Abstract
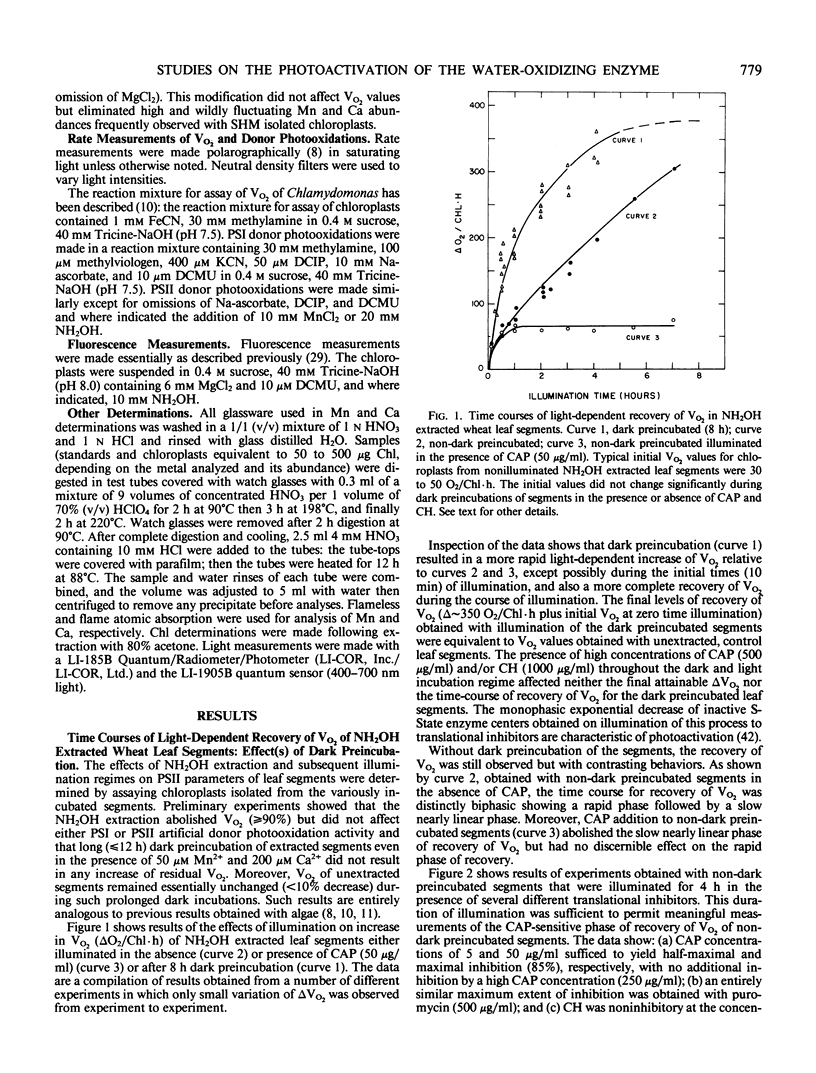
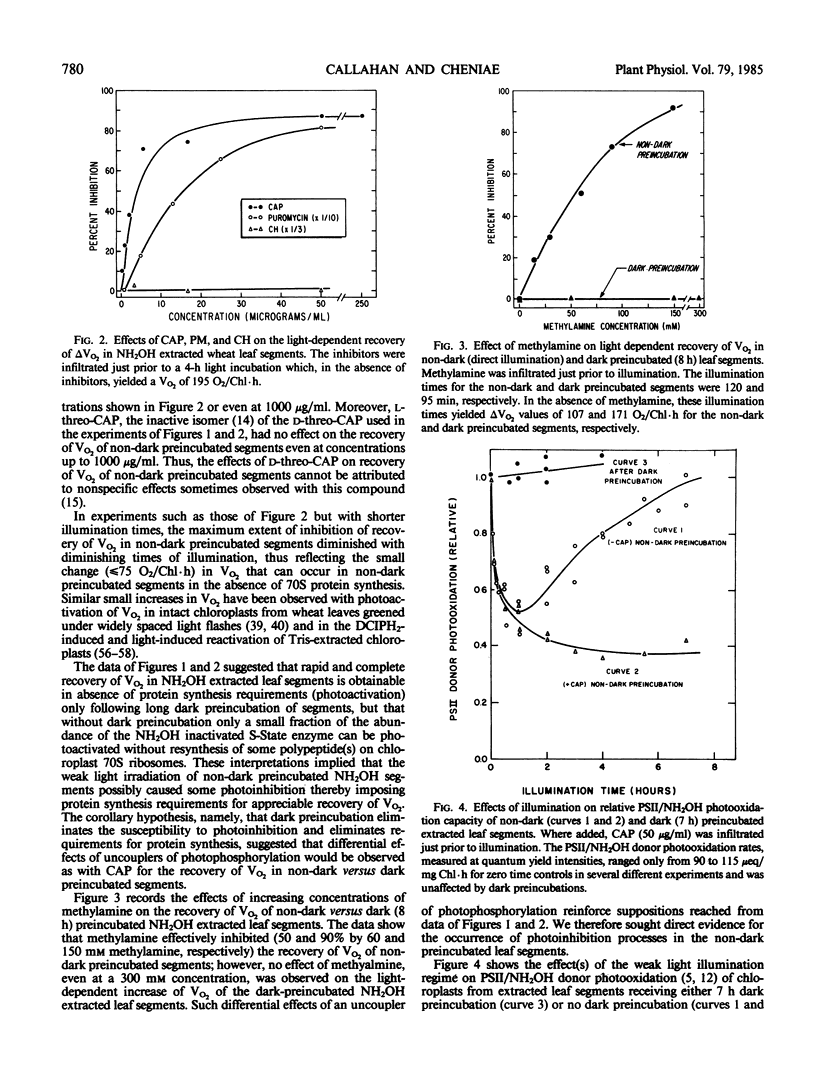
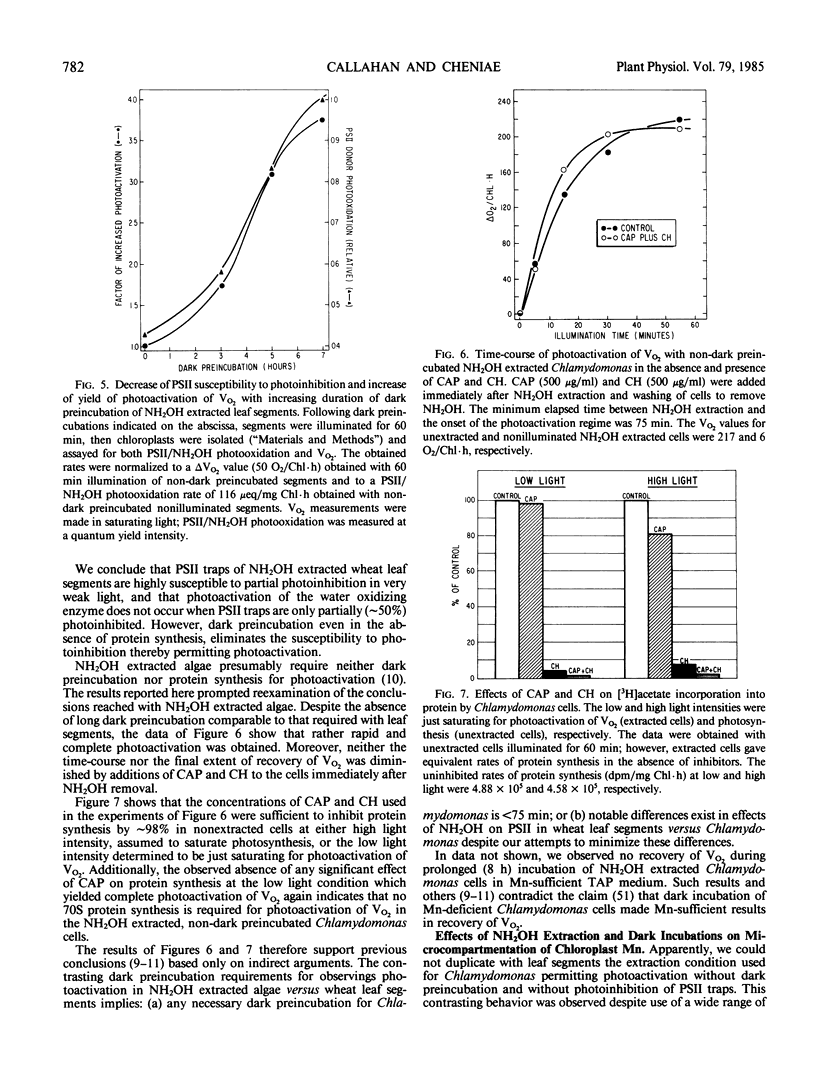
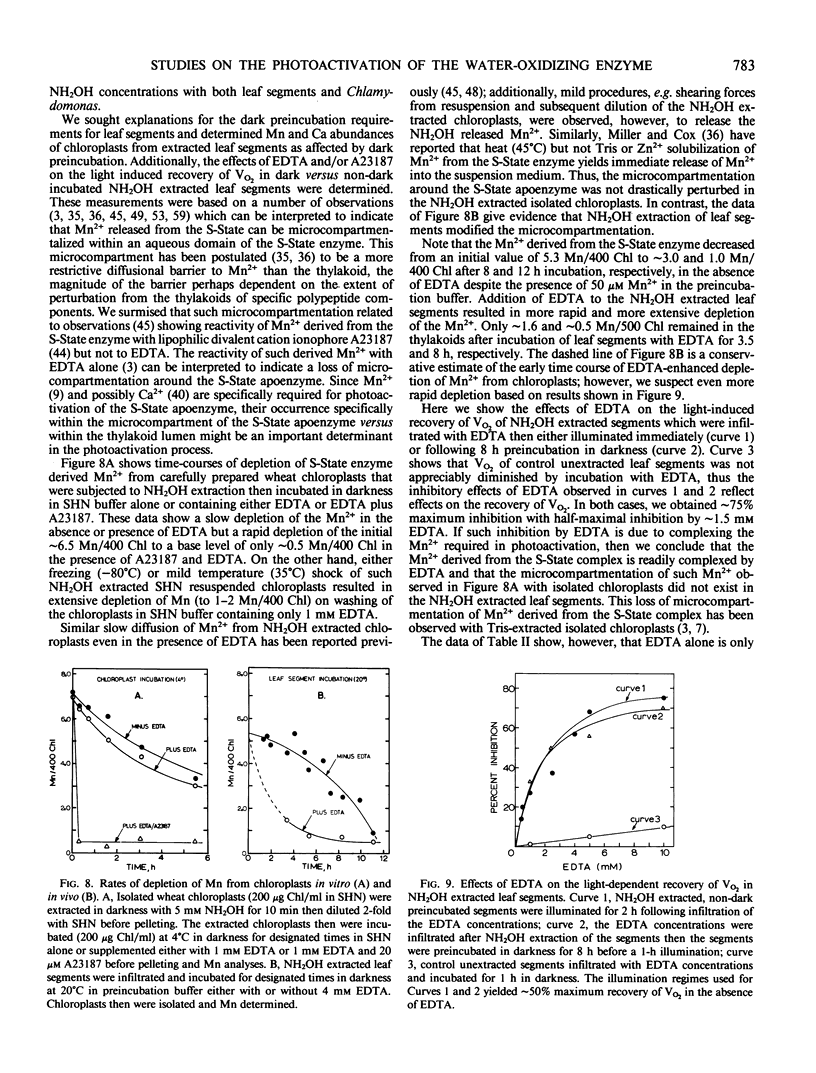
In weak yet optimal light intensity, complete photoactivation of the water-oxidizing enzyme in NH2OH-extracted wheat (Triticum aestivum, var Oasis) leaf segments could be obtained only after long dark preincubation. Photoactivation was not affected by ethylenediaminetetraacetate or inhibitors of photophosphorylation and protein synthesis, but was partially inhibited by a divalent cation ionophore. Complete photoactivation required ligation of ∼4 Mn by the water oxidizing enzyme.
