doi: 10.1016/j.neuropharm.2020.108104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sayas CL, Ávila J. GSK-3 and Tau:a key duet in Alzheimer's disease. Cells. (2021);10:721. doi: 10.3390/cells10040721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scoles DR, Minikel EV, Pulst SM. Antisense oligonucleotides:a primer. Neurol Genet. (2019);5:e323. doi: 10.1212/NXG.0000000000000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharma K. Cholinesterase inhibitors as Alzheimer's therapeutics. Mol Med Rep. (2019);20:1479–1487. doi: 10.3892/mmr.2019.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silva M, Nandi GA, Tentarelli S, Gurrell IK, Jamier T, Lucente D, Dickerson BC, Brown DG, Brandon NJ, Haggarty SJ. Prolonged tau clearance and stress vulnerability rescue by pharmacological activation of autophagy in tauopathy neurons. Nat Commun. (2020);11:1–18. doi: 10.1038/s41467-020-16984-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Snitow ME, Bhansali RS, Klein PS. Lithium and therapeutic targeting of GSK-3. Cells. (2021);10:255. doi: 10.3390/cells10020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soeda Y, Saito M, Maeda S, Ishida K, Nakamura A, Kojima S, Takashima A. Methylene blue inhibits formation of tau fibrils but not of granular tau oligomers:a plausible key to understanding failure of a clinical trial for Alzheimer's disease. J Alzheimers Dis. (2019);68:1677–1686. doi: 10.3233/JAD-181001. [DOI] [PubMed] [Google Scholar]
- 77.Soeda Y, Takashima A. New insights into drug discovery targeting tau protein. Front Mol Neurosci. 13. (2020):590896. doi: 10.3389/fnmol.2020.590896. (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stang CD, Turcano P, Mielke MM, Josephs KA, Bower JH, Ahlskog JE, Boeve BF, Martin PR, Upadhyaya SG, Savica R. Incidence and trends of progressive supranuclear palsy and corticobasal syndrome:a population-based study. J Parkinsons Dis. (2020);10:179–184. doi: 10.3233/JPD-191744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taleski G, Sontag E. Protein phosphatase 2A and tau:an orchestrated 'Pas de Deux'. FEBS Lett. (2018);592:1079–1095. doi: 10.1002/1873-3468.12907. [DOI] [PubMed] [Google Scholar]
- 80.Theunis C, Crespo-Biel N, Gafner V, Pihlgren M, López-Deber MP, Reis P, Hickman DT, Adolfsson O, Chuard N, Ndao DM. Efficacy and safety of a liposome-based vaccine against protein Tau, assessed in tau. P301L mice that model tauopathy. PLoS One. (2013);8:e72301. doi: 10.1371/journal.pone.0072301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thoenen H, Sendtner M. Neurotrophins:from enthusiastic expectations through sobering experiences to rational therapeutic approaches. Nat Neurosci. (2002);5:1046–1050. doi: 10.1038/nn938. [DOI] [PubMed] [Google Scholar]
- 82.Tsai RM, Miller Z, Koestler M, Rojas JC, Ljubenkov PA, Rosen HJ, Rabinovici GD, Fagan AM, Cobigo Y, Brown JA. Reactions to multiple ascending doses of the microtubule stabilizer TPI-287 in patients with Alzheimer disease, progressive supranuclear palsy and corticobasal syndrome:a randomized clinical trial. JAMA Neurol. (2020);77:215–224. doi: 10.1001/jamaneurol.2019.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Turcano P, Stang CD, Mielke MM, Martin PR, Upadhyaya SG, Josephs KA, Boeve BF, Knopman DS, Petersen RC, Savica R. Incidence of frontotemporal disorders in Olmsted County:a population-based study. Alzheimers Dement. (2020);16:482–490. doi: 10.1016/j.jalz.2019.08.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.VandeVrede L, Dale ML, Fields S, Frank M, Hare E, Heuer HW, Keith K, Koestler M, Ljubenkov PA, McDermott D. Open-label phase 1 futility studies of salsalate and young plasma in progressive supranuclear palsy. Mov Disord Clin Pract. (2020);7:440–447. doi: 10.1002/mdc3.12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang L, Kumar R, Pavlov PF, Winblad B. Small molecule therapeutics for tauopathy in Alzheimer's disease:walking on the path of most resistance. Eur J Med Chem. (2020);209:112915. doi: 10.1016/j.ejmech.2020.112915. [DOI] [PubMed] [Google Scholar]
