Abstract
Acne vulgaris is an inflammatory skin condition that affects virtually everyone at some point. Papules, comedones, pustules, scarring, and nodules are standard features of the disease and can have a detrimental social and psychological impact on an individual. Although allopathic acne treatments are available, they have adverse side effects, are expensive, and are prone to cause antibiotic resistance. The present study is aimed at formulating and evaluating topical gels containing Aloe vera, Allium cepa, and Eucalyptus globulus extracts as potential antiacne drugs. Six formulations containing the herbal extracts were prepared using 1% Carbopol 940 as a gelling agent. The phytochemical composition of the plant extracts was determined. The extracts and gels' minimum inhibitory concentration (MIC) was assessed using the microbroth dilution method. The physicochemical properties of the formulated gels, such as homogeneity, colour, texture, odour, grittiness, spreadability, extrudability, viscosity, pH, and drug content, were evaluated. All the plant extracts contained alkaloids, flavonoids, tannins, triterpenoids, and coumarins. The gel formulations showed varying activity against Staphylococcus epidermidis, Staphylococcus aureus, Escherichia coli, Candida albicans, and Pseudomonas aeruginosa at various concentrations. The phytochemical components of the plant extracts are probably responsible for the antimicrobial activity of the gel formulations. The 5% Aloe vera-Allium cepa (1 : 1) combination gel formulation showed excellent activity against Staphylococcus epidermidis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Candida albicans, with MICs of 12.50, 25.00, 6.25, 25.00, and 12.50 mg/mL, respectively. The gels generally had good physicochemical and antimicrobial properties and could be used as antiacne remedies.
1. Introduction
Acne vulgaris, or acne, is a skin condition that affects many people and influences almost every individual [1]. It generally impacts individuals in their adolescence and young grown-ups and is stimulated by male chemicals brought about by the adrenal glands of both genders. It is, for the most part, observed on the face, chest, and back. Symptoms include pain, pustules (pimples), papules, tenderness, erythema, and loss of function [2].
Acne influenced around 650 million individuals worldwide in 2015 and was appraised as the eighth-most regular sickness universally [3–5]. Nationalities with hazier skin are more inclined to postprovocative hyperpigmentation, and it is more extreme in those with a positive family ancestry. Scars are known to lessen with age and time, yet this is very dubious [6]. Acne accounted for 5.3 percent of all skin diagnoses in a recent survey and is well-known as the second most common of all skin conditions [1, 7]. During puberty, it is more common in boys than girls; however, it is more prevalent in ladies than men during adulthood. Acne prevalence is related to diet [8, 9], weight, hereditary commitment, hormonal changes, and feelings of anxiety [10–12].
Pimples may happen when the sebaceous organs associated with pores liable for moving dead cells to the surface space of the skin get hindered. This blockade usually results in bacterial colonization and attack on the sebum, resulting in whiteheads, blackheads, and finally, inflammation and scars when the body's mechanism tries to fight back. Propionibacterium acnes and Staphylococcus epidermidis assume significant parts concerning inflammatory acne and shallow disease by utilizing sebaceous fatty substances into unsaturated fats, to which neutrophils are pulled in [13, 14].
Medicinal plants have the advantages of patient tolerance and wide acceptability [15]. For example, Azadirachta indica and Citrus aurantifolia have been folklorically used as antiacne agents [16–18]. Aloe vera, Allium cepa, and Eucalyptus globulus have antimicrobial, anti-inflammatory, and antioxidant activities that significantly treat skin infections. They are additionally known to have nutrients and minerals to improve the general strength of the skin. Acne accounted for 5.3% of all skin diagnoses reported, and acne vulgaris was the second most common by gender [1, 19, 20].
In Ghana, only some acne measures are available since many individuals get their acne treated at their local pharmacy and buy over-the-counter acne treatments, particularly topical skincare medications. As a result, there needs to be more data on acne. The method of acne treatment is gradually shifting away from allopathic prescriptions, which are expensive when considering the total cost of treatment and are also known to elicit bacterial resistance [15]. People who suffer from acne want quick remedies to boost their self-esteem. This study is aimed at formulating and evaluating the effectiveness of an active antiacne gel against P. aeruginosa and Staph. epidermidis with excellent synergistic effect against other microbial strains [17, 21–26].
2. Materials and Methods
2.1. Materials
Eucalyptus globulus (family: Myrtaceae) oil, Aloe vera (family: Liliaceae) leaves, and Allium cepa (family: Liliaceae) bulbs (Allium cepa) were purchased from Adum, Kumasi, Ghana. The plant materials were authenticated at the Department of Herbal Medicine, KNUST, Kumasi, Ghana, by Mr Clifford Asare. Staphylococcus epidermidis (clinical strain), Staphylococcus aureus (ATCC 25923), Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 4853), and Candida albicans (clinical strain) were obtained as pure isolate cultures (KCCR, KNUST) and used as test microorganisms. The Mueller-Hinton broth (Oxoid Ltd., Basingstoke, UK), 0.5 McFarland (Hardy Diagnostics), diethyl ether, ethanol, and distilled water were obtained from the Department of Pharmaceutics, KNUST, Ghana.
2.2. Methods
2.2.1. Preparation of Aloe vera and Allium cepa Extracts
(1) Aloe vera Extract. The matured leaves were washed with diethyl ether and distilled water. The gel was drained when the parenchymatous layer of the leaves was peeled away. A mortar and pestle were used to create the slurry. After that, the new gel was drained, and the weight was recorded. The gel was utilized in the formulation [27]. This procedure was carried out in triplicate.
