Abstract
Background
Seroprevalence and the proportion of people with neutralizing activity (functional immunity) against SARS-CoV-2 variants were high in early 2022. In this prospective, population- based, multi-region cohort study, we assessed the development of functional and hybrid immunity (induced by vaccination and infection) in the general population during this period of high incidence of infections with Omicron variants.
Methods
We randomly selected and assessed individuals aged ≥16 years from the general population in southern (n = 739) and north-eastern (n = 964) Switzerland in March 2022. We assessed them again in June/July 2022, supplemented with a random sample from western (n = 850) Switzerland. We measured SARS-CoV-2 specific IgG antibodies and SARS-CoV-2 neutralizing antibodies against three variants (ancestral strain, Delta, Omicron).
Results
Seroprevalence remained stable from March 2022 (97.6%, n = 1894) to June/July 2022 (98.4%, n = 2553). In June/July, the percentage of individuals with neutralizing capacity against ancestral strain was 94.2%, against Delta 90.8% and against Omicron 84.9%, and 50.6% developed hybrid immunity. Individuals with hybrid immunity had highest median levels of anti-spike IgG antibodies titres [4518 World Health Organization units per millilitre (WHO U/mL)] compared with those with only vaccine- (4304 WHO U/mL) or infection- (269 WHO U/mL) induced immunity, and highest neutralization capacity against ancestral strain (hybrid: 99.8%, vaccinated: 98%, infected: 47.5%), Delta (hybrid: 99%, vaccinated: 92.2%, infected: 38.7%) and Omicron (hybrid: 96.4%, vaccinated: 79.5%, infected: 47.5%).
Conclusions
This first study on functional and hybrid immunity in the Swiss general population after Omicron waves showed that SARS-CoV-2 has become endemic. The high levels of antibodies and neutralization support the emerging recommendations of some countries where booster vaccinations are still strongly recommended for vulnerable persons but less so for the general population.
Keywords: SARS-CoV-2, COVID-19, hybrid immunity, functional immunity, seroprevalence, neutralization, vaccination, infection, cohort, population-based
Key Messages.
Our study is one of the first that assessed infection-induced, vaccine-induced and hybrid immunity and neutralizing activity of antibodies against SARS-CoV-2 in a larger population-based sample.
The population-based cohort study showed that by mid-2022, SARS-CoV-2 has become endemic and the levels of antibodies and neutralization against the ancestral strain, Delta, and Omicron variants were very high in the general Swiss population.
Hybrid immunity confers higher levels of neutralizing activity compared with both vaccine-induced and infection-induced immunity.
The high levels of antibodies and neutralization support the emerging recommendations of some countries where booster vaccinations are still strongly recommended for vulnerable persons but less strongly recommended for the general population.
Introduction
The World Health Organization (WHO) emphasized in June 2022 the key importance of continuous monitoring of immunity against SARS-CoV-2 and its variants of concern (VOC) in the general population, to inform public health measures and vaccination strategies.1 Early seroprevalence studies in spring 2020 showed that up to 10% of the general population had already developed antibodies against SARS-CoV-2 after the first wave in Europe and North America.2–5 Up to the point where vaccinations were approved in late 2020, seroprevalence increased to, on average, 25% as a consequence of SARS-CoV-2 infections, but with great variations within and across countries.1,6,7 Following the introduction of vaccines, seroprevalence quickly increased to around 50% in the general population worldwide and to above 90% in high-income countries.6,8 The lower rate of severe COVID-19 in vaccinated individuals provides strong support for the effectiveness of vaccines. However, although vaccines confer very high individual protection against COVID-19 symptoms, hospitalization and deaths, protection against new infections is partial and may decrease over time and with the emergence of new VOCs that can escape previously induced immunity.9–11
The rise of the highly infectious Omicron VOCs in early 2022 caused many infections in fully vaccinated or boosted persons. This led to a high seroprevalence and functional immunity in the general Swiss population, as measured by neutralizing activity of antibodies in serum.12 Functional immunity contributes to protection from severe courses of COVID-19 and is stronger if induced by both vaccinations and infections than by either alone (i.e. hybrid immunity).13–15 To inform public health measures and further booster vaccine strategies, it is important to assess population levels of seroprevalence and durability of functional and hybrid immunity developed during a time of high incidence of Omicron infections. The aim of this study was to assess the trajectory of anti-SARS-CoV-2 antibody titres and functional and hybrid immunity in the general population, and to compare such trajectories across age groups and three cantons, i.e. federal states of the Swiss confederation, covering the three main regions in Switzerland.
