Abstract
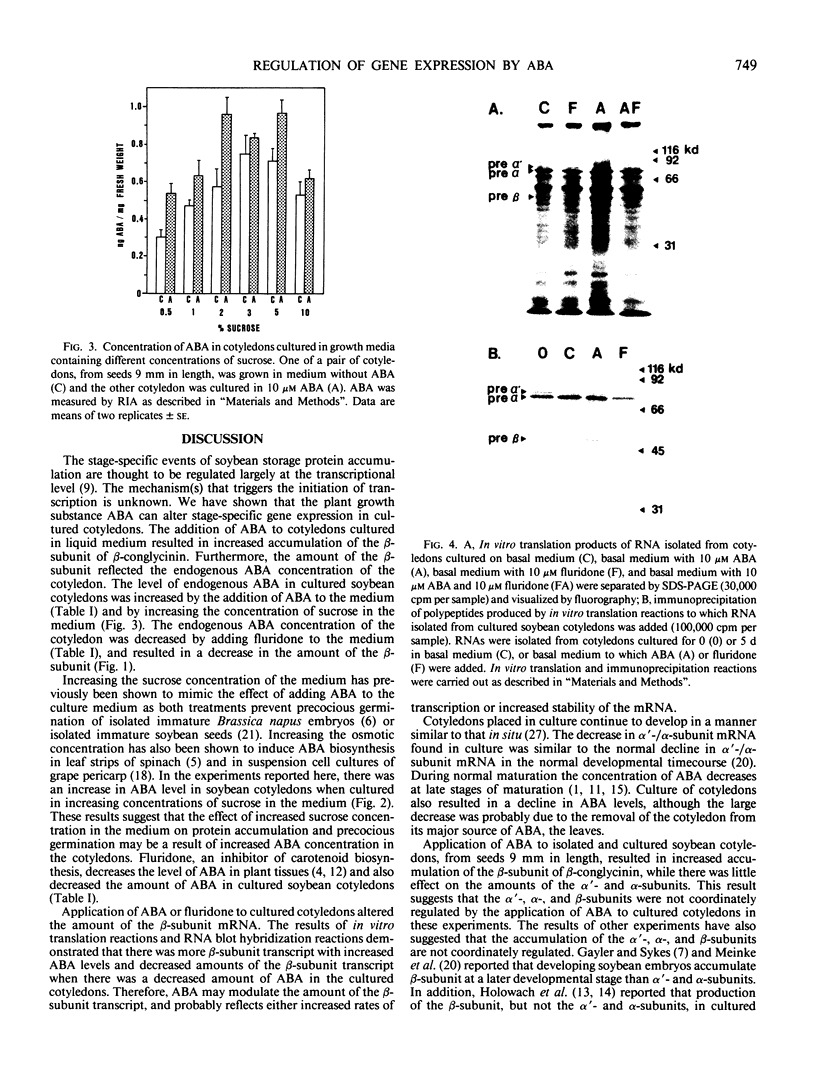
The regulation of cotyledon-specific gene expression by exogenously applied abscisic acid (ABA) was studied in developing cultured cotyledons of soybean (Glycine max L. Merr. cv Provar). When immature cotyledons were cultured in modified Thompson's medium, the addition of ABA resulted in an increased concentration of the β-subunit of β-conglycinin, one of the major storage proteins of soybean seeds. The amount of the α′-and α-subunits of β-conglycinin was relatively unaffected by the ABA treatment. When fluridone, an inhibitor of carotenoid biosynthesis that has been shown to decrease ABA levels in plant tissues, was added to the medium the level of ABA and the β-subunit decreased in the cotyledons. Increasing the concentration of sucrose in the culture medium caused an increase in the concentration of ABA and β-subunit in the cotyledons. When in vitro translation products from RNA isolated from cotyledons cultured with ABA were immunoprecipitated with antiserum against β-conglycinin, there was an increased amount of pre-β-subunit polypetide compared to the translation products from RNA isolated from control cotyledons. The pre-β-subunit polypeptide was not detected in translation products from RNA isolated from fluridone-treated cotyledons. Nucleic acid hybridization reactions showed that the level of β-subunit mRNA was higher in ABA-treated cotyledons compared to the control, and was lower in the fluridone-treated cotyledons. We have shown that exogenous ABA is able to modulate the accumulation of the β-subunit of β-conglycinin in developing cultured soybean cotyledons.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Straus J. W., Dudock B. S. Preparation of a cell-free protein-synthesizing system from wheat germ. Methods Enzymol. 1983;101:635–644. doi: 10.1016/0076-6879(83)01044-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Creelman R. A., Zeevaart J. A. Abscisic Acid accumulation in spinach leaf slices in the presence of penetrating and nonpenetrating solutes. Plant Physiol. 1985 Jan;77(1):25–28. doi: 10.1104/pp.77.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayler K. R., Sykes G. E. beta-Conglycinins in Developing Soybean Seeds. Plant Physiol. 1981 May;67(5):958–961. doi: 10.1104/pp.67.5.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R. B., Hoschek G., Ditta G. S., Breidenbach R. W. Developmental regulation of cloned superabundant embryo mRNAs in soybean. Dev Biol. 1981 Apr 30;83(2):218–231. doi: 10.1016/0012-1606(81)90468-1. [DOI] [PubMed] [Google Scholar]