(2) Allium cepa Extract. About 165 bulbs of Allium cepa were peeled, washed, crushed, and homogenized into smaller pieces and air-dried at 26 ± 1°C. About 637.61 g of the pulverized Allium cepa was weighed and soaked in 4 L of ethanol for 168 hours, filtered, and evaporated to dryness using the water bath to obtain a concentrate [21, 28]. The extractive procedure was repeated, and the weights of the concentrates were recorded.
2.2.2. Identification and Chemical Composition of Eucalyptus globulus Oil
The volatile oil of Eucalyptus globulus was stored at room temperature in an amber-coloured bottle to avoid rancidity. An identification and chemical composition test was performed on the oil to ascertain its quality and chemical constituents using gas-liquid chromatography [23, 29, 30].
2.2.3. Determination of Percentage Yield
An analytical balance (model Kern PCB 1000-2) was used to determine the total weight of raw material before extraction and the weight of extract after extraction. From these extracts, the percentage yield was calculated using the following formula:
| (1) |
2.2.4. Phytochemical Analysis
Phytochemical screening was performed to determine the secondary metabolites in the various plant materials. Using previously established protocols, the secondary metabolites investigated were tannins, alkaloids, flavonoids, triterpenoids, sterols, glycosides, coumarins, and saponins [27, 31, 32].
2.2.5. Preparation of Mueller-Hinton Broth (Single and Double Strength)
For single strength, 2.1 g Mueller-Hinton broth was dissolved in 100 mL sterile water, transferred to 10 mL test tubes, and sterilized for 30 minutes in an autoclave at 121°C [33]. For double-strength, 4.2 g was weighed and dissolved in 100 mL sterile water, transferred into test tubes of 10 mL each, and sterilized in an autoclave at 121°C for 30 minutes, cooled, and stored at room temperature; 25°C.
2.2.6. Subculture of Microorganisms
Staphylococcus epidermidis and Staphylococcus aureus (gram-positive), Escherichia coli and Pseudomonas aeruginosa (gram-negative), and Candida albicans (fungi) are the organisms used. The Mueller-Hinton broth test tube was sterilized before use by flaming the mouth with a Bunsen burner under a laminar flow cabinet. Then, 1 mL of the pure isolate was introduced into the broth, flamed again, and capped with a cork to be incubated at 37°C for 24 hours [27, 33].
(1) Streaking of the Subcultured Organisms to Obtain Pure Isolates. The agar was prepared, transferred into test tubes, and sterilized in an autoclave for 30 minutes at 121°C. It was poured onto a petri dish and cooled in the safety cabinet. The subcultured organisms were streaked over the surface of the agar and incubated for 24 hours with a sterile inoculum loop [27, 33].
2.2.7. Estimation of Minimum Inhibitory Concentrations (MIC) of Plant Materials
The microbroth dilution technique was employed to estimate the MIC. The microtiter plate was filled with appropriate and calculated amounts of the Mueller-Hinton nutritious broth, sterile water, distinct plant materials concentrations, and microorganisms (as compared to the McFarland standard) and incubated at 37°C for 24 hours. The MIC was determined using test formulations with the lowest dilution concentration and no apparent growth [27, 34–38].
2.2.8. Formulation of Individual Gels
Gels of the samples (Eucalyptus and Allium cepa) and aloe gel were prepared at a concentration of 5% after preformulation trials (Table 1). Carbopol 940 was placed in distilled water in a separate beaker with constant stirring. Propylparaben and methylparaben were dissolved in 5 mL of distilled water in another beaker. The extracts were added to the solution and thoroughly levigated. Afterward, the combination above was added to the carbopol mixture and mixed well. Finally, with steady and continuous stirring, propylene glycol and triethanolamine were added to the dispersion in drops, and the pH was adjusted to 6.8-7.4 (neutralized) [17, 39].
Table 1.
Constituents for the formulation of gels.
| Ingredient | Part used | Concentration (%) | Use/function |
|---|---|---|---|
| Ethanolic extract of Allium cepa | Bulb | 5 | Antiscarring, anti-inflammatory |
| A slurry of Aloe vera | Large leaves | 5 | Kills acne-causing bacteria, scars, moisturizer |
| Extract of Eucalyptus globulus by hydro distillation | Leaves | 5 | Antiacne agent |
| Carbopol 940 | — | 1 | Gelling agent |
| Methylparaben | — | 0.1 | Preservative |
| Propylparaben | — | 0.1 | Preservative |
| Triethanolamine | — | 2 | Neutralizer |
| Propylene glycol | — | 2 | Humectant |
| Distilled water | — | QS | Vehicle |
2.2.9. Formulation of Combination Gels
Combination gels were prepared at the same concentration of 5%, which corresponded to 10 times the minimum inhibitory concentration values. Carbopol 940 was distributed uniformly in a separate beaker in water with continuous stirring. Propylparaben and methylparaben were dissolved in 5 mL of water in another beaker. To the solution, the extracts were added and levigated well. The above mixture was then added to the carbopol mixture and stirred well. Finally, propylene glycol and triethanolamine were added in drops to the dispersion with constant and continuous stirring, and also the pH was adjusted to 6.8-7.4 (neutralized) [17].
2.2.10. Physicochemical Evaluation of Individual and Combination Gels
Official techniques were used to assess pH, colour, odour, consistency, texture, greasiness, homogeneity, grittiness, compatibility, drug content, viscosity, extrudability, and spreadability [17, 40–43].