Methods
Study design, sampling, and participants
This prospective, population-based, multi-region cohort study is part of the Corona Immunitas research programme in Switzerland,16,17 for which we had completed four Phases of seroprevalence studies between April 2020 and October 2021 using a standardized protocol (study registration: ISRCTN registry 18181860). The current study includes results from Phases 5 and 6, for which assessments were conducted between 1 March and 1 April 2022, and 30 May and 11 July 2022, respectively (detailed results of Phase 5 published elsewhere).12
In Phase 5, we randomly selected individuals from the general population in southern (canton of Ticino) and north-eastern (canton of Zurich) Switzerland, who were assessed again in Phase 6. For cross-sectional analyses in Phase 6, we supplemented the southern and eastern Switzerland sample with a random sample from the general population in western Switzerland (canton of Vaud). Due to another seroprevalence study requested by the cantonal health authorities of Vaud, which took place in the autumn of 2021, the canton of Vaud only participated in Phase 6; it was not feasible to conduct an additional assessment between autumn 2021 and June 2022. The three Swiss cantons differ across demographic, socio-cultural and linguistic aspects and climate, all of which may impact on the dynamics of the pandemic.18 However, they are fairly representative for their language region (Italian, German, and French; map of Switzerland for overview see Supplementary Figure S1, available as Supplementary data at IJE online). The Swiss Federal Office of Statistics provided random samples of the general population in age-stratified (16–29, 30–44, 45–64 and ≥65 years) groups, separately for the cantons of Ticino, Vaud and Zurich. We selected these groups after consultation with the Swiss Federal Office of Public Health to adequately account for the potential impact on seroprevalence of social behaviour, adherence to public health measures and vaccination uptake, all of which differ across these age groups.19 The target sample size was 200 for each age stratum in the three cantons (i.e. total planned sample size of 2400). Based on the framework proposed by Larremore et al.,20 we deemed 200 participants to provide precise estimates; given a sensitivity of 97% and a specificity of 99% for the serological test we have used,21 a population of 200 persons with 180 positive tests and 20 negative tests (observed prevalence 90.0%) yields a posterior prevalence of 92.3% with 90% credible intervals of 88.5 and 95.8 (Supplementary Figure S2, available as Supplementary data at IJE online). All Phase 5 participants in Ticino and Zurich were invited to participate in the Phase 6 blood sampling.
Data collection
We invited participants to in-person study visits at a health care facility to provide a blood sample. People who were not able or willing to travel were offered home visits. For each participant, trained personnel collected venous blood samples, according to clinical standards and COVID-19 hygiene measures. Before the first study visit, all participants completed a baseline questionnaire including information regarding sociodemographics, vaccinations, SARS-CoV-2 infections, hospital and intensive care unit (ICU) admissions, symptoms in case of infections and past medical history, using the secure, web-based Research Electronic Data Capture platform (REDCap) for data collection and management.22,23 They also had the possibility to fill in the questionnaire in a paper/pencil version. Participants from the cantons of Ticino and Zurich who were recruited in Phase 5 were invited for a second study visit and blood sampling in Phase 6, 3 to 4 months later. Before this second study visit, participants filled in another questionnaire targeting the time between the first and second blood sampling including questions on new self-reported SARS-CoV-2 infections, symptoms and vaccinations.
Laboratory assays for SARS-CoV-2 antibodies and neutralizing capacity against SARS-CoV-2 variants
We assessed SARS-CoV-2 specific antibodies against the spike and nucleocapsid proteins using Sensitive Anti-SARS-CoV-2 Spike Trimer Immunoglobulin Serological (SenASTrIS), a Luminex binding assay.21 The assay measures binding of IgG antibodies to the trimeric SARS-CoV-2 spike and the nucleocapsid proteins. The test has a high specificity (99%) and sensitivity (97%), has been validated in samples of the general population and in specific subgroups21 and results in semi-quantitative median fluorescence intensity (MFI) values. The MFI values have additionally been translated to the WHO units per millilitre (U/mL) scale as measured by the Elecsys Anti-SARS-CoV-2 immunoassay by Roche.12 We also assessed the presence of SARS-CoV-2 neutralizing antibodies against three variants (ancestral strain, Delta and Omicron) that were dominant in Switzerland in 2022, using a cell- and virus-free assay.24 This assay measures the proportion of antibodies that block the interaction of the angiotensin-converting enzyme 2 receptor (ACE2r) with the receptor-binding domain of the trimer spike protein of the ancestral strain and variants of concern. All analyses were performed in the laboratory of Immunology of the Lausanne University Hospital (CHUV).
Outcome definition
We defined seropositivity based on the presence of anti-spike IgG antibodies according to the threshold of SenASTrIS test positivity with MFI ≥6 (levels categorized: ≥6 and <12 low, ≥12 and <40 middle, ≥40 high) and neutralization capacity based on the cut-off value of the cell- and virus-free assay of 50. Functional immunity was defined based on neutralization capacity of the cell- and virus-free assay above the threshold value of 50. This was determined independently for each variant spike