- 86.Wang X, Li W, Marcus J, Pearson M, Song L, Smith K, Terracina G, Lee J, Hong KLK, Lu SX. MK-∏ a novel and selective O-GlcNAcase inhibitor that reduces the formation of pathological tau and ameliorates neurodegeneration in a mouse model of tauopathy. J Pharmacol Exp Ther. (2020);374:252–263. doi: 10.1124/jpet.120.266122. [DOI] [PubMed] [Google Scholar]
- 87.Wang Y, Mandelkow E. Tau in physiology and pathology. Nat Rev Neurosci. (2016);17:22–35. doi: 10.1038/nrn.2015.1. [DOI] [PubMed] [Google Scholar]
- 88.Wang Y, Nguyen K, Spitale RC, Chaput JC. A biologically stable DNAzyme that efficiently silences gene expression in cells. Nat Chem. (2021);13:319–326. doi: 10.1038/s41557-021-00645-x. [DOI] [PubMed] [Google Scholar]
- 89.Wei H, Zhang HL, Wang XC, Xie JZ, An DD, Wan L, Wang JZ, Zeng Y, Shu XJ, Westermarck J, Lu YM, Ohlmeyer M, Liu R. Direct activation of protein phosphatase 2A (PP2A) by tricyclic sulfonamides ameliorates Alzheimer's disease pathogenesis in cell and animal models. Neurotherapeutics. (2020);17:1087–1103. doi: 10.1007/s13311-020-00841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wei W, Liu Y, Dai CL, Baazaoui N, Tung YC, Liu F, Iqbal K. Neurotrophic treatment initiated during early postnatal development prevents the Alzheimer-like behavior and synaptic dysfunction. J Alzheimers Dis. (2021);82:631–646. doi: 10.3233/JAD-201599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wurm R, Klotz S, Rahimi J, Katzenschlager R, Lindeck-Pozza E, Regelsberger G, Danics K, Kapas I, Bíró Z, Stögmann E. Argyrophilic grain disease in individuals younger than 75 years:clinical variability in an under-recognized limbic tauopathy. Eur J Neurol. (2020);27:1856–1866. doi: 10.1111/ene.14321. [DOI] [PubMed] [Google Scholar]
- 92.Xu H, Rösler TW, Carlsson T, de Andrade A, Fiala O, Hollerhage M, Oertel WH, Goedert M, Aigner A, Höglinger GU. Tau silencing by siRNA in the P301S mouse model of tauopathy. Curr Gene Ther. (2014);14:343–351. doi: 10.2174/156652321405140926160602. [DOI] [PubMed] [Google Scholar]
- 93.Yadikar H, Torres I, Aiello G, Kurup M, Yang Z, Lin F, Kobeissy F, Yost R, Wang KK. Screening of tau protein kinase inhibitors in a tauopathy-relevant cell-based model of tau hyperphosphorylation and oligomerization. PLoS One. (2020);15:e0224952. doi: 10.1371/journal.pone.0224952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yoshiyama Y, Kojima A, Ishikawa C, Arai K. Anti-inflammatory action of donepezil ameliorates tau pathology, synaptic loss and neurodegeneration in a tauopathy mouse model. J Alzheimers Dis. (2010);22:295–306. doi: 10.3233/JAD-2010-100681. [DOI] [PubMed] [Google Scholar]
- 95.You G, Yao J, Liu Q, Li N. The strategies for treating “Alzheimer's disease”:insulin signaling may be a feasible target. Curr Issues Mol Biol. (2022);44:6172–6188. doi: 10.3390/cimb44120421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu KC, Kwan P, Cheung SK, Ho A, Baum L. Effects of resveratrol and morin on insoluble tau in tau transgenic mice. Transl Neurosci. (2018);9:54–60. doi: 10.1515/tnsci-2018-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang B, Yao Y, Cornec AS, Oukoloff K, James MJ, Koivula P, Trojanowski JQ, Smith AB, Lee VMY, Ballatore C. A brain-penetrant triazolopyrimidine enhances microtubule-stability, reduces axonal dysfunction and decreases tau pathology in a mouse tauopathy model. Mol Neurodegener. (2018);13:59. doi: 10.1186/s13024-018-0291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang H, Cao Y, Ma L, Wei Y, Li H. Possible mechanisms of tau spread and toxicity in Alzheimer's disease. Front Cell Dev Biol. (2021);9:707268. doi: 10.3389/fcell.2021.707268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou Y, Shi J, Chu D, Hu W, Guan Z, Gong CX, Iqbal K, Liu F. Relevance of phosphorylation and truncation of tau to the etiopathogenesis of Alzheimer's disease. Front Aging Neurosci. (2018);10:27. doi: 10.3389/fnagi.2018.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]