2.2.11. Estimation of MIC of Formulated Gels
The microbroth dilution technique was employed to estimate the MIC. The microtiter plate was filled with appropriate and calculated amounts of the Mueller-Hinton nutritious broth, sterile water, distinct concentrations of the formed gels, and microorganisms (compared to the McFarland standard) and incubated at 37°C for 24 hours. Three wells of the microtiter plate were used for each test organism. A test tube with extract/oil was used as a positive control, and no organism was present in the negative control. The MIC was determined using test formulations with the lowest dilution concentration and no apparent growth [27, 34–38].
3. Results and Discussion
3.1. Identification and Chemical Composition of Eucalyptus Oil
The chemical composition of Eucalyptus globulus oil was determined using gas chromatography-mass spectroscopy. Eucalyptus plants produce terpenoid hydrocarbons and essential oils (eucalyptus oils), and they can be categorized as medicinal, industrial, or perfumery, depending on their chemical makeup. The eucalyptus product's GC/MS total ion chromatogram was created under the conditions described above, and the results are presented in Figure 1. The softcopy findings of GC/MS Turbo Mass utilizing peak area normalization measurements were used to determine the concentration of all the detected chemicals based on peak area/peak height. The two major detected peaks and chemical constituents were those of 1, 8-cineole, and terpinene (Table 2). Eucalyptus oil is deemed therapeutic if it contains not less than 70% of 1, 8-cineole [44–46].
Figure 1.
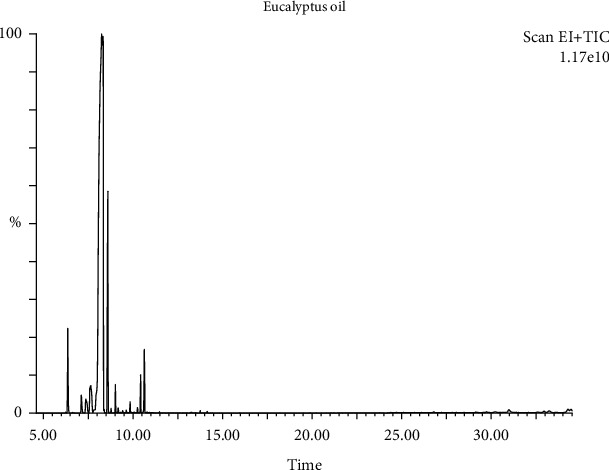
Identification test on eucalyptus oil using GC-MS.
Table 2.
Chemical composition of eucalyptus oil determined by GC-MS.
| No. | Chemical compound | Chemical content (%) |
|---|---|---|
| 1. | 1,8-Cineole | 78.13-78.35 |
| 2. | Terpinene | 7.30-9.35 |
| 3. | Pinocarveol | 0.26-0.34 |
| 4. | α-Terpineol | 1.98-2.53 |
| 5. | Terpinyl acetate | 0.60-0.76 |
| 6. | Terpinene-4-ol | 0.89-1.14 |
| 7. | Linoleic acid | 0.43-0.55 |
| 8. | α-Pinene | 2.34-2.98 |
| 9. | β-Pinene | 0.71-0.91 |
| 10. | Cyclycloeucalenol | 0.19-0.24 |
| 11. | α-Myrcene | 1.09-1.40 |
| 12. | α-Amyrin | 0.38-0.48 |
| 13. | Minolinolenin | 0.22-0.28 |
| 14. | Squalene | 0.35-0.45 |
The eucalyptus oil studied contained more significant than 70% of 1,8-cineole, suggesting that the oil may be utilized for therapeutic purposes [46, 47]. Genetic factors determine the chemical composition of essential oils. However, other variables, including location, the vegetative cycle, the method of production, and environmental elements, including soil characteristics, relative humidity, solar radiation, temperature, and hydric stress, can significantly alter chemical constituent production [46, 48].
3.2. Phytochemical Composition of Plant Materials
Phytochemicals have pharmacological properties that can treat several ailments, including bacterial and fungal infections. The bioactive chemicals saponins, glycosides, flavonoids, alkaloids, and tannins were discovered due to the phytochemical screening (Table 3). Secondary metabolites found in plant materials have been shown to have antimicrobial activity against pathogenic bacteria. Phenolic compounds (tannins) and alkaloids are known to inhibit the activities of pathogenic organisms and therefore are responsible for the inhibitory activities observed by the gel. [27, 31, 32].
Table 3.
Phytochemical constituents of plant materials.
| Extract | Tan | Gly | Sap | Flav | Alka | Trit | Ste | Coum |
|---|---|---|---|---|---|---|---|---|
| ∗Eucalyptus | + | + | + | + | + | + | - | + |
| Allium cepa | + | + | + | + | + | + | + | + |
| Aloe | + | - | - | + | + | + | - | + |
Key: absent (-); present (+); Tan = tannins; Gly = glycosides; Sap = saponnins; Flav = flavonoids; Alka = alkaloids; Trit = triterpenoids; Ste = sterols; Coum = coumarins; ∗oil.
Plant-derived biologically active molecules are often considered more acceptable and less hazardous than synthetic substances, providing many potential disease-controlling drugs. Secondary metabolites can control pathogenic organisms, overcoming the problems associated with produced chemicals, according to research into plant biochemistry, physiology, and natural product chemistry. As a result, there is rising interest in developing alternative microbial contamination control strategies that reduce or eliminate the need for antibiotics [27, 31, 32].
3.3. Microbiological Evaluation of Extracts and Formulated Gels
The MIC of the extracts against the tested organisms is required to calculate the dose in the formula. This MIC was then increased by ten to generate the dose, which was then utilized to calculate the amount of extract used in the gel formulations. The MIC results are shown in Tables 4 and 5. The antibacterial properties of the plant extracts were tested using the microbroth dilution technique. The findings of this study showed that the plant extracts were active against the test organisms.
Table 4.