Without dark preincubation, photosystem II (PSII) was susceptible to weak light photoinhibition resulting in: (a) 50% maximum decrease in photooxidation of artificial electron donors by PSII: (b) increased times for the variable fluorescence rise (with 3-(3,4-dichlorophenyl)-1,1-dimethyl urea): (c) abolishment of photoactivation: and (d) the imposition of sensitivity to inhibitors of photophosphorylation and 70S but not 80S protein synthesis on subsequent light-dependent recovery from photoinhibition and recovery of O2 evolution. Decrease in susceptibility to photoinhibition and increase in rates of photoactivation resulting from dark preincubations proved closely correlated. Neither protein synthesis nor increases in abundances of thylakoid Mn2+ and Ca2+ were required for escape from photoinhibition. However, photoactivation of the wateroxidizing enzyme in NH2OH-extracted Chlamydomonas occurred in absence of dark preincubation and protein synthesis. Results are discussed in the context of disassembly/reassembly/resynthesis of specific PSII polypeptides.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blankenship R. E., Sauer K. Manganese in photosynthetic oxygen evolution. I. Electron paramagnetic resonance study of the environment of manganese in Tris-washed chloroplasts. Biochim Biophys Acta. 1974 Aug 23;357(2):252–266. doi: 10.1016/0005-2728(74)90065-6. [DOI] [PubMed] [Google Scholar]
- Bouges-Bocquet B. Kinetic models for the electron donors of photosystem II of photosynthesis. Biochim Biophys Acta. 1980 Dec;594(2-3):85–103. doi: 10.1016/0304-4173(80)90006-3. [DOI] [PubMed] [Google Scholar]
- Bouges B. Action de faibles concentrations d'hydroxylamine sur l'émission d'oxygène des algues Chlorella et des chloroplastes d'épinards. Biochim Biophys Acta. 1971 Apr 6;234(1):103–112. doi: 10.1016/0005-2728(71)90135-6. [DOI] [PubMed] [Google Scholar]
- Cheniae G. M., Martin I. F. Effects of Hydroxylamine on Photosystem II: I. Factors Affecting the Decay of O(2) Evolution. Plant Physiol. 1971 Apr;47(4):568–575. doi: 10.1104/pp.47.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheniae G. M., Martin I. F. Effects of Hydroxylamine on Photosystem II: II. Photoreversal of the NH(2)OH Destruction of O(2) Evolution. Plant Physiol. 1972 Jul;50(1):87–94. doi: 10.1104/pp.50.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheniae G. M., Martin I. F. Photoactivation of the manganese catalyst of O 2 evolution. I. Biochemical and kinetic aspects. Biochim Biophys Acta. 1971 Nov 2;253(1):167–181. doi: 10.1016/0005-2728(71)90242-8. [DOI] [PubMed] [Google Scholar]
- Dismukes G. C., Siderer Y. Intermediates of a polynuclear manganese center involved in photosynthetic oxidation of water. Proc Natl Acad Sci U S A. 1981 Jan;78(1):274–278. doi: 10.1073/pnas.78.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. J. [Chloroplast ribosomes: stereospecificity of inhibition by chloramphenicol]. Science. 1969 Jan 31;163(3866):477–478. doi: 10.1126/science.163.3866.477. [DOI] [PubMed] [Google Scholar]
- Golbeck J. H., Martin I. F., Fowler C. F. Mechanism of Linolenic Acid-induced Inhibition of Photosynthetic Electron Transport. Plant Physiol. 1980 Apr;65(4):707–713. doi: 10.1104/pp.65.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann P. H. Studies on the manganese of the chloroplast. Plant Physiol. 1967 Jul;42(7):997–1007. doi: 10.1104/pp.42.7.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P., Croze E. The relationship between the activity of chloroplast photosystem II and the midpoint oxidation-reduction potential of cytochrome b-559. Biochim Biophys Acta. 1977 Oct 12;462(1):86–101. doi: 10.1016/0005-2728(77)90191-8. [DOI] [PubMed] [Google Scholar]