- Haffner M. H., Chin M. B., Lane B. G. Wheat embryo ribonucleates. XII. Formal characterization of terminal and penultimate nucleoside residues at the 5'-ends of "capped" RNA from imbibing wheat embryos. Can J Biochem. 1978 Jul;56(7):729–733. doi: 10.1139/o78-109. [DOI] [PubMed] [Google Scholar]
- Hein M. B., Brenner M. L., Brun W. A. Concentrations of Abscisic Acid and Indole-3-Acetic Acid in Soybean Seeds during Development. Plant Physiol. 1984 Dec;76(4):951–954. doi: 10.1104/pp.76.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowach L. P., Thompson J. F., Madison J. T. Effects of exogenous methionine on storage protein composition of soybean cotyledons cultured in vitro. Plant Physiol. 1984 Mar;74(3):576–583. doi: 10.1104/pp.74.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowach L. P., Thompson J. F., Madison J. T. Storage Protein Composition of Soybean Cotyledons Grown In Vitro in Media of Various Sulfate Concentrations in the Presence and Absence of Exogenous l-Methionine. Plant Physiol. 1984 Mar;74(3):584–589. doi: 10.1104/pp.74.3.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu F. C. Abscisic Acid Accumulation in Developing Seeds of Phaseolus vulgaris L. Plant Physiol. 1979 Mar;63(3):552–556. doi: 10.1104/pp.63.3.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lingappa V. R., Lingappa J. R., Prasad R., Ebner K. E., Blobel G. Coupled cell-free synthesis, segregation, and core glycosylation of a secretory protein. Proc Natl Acad Sci U S A. 1978 May;75(5):2338–2342. doi: 10.1073/pnas.75.5.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obendorf R. L., Wettlaufer S. H. Precocious Germination during In Vitro Growth of Soybean Seeds. Plant Physiol. 1984 Dec;76(4):1024–1028. doi: 10.1104/pp.76.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quebedeaux B., Sweetser P. B., Rowell J. C. Abscisic Acid Levels in Soybean Reproductive Structures during Development. Plant Physiol. 1976 Sep;58(3):363–366. doi: 10.1104/pp.58.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler M. A., Schmitt E. S., Beachy R. N. Closely related families of genes code for the alpha and alpha' subunits of the soybean 7S storage protein complex. Nucleic Acids Res. 1982 Dec 20;10(24):8225–8244. doi: 10.1093/nar/10.24.8225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schussler J. R., Brenner M. L., Brun W. A. Abscisic Acid and its relationship to seed filling in soybeans. Plant Physiol. 1984 Oct;76(2):301–306. doi: 10.1104/pp.76.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]