Mean MICs (mg/mL) of the different plant materials against test organisms.
| Plant materials | Staphylococcus epidermidis | Staphylococcus aureus | Escherichia coli | Pseudomonas aeruginosa | Candida albicans |
|---|---|---|---|---|---|
| Aloe gel | 0.625 ± 0.003 | 2.500 ± 0.040 | - | - | 1.250 ± 0.005 |
| Eucalyptus oil | 0.625 ± 0.005 | 2.500 ± 0.050 | 2.500 ± 0.060 | - | 1.250 ± 0.020 |
| Allium extract | 0.156 ± 0.002 | 0.625 ± 0.010 | 0.313 ± 0.002 | 2.500 ± 0.007 | 1.250 ± 0.030 |
| Allium juice | 2.500 ± 0.050 | 10.000 ± 0.030 | 40.000 ± 0.100 | - | 10.000 ± 0.009 |
Key: (-) no inhibition.
Table 5.
Mean MICs (mg/mL) of the different gel formulations against test organisms.
| Gel formulations | Staphylococcus epidermidis | Staphylococcus aureus | Escherichia coli | Pseudomonas aeruginosa | Candida albicans |
|---|---|---|---|---|---|
| 5% Aloe vera gel | 6.25 ± 0.02 | 25.00 ± 0.05 | - | - | 12.50 ± 0.11 |
| 5% Eucalyptus globulus gel | 6.25 ± 0.06 | 25.00 ± 0.20 | 25.00 ± 0.13 | - | 12.50 ± 0.21 |
| 5% Allium cepa gel | 12.50 ± 0.07 | 6.25 ± 0.31 | 3.13 ± 0.15 | 25.00 ± 0.22 | 12.50 ± 0.22 |
| 5% Allium cepa and Aloe vera gel (1 : 1) | 12.50 ± 0.10 | 25.00 ± 0.22 | 6.25 ± 0.21 | 25.00 ± 0.10 | 12.50 ± 0.08 |
| 5% Allium cepa and Eucalyptus gel (1 : 1) | 25.00 ± 0.12 | 12.50 ± 0.12 | 25.00 ± 0.50 | - | 25.00 ± 0.35 |
| 5% Allium cepa, Eucalyptus, and Aloe vera gel (1 : 1 : 1) | 25.00 ± 0.13 | 25.00 ± 0.08 | 25.00 ± 0.31 | - | 6.25 ± 0.43 |
Key: (-) no inhibition.
The plant materials exerted antimicrobial action at different concentrations against the organisms tested. Allium cepa extract showed activity against all the test organisms. Eucalyptus globulus oil and Aloe vera gel also exhibited activity against all the organisms except Pseudomonas aeruginosa (Aloe vera and eucalyptus oil) and E. coli (Aloe vera) (Table 4), indicating that products derived from the plant extracts could be beneficial in treating infections caused by these microorganisms. Allium cepa juice was not used in the gel formulation because of its weak activity compared to the ethanolic extract. Crude extract activity is deemed significant if MIC values are less than 100 μg/mL, moderate when 100 μg/mL to 625 μg/mL, or low when 625 μg/mL or above [49, 50]. Based on this information, the extracts and oil possess low activity.
The different gel formulations showed inhibitory effects against Staphylococcus epidermidis and Staphylococcus aureus at 6.25, 12.5, and 25 mg/mL (Table 5). Only formulations containing Aloe vera only and eucalyptus oil exhibited the most antibacterial effect against Staph epidermidis. This finding is comparable to previous studies [44, 51, 52]. Nonetheless, Allium cepa only formulation was most active against Staph aureus. This result may explain why the three plant extracts treat many diseases [25].
Pseudomonas aeruginosa has been linked to skin infections, especially at exposed sites like wounds and pressure sores, and can also affect burst and infected nodules and papules. In this current study, Allium cepa only and Allium cepa in combination with Aloe vera were the most active against Pseudomonas with an inhibitory value of 25 mg/mL. Aloe vera alone was not active against Pseudomonas, as reported by Goudarzi et al. [53]. The inhibitory action of these gels against P. aeruginosa explains their potential advantage in treating skin infections with such organisms implicated.
The gel formulations exerted broad antimicrobial action against gram-positive and gram-negative bacteria and the fungus. The growth of Candida albicans was likewise suppressed most by the Allium cepa, Aloe vera, and Eucalyptus combination gel with an inhibitory value of 6.25 mg/mL. Aloe vera having inhibition against Candida albicans was similar to that reported by Stanley et al. [25]. There are several clinical manifestations of candidiasis, but most commonly involve mucosa surfaces and deep-seated infections. This study contradicts [54], who reported that Aloe vera gel had no inhibitory effects against Candida albicans. All gel formulations inhibited Escherichia coli with different MICs of 3.125, 6.25, and 25 mg/mL except the Aloe vera gel formulation, which contradicts the findings of Stanley et al. [25], who observed inhibition in their study. Allium cepa-only gel formulation showed the most significant inhibition. Eucalyptus oil was active against E. coli (gram-negative organism), which aligns with previous findings [48, 50, 53].
The antimicrobial property of Allium cepa-Aloe vera combination gel had a four (4) fold increase in activity against S.aureus compared to Aloe vera gel and a two (2) fold activity against E.coli. The increased antimicrobial activity of the Allium cepa-Aloe vera combination gel may be due to the synergistic activity of the Allium cepa and Aloe vera gels (Table 5). Allium cepa-Eucalyptus gel combinations had a four (4) fold increase in antimicrobial activity against S.epidermidis compared to Eucalyptus gel alone (Table 5). Table 5 further revealed that some formulations were bacteriostatic while others were bactericidal on the test organisms. The current research reveals the formulations' therapeutic potential in antibacterial and antioxidant properties. According to the results of the tests mentioned above, the extracts have a significant growth inhibitory action on the organisms. The efficacy of these formulations based on MIC values supports their use in the prevention and treatment of bacterial infections caused by a variety of pathogenic bacteria with antibiotic resistance, most importantly, acne [55].