- Jursinic P., Stemler A. Changes in [C]Atrazine Binding Associated with the Oxidation-Reduction State of the Secondary Quinone Acceptor of Photosystem II. Plant Physiol. 1983 Nov;73(3):703–708. doi: 10.1104/pp.73.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin S., Kok B. Fluorescence induction studies in isolated chloroplasts. I. Number of components involved in the reaction and quantum yields. Biochim Biophys Acta. 1966 Nov 8;126(3):413–432. doi: 10.1016/0926-6585(66)90001-x. [DOI] [PubMed] [Google Scholar]
- Margulies M. M. Effect of cold-storage of bean leaves on photosynthetic reactions of isolated chloroplasts. Inability to donate electrons to photosystem II and relation to manganese content. Biochim Biophys Acta. 1972 Apr 20;267(1):96–103. doi: 10.1016/0005-2728(72)90141-7. [DOI] [PubMed] [Google Scholar]
- Michalski W. P., Kaniuga Z. Photosynthetic apparatus in chilling-sensitive plants. VII. Comparison of the effect of galactolipase treatment of chloroplasts and cold-dark storage of leaves on photosynthetic electron flow. Biochim Biophys Acta. 1980 Jan 4;589(1):84–99. doi: 10.1016/0005-2728(80)90134-6. [DOI] [PubMed] [Google Scholar]
- Ono T. A., Inoue Y. Photoactivation of the Water-Oxidation System in Isolated Intact Chloroplasts Prepared from Wheat Leaves Grown under Intermittent Flash Illumination. Plant Physiol. 1982 Jun;69(6):1418–1422. doi: 10.1104/pp.69.6.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort D. R., Izawa S. Studies on the Energy-coupling Sites of Photophosphorylation: V. Phosphorylation Efficiencies (P/e(2)) Associated with Aerobic Photooxidation of Artificial Electron Donors. Plant Physiol. 1974 Mar;53(3):370–376. doi: 10.1104/pp.53.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- Robinson H. H., Sharp R. R., Yocum C. F. Topology of NH2OH induced Mn(II) release from chloroplast thylakoid membranes. Biochim Biophys Acta. 1981 Jul;636(2):144–152. doi: 10.1016/0005-2728(81)90087-6. [DOI] [PubMed] [Google Scholar]
- Sharp R. R., Yocum C. F. Factors influencing hydroxylamine inactivation of photosynthetic water oxidation. Biochim Biophys Acta. 1981 Mar 12;635(1):90–104. doi: 10.1016/0005-2728(81)90010-4. [DOI] [PubMed] [Google Scholar]
- Sharp R. R., Yocum C. F. Field-dispersion profiles of the proton spin-lattice relaxation rate in chloroplast suspensions. Effect of manganese extraction by EDTA, Tris, and hydroxylamine. Biochim Biophys Acta. 1980 Aug 5;592(1):185–195. doi: 10.1016/0005-2728(80)90124-3. [DOI] [PubMed] [Google Scholar]
- Teichler-Zallen D. The effect of manganese on chloroplast structure and photosynthetic ability of Chlamydomonas reinhardi. Plant Physiol. 1969 May;44(5):701–710. doi: 10.1104/pp.44.5.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wydrzynski T., Sauer K. Periodic changes in the oxidation state of manganese in photosynthetic oxygen evolution upon illumination with flashes. Biochim Biophys Acta. 1980 Jan 4;589(1):56–70. doi: 10.1016/0005-2728(80)90132-2. [DOI] [PubMed] [Google Scholar]
- Yocum C. F., Yerkes C. T., Blankenship R. E., Sharp R. R., Babcock G. T. Stoichiometry, inhibitor sensitivity, and organization of manganese associated with photosynthetic oxygen evolution. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7507–7511. doi: 10.1073/pnas.78.12.7507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Haan G. A., Duysens L. N., Egberts D. J. Fluorescence yield kinetics in the microsecond-range in Chlorella pyrenoidosa and spinach chloroplasts in the presence of hydroxylamine. Biochim Biophys Acta. 1974 Dec 19;368(3):409–421. doi: 10.1016/0005-2728(74)90186-8. [DOI] [PubMed] [Google Scholar]