3.4. Quality Control Tests on Formulated Topical Gels
3.4.1. Organoleptic Characteristics of the Gels
Physical appearance, pH, colour, odour, consistency, greasiness, grittiness, homogeneity, texture, compatibility, viscosity, spreadability, drug content, and extrudability were examined for the six gel formulations. The study's findings were within the ICH standards' allowed limits, and the specifics are reported in Tables 6 and 7. The produced gels were homogeneous and uniform in appearance, odour, and consistency. All formulations had pH values close to neutral (6.80-7.49), indicating they may not cause skin irritation. The gel formulations were visually examined for colour using a colour codebook. The produced formulations were transparent without the active components in the preformulation studies but exhibited different colours in the formulation studies (Table 7). All the developed gel formulations were homogeneous and free of lumps.
Table 6.
Organoleptic characteristics of carbopol (gelling agent) and formulated gels.
| Gel | Conc (% w/v) | Colour | Odour | Consistency | Greasiness | Grittiness | Homogeneity | pH | Texture | Patient acceptability |
|---|---|---|---|---|---|---|---|---|---|---|
| Carbopol | 1 | Colourless | Characteristic | Very consistent | Nongreasy | Nongritty | No lump | 6.81 ± 0.11 | Smooth | Acceptable |
| Allium cepa | 5 | #FEB475 (soft orange) | Characteristic odour | Consistent | Nongreasy | Nongritty | No lump | 7.16 ± 0.30 | Smooth | Acceptable |
| Eucalyptus | 5 | 255,255,255 (white) | Minty | Consistent | Nongreasy | Nongritty | No lump | 7.35 ± 0.21 | Smooth | Acceptable |
| Aloe | 5 | A8003-0 (clear) | Characteristic odour | Consistent | Nongreasy | Nongritty | No lump | 7.15 ± 0.10 | Smooth | Acceptable |
| Allium cepa and Eucalyptus | 5 (1 : 1) | #FCDE9C (very soft orange) | Characteristic minty odour | Consistent | Nongreasy | Nongritty | No lump | 7.18 ± 0.08 | Smooth | Acceptable |
| Allium cepa and Aloe | 5 (1 : 1) | #EEB479 (soft orange) | Characteristic odour | Consistent | Nongreasy | Nongritty | No lump | 6.85 ± 0.13 | Smooth | Acceptable |
| Allium cepa, Aloe, and Eucalyptus | 5 (1 : 1 : 1) | #FFF3B2 (pale yellow) | Characteristic minty odour | Consistent | Nongreasy | Nongritty | No lump | 7.43 ± 0.11 | Smooth | Acceptable |
The numbers in parentheses are ratios of the plant materials used.
Table 7.
Physicochemical properties of carbopol (gelling agent) and formulated gels.
| Gel formulation | Concentration (% w/v) | Spreadability (50 g) (cm) | Extrudability (%) | Viscosity (Cp) (speed 6) | Active plant content (%) |
|---|---|---|---|---|---|
| Carbopol | 1 | 0.9 ± 0.006 | 94.75 ± 0.026 | 795.0 ± 0.653 | — |
| Allium cepa | 5 | 1.2 ± 0.017 | 95.60 ± 0.065 | 140.0 ± 0.364 | 99.83 ± 0.020 |
| Eucalyptus | 5 | 0.7 ± 0.033 | 80.70 ± 0.392 | 630.0 ± 0566 | 97.91 ± 0.006 |
| Aloe | 5 | 1.1 ± 0.011 | 93.45 ± 0.041 | 306.7 ± 0.173 | 99.29 ± 0.053 |
| Allium cepa and Eucalyptus (1 : 1) | 5 | 1.3 ± 0.011 | 84.00 ± 0.065 | 216.7 ± 0.493 | 90.68 ± 0.052 |
| Allium cepa and Aloe (1 : 1) | 5 | 1.4 ± 0.007 | 95.35 ± 0.041 | 125.0 ± 0.000 | 99.32 ± 0.131 |
| Allium cepa, Eucalyptus, and Aloe (1:1 : 1) | 5 | 1.2 ± 0.017 | 87.95 ± 0.043 | 148.3 ± 0.196 | 99.52 ± 0.011 |
3.4.2. Physicochemical Properties of Carbopol (Gelling Agent) and Formulated Gels
The spreadability of gels is crucial because it shows how the gel acts once removed from the tube. According to the spreadability parameters (Table 7) [42], the gel formulations are easily spreadable and can be easily applied to the skin after removal from the primary packaging material. Also, more than 90% of the contents of most of the gel formulations were extrudable, indicating excellent extrudability. The gel can, therefore, be easily removed from its primary package with minimal stress/force. A few had greater than 80% extrudability (Table 7) (extrudability: >90%: excellent; >80%: acceptable; >70%: fair) [40]. The viscosity of gels is known to impart the spreadability and extrudability of gels. Therefore, the viscosity of formulated gels should be characterized and ensured that they produce gels with optimum spread and extrudability [39, 40]. The spreadability and extrudability results corroborate that the formulated gels had suitable viscosities (Table 7). The percentage of active plant content also fell within the range of 90.68 to 99.83% and conformed to the USP limit for drug content. The values indicate that the gels contained uniform amounts of active herbal constituents (Table 7) and will be able to deliver the expected amount of active constituents to the site of action [42, 56].
4. Conclusion
Acne vulgaris is a reasonably prevalent condition that affects virtually everyone at some point. Herbal medications are considered safer than allopathic drugs; therefore, the current formulations can be recommended as an effective tool for managing and treating acne. According to the findings from this study, varying combinations of Aloe vera leaf extracts, Eucalyptus globulus oil, and Allium cepa bulb extracts have effective synergistic therapeutic characteristics for managing acne vulgaris. The 5% Aloe vera-Allium cepa (1 : 1) combination gel was most active against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Candida albicans and possessed the requisite physicochemical properties for use in acne vulgaris.
Acknowledgments
The authors are grateful to the technicians of the Department of Pharmaceutics, Faculty of Pharmacy and Pharmaceutical Sciences, KNUST, Kumasi, Ghana, for their technical assistance.
Data Availability
The data used to support the findings of this study are included in the article.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this study.
References
- 1.Rosenbaum B. E., Klein R., Hagan P. G., et al. Dermatology in Ghana: a retrospective review of skin disease at the Korle bu teaching hospital dermatology clinic. The Pan African Medical Journal . 2017;26:1–9. doi: 10.11604/pamj.2017.26.125.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagatin E., Freitas T. H. P. D., Rivitti-Machado M. C., Ribeiro B. M., Nunes S., Rocha M. A. D. D. Adult female acne: a guide to clinical practice. Anais Brasileiros de Dermatologia . 2019;94(1):62–75. doi: 10.1590/abd1806-4841.20198203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flohr C., Hay R. Putting the burden of skin diseases on the global map. British Journal of Dermatology . 2021;184(2):189–190. doi: 10.1111/bjd.19704. [DOI] [PubMed] [Google Scholar]
- 4.Soriano J. B., Abajobir A. A., Abate K. H., et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet Respiratory Medicine . 2017;5(9):691–706. doi: 10.1016/S2213-2600(17)30293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan J. K. L., Bhate K. A global perspective on the epidemiology of acne. British Journal of Dermatology . 2015;172(S1):3–12. doi: 10.1111/bjd.13462. [DOI] [PubMed] [Google Scholar]
- 6.Okoro E., Ogunbiyi A., George A. Prevalence and pattern of acne vulgaris among adolescents in Ibadan, South-West Nigeria. Journal of the Egyptian Women's Dermatologic Society . 2016;13(1):7–12. doi: 10.1097/01.EWX.0000470561.85599.0d. [DOI] [Google Scholar]
- 7.Banner A., Dinsey M., Ezzedine K., Dadzie O. E. The spectrum of skin diseases occurring in a multiethnic population in North-West London, U.K.: findings from a cross-sectional descriptive study. British Journal of Dermatology . 2017;176(2):523–525. doi: 10.1111/bjd.14824. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhary M. K., Chaudhary M. A review of treatment options for acne vulgaris. World Journal of Pharmacy and Pharmaceutical Sciences . 2016;5(7):524–545. doi: 10.20959/wjpps20167-7135. [DOI] [Google Scholar]
- 9.Aghasi M., Golzarand M., Shab-Bidar S., Aminianfar A., Omidian M., Taheri F. Dairy intake and acne development: a meta-analysis of observational studies. Clinical Nutrition . 2019;38(3):1067–1075. doi: 10.1016/j.clnu.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Berry S. An Assessment of Acne, Stress, and Psychological Symptoms in College Students: A Daily Diary Study . 2020. Honors Theses, https://egrove.olemiss.edu/hon_thesis/1371.
- 11.Dixon L. J., Witcraft S. M., McCowan N. K., Brodell R. T. Stress and skin disease quality of life: the moderating role of anxiety sensitivity social concerns. British Journal of Dermatology . 2018;178(4):951–957. doi: 10.1111/bjd.16082. [DOI] [PubMed] [Google Scholar]
- 12.Jović A., Marinović B., Kostović K., Čeović R., Basta-Juzbašić A., Mokos Z. B. The impact of psychological stress on acne. Acta Dermatovenerologica Croatica . 2017;25(2):133–141. [PubMed] [Google Scholar]
- 13.Dreno B., Gollnick H. P. M., Kang S., et al. Understanding innate immunity and inflammation in acne: implications for management. Journal of the European Academy of Dermatology and Venereology . 2015;29(S4):3–11. doi: 10.1111/jdv.13190. [DOI] [PubMed] [Google Scholar]
- 14.Lee I. S., Lee A. R., Lee H., et al. Psychological distress and attentional bias toward acne lesions in patients with acne. Psychology, Health & Medicine . 2014;19(6):680–686. doi: 10.1080/13548506.2014.880493. [DOI] [PubMed] [Google Scholar]
- 15.Rafieian-Kopaei M. Medicinal plants and the human needs. Journal of HerbMed Pharmacology . 2012;1(1):1–2. [Google Scholar]
- 16.Kusuma S. A. F., Abdassah M., Valas B. E. Formulation and evaluation of anti acne gel containing Citrus aurantifolia fruit juice using carbopol as gelling agent. International Journal of Applied Pharmaceutics . 2018;10(4):147–152. doi: 10.22159/ijap.2018v10i4.26788. [DOI] [Google Scholar]
- 17.Patil A. C., Patil A. R., Patil A. A. C., et al. Formulation and evaluation of polyherbal anti-acne gel. Research Journal of Topical and Cosmetic Sciences . 2017;8(2):p. 61. doi: 10.5958/2321-5844.2017.00007.3. [DOI] [Google Scholar]
- 18.Yamini K., Onesimus T. Preparation and evaluation of herbal anti-acne gel. International Journal of Pharma and Bio Sciences . 2013;4(2):956–960. [Google Scholar]
- 19.Heng A. H. S., Chew F. T. Systematic review of the epidemiology of acne vulgaris. Scientific Reports . 2020;10(1):1–29. doi: 10.1038/s41598-020-62715-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tayel K., Attia M., Agamia N., Fadl N. Acne vulgaris: prevalence, severity, and impact on quality of life and self-esteem among Egyptian adolescents. Journal of the Egyptian Public Health Association . 2020;95(1) doi: 10.1186/s42506-020-00056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bakht J., Khan S., Shafi M. In vitro antimicrobial activity of Allium cepa (dry bulbs) against gram-positive and gram-negative bacteria and fungi. Pakistan Journal of Pharmaceutical Sciences . 2014;27(1):139–145. [PubMed] [Google Scholar]
- 22.Denloye A. A. Bioactivity of Powder and Extracts from Garlic, Allium sativum L. (Alliaceae) and Spring Onion, Allium fistulosum L. (Alliaceae) against Callosobruchus maculatus F. (Coleoptera: Bruchidae) on Cowpea, Vigna unguiculata (L.) Walp (Leguminosae) Seeds. Psyche: A Journal of Entomology . 2010;2010:15. doi: 10.1155/2010/958348.958348 [DOI] [Google Scholar]
- 23.Djelloul R., Mokrani K., Hacini N. Study of the antibacterial activity of the extract from the essential oil of Eucalyptus globulus and Rosmarinus officinalis on three bacterial strains. International Journal of Applied Environmental Sciences . 2017;12(1):47–56. [Google Scholar]
- 24.Enejiyon S. O., Abdulrahman A. A., Adedeji A. S. Antibacterial activities of the extracts of Allium sativum (garlic) and Allium cepa (Allium cepa) against selected pathogenic bacteria. Tanzania Journal of Science . 2020;46(3):914–922. [Google Scholar]
- 25.Stanley M. C., Ifeanyi O. E., Nwakaego C. C., Esther I. O. Antimicrobial effects of Aloe vera on some human pathogens. International Journal of Current Microbiology and Applied Sciences . 2014;3(3):1022–1028. [Google Scholar]
- 26.Varshney A. K. Research gate; 2015. Aloe vera: Development of Gel Extraction Process for Aloe vera Leaves Chapter No. Contents Acknowledgement List of Abbreviations List of Nomenclature Introduction Review of Literature Materials and Methods Results and Discussion Summary and Conclusion. December 2012 . [Google Scholar]
- 27.Yebpella G. G., Adeyemi Hassan M. M., Hammuel C., Magomya A. M., Agbaji A. S., Okonkwo E. M. Phtyochemical screening and comparative study of antimicrobial activity of Aloe vera various extracts. African Journal of Microbiology Research . 2011;5(10):1182–1187. doi: 10.5897/AJMR10.818. [DOI] [Google Scholar]
- 28.Prasetyaningrum A., Rokhati N., Dharmawan Y., Prinanda G. R. Comparison study for extraction of bioactive flavonoids from moringa oleifera, apple, onion, and ascorbic acid (orange) by using microwave-assisted, ultrasound-assisted and maceration methods. IOP Conference Series: Materials Science and Engineering . 2021;1053(1, article 012123) doi: 10.1088/1757-899x/1053/1/012123. [DOI] [Google Scholar]
- 29.Vora J., Srivastava A., Modi H. Antibacterial and antioxidant strategies for acne treatment through plant extracts. Informatics in Medicine Unlocked . 2018;13:128–132. doi: 10.1016/j.imu.2017.10.005. [DOI] [Google Scholar]
- 30.Nurzyńska-Wierdak R., Pietrasik D., Walasek-Janusz M. Essential oils in the treatment of various types of acne—a review. Plants . 2023;12(1):p. 90. doi: 10.3390/plants12010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dissanayake D. M. I. H., Perera D. D. B. D., Keerthirathna L. R., et al. Antimicrobial activity of Plumbago indica and ligand screening of plumbagin against methicillin-resistant Staphylococcus aureus. Journal of Biomolecular Structure and Dynamics . 2022;40(7):3273–3284. doi: 10.1080/07391102.2020.1846622. [DOI] [PubMed] [Google Scholar]
- 32.Prod J. N., Resour P., Zohra S. F., Meriem B., Samira S., Muneer M. A. Phytochemical screening and identification of some compounds from Mallow. Journal of Natural Product and Plant Resources . 2012;2(4):512–516. [Google Scholar]
- 33.Hossain M. S., Balakrishnan V., Rahman N. N. N. A., Sarker M. Z. I., Kadir M. O. A. Treatment of clinical solid waste using a steam autoclave as a possible alternative technology to incineration. International Journal of Environmental Research and Public Health . 2012;9(3):855–867. doi: 10.3390/ijerph9030855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobina E. Susceptibility pattern of some bacterial isolates from chronic wounds to selected local brands of antibiotics . Research gate; 2018. [DOI] [Google Scholar]
- 35.Kavanagh A., Ramu S., Gong Y., Cooper M. A., Blaskovich M. A. T. Effects of microplate type and broth additives on microdilution MIC susceptibility assays. Antimicrobial Agents and Chemotherapy . 2019;63(1) doi: 10.1128/AAC.01760-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Metzler K. The Mutant-Prevention Concentration Concept and Its Application to Staphylococcus Aureus . Semantic scholar; 2004. [Google Scholar]
- 37.Paul S., Modak D., Chattaraj S., et al. Aloe vera gel homogenate shows anti-inflammatory activity through lysosomal membrane stabilization and downregulation of TNF-α and Cox-2 gene expressions in inflammatory arthritic animals. Future Journal of Pharmaceutical Sciences . 2021;7(1):1–8. doi: 10.1186/s43094-020-00163-6. [DOI] [Google Scholar]
- 38.Wiegand I., Hilpert K., Hancock R. E. W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nature Protocols . 2008;3(2):163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 39.Ayoub R. K., Murtaza G., Imran M., et al. Formulation and permeation kinetic studies of flurbiprofen gel. Tropical Journal of Pharmaceutical Research . 2015;14(2):195–203. doi: 10.4314/tjpr.v14i2.2. [DOI] [Google Scholar]
- 40.Aiyalu R., Govindarjan A., Ramasamy A. Formulation and evaluation of topical herbal gel for the treatment of arthritis in animal model. Brazilian Journal of Pharmaceutical Sciences . 2016;52(3):493–507. doi: 10.1590/s1984-82502016000300015. [DOI] [Google Scholar]
- 41.Baviskar D. T., Biranwar Y. A., Bare K. R., Parik V. B., Sapate M. K., Jain D. K. In vitro and in vivo evaluation of diclofenac sodium gel prepared with cellulose ether and carbopol 934P. Tropical Journal of Pharmaceutical Research . 2013;12(4):489–494. doi: 10.4314/tjpr.v12i4.7. [DOI] [Google Scholar]
- 42.Helal D. A., Abd El-Rhman D., Abdel-Halim S. A., El-Nabarawi M. A. Formulation and evaluation of fluconazole topical gel. International Journal of Pharmacy and Pharmaceutical Sciences . 2012;4(Supplement 5):176–183. [Google Scholar]
- 43.Kasar P. M., Kale K., Phadtare D. G. Formulation and evaluation of topical antifungal gel containing itraconazole. International Journal of Current Pharmaceutical Research . 2018;10(4):p. 71. doi: 10.22159/ijcpr.2018v10i4.28470. [DOI] [Google Scholar]
- 44.Djenane D., Yangüela J., Amrouche T., Boubrit S., Boussad N., Roncalés P. Chemical composition and antimicrobial effects of essential oils of Eucalyptus globulus, Myrtus communis and Satureja hortensis against Escherichia coli O157:H7 and Staphylococcus aureus in minced beef. Food Science and Technology International . 2011;17(6):505–515. doi: 10.1177/1082013211398803. [DOI] [PubMed] [Google Scholar]
- 45.Islam M., Khalid S., Ahmad I., Zuberi S. A., Fatima K. Review article essential oils: pharmacopeial. Baqai Journal of Health Sciences . 2018;21(1):49–67. [Google Scholar]
- 46.Joshi A., Sharma A., Bachheti R. K., Pandey D. P. A comparative study of the chemical composition of the essential oil from Eucalyptus globulus growing in Dehradun (India) and around the world. Oriental Journal of Chemistry . 2016;32(1):331–340. doi: 10.13005/ojc/320137. [DOI] [Google Scholar]
- 47.Subramanian P. A., Gebrekidan A., Nigussie K. Yield, contents and chemical composition variations in the essential oils of different Eucalyptus globulus trees from Tigray, northern Ethiopia. Journal of Pharmaceutical and Biomedical Sciences . 2012;17(11):p. 17. [Google Scholar]
- 48.Chaves T. P., Pinheiro R. E., Melo E. S., et al. Essential oil of Eucalyptus camaldulensis Dehn potentiates β-lactam activity against Staphylococcus aureus and Escherichia coli resistant strains. Industrial Crops and Products . 2018;112:70–74. doi: 10.1016/j.indcrop.2017.10.048. [DOI] [Google Scholar]
- 49.Bandara K. R., Padumadasa C., Peiris D. C. Potent antibacterial, antioxidant and toxic activities of extracts from Passiflora suberosa L. leaves. PeerJ . 2018;6, article e4804 doi: 10.7717/peerj.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuete V. Potential of Cameroonian plants and derived products against microbial infections: a review. Planta Medica . 2010;76(14):1479–1491. doi: 10.1055/s-0030-1250027. [DOI] [PubMed] [Google Scholar]
- 51.El Amraoui B., El Amraoui M., Cohen N., Fassouane A. Antifungal and antibacterial activity of marine microorganisms. Annales Pharmaceutiques Francaises . 2014;72(2):107–111. doi: 10.1016/j.pharma.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 52.Sabo V. A., Knezevic P. Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: a review. Industrial Crops and Products . 2019;132:413–429. doi: 10.1016/j.indcrop.2019.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goudarzi M., Fazeli M., Azad M., Seyedjavadi S. S., Mousavi R. Aloe vera gel: effective therapeutic agent against multidrug-resistant Pseudomonas aeruginosa isolates recovered from burn wound infections. Chemotherapy Research and Practice . 2015;2015:5. doi: 10.1155/2015/639806.639806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Danish P., Ali Q., Hafeez M. M., Malik A. Antifungal and antibacterial activity of aloe vera plant extract. Biological and Clinical Sciences Research Journal . 2020;2020(1) doi: 10.54112/bcsrj.v2020i1.4. [DOI] [Google Scholar]
- 55.Adnan M. Bioactive potential of essential oil extracted from the leaves of Eucalyptus globulus (Myrtaceae) Journal of Pharmacognosy and Phytochemistry . 2019;8(1):213–216. [Google Scholar]
- 56.USP38/NF33. Validation of Compendial Methods Section . The United States Pharmacopeial/National Formulary; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included in the article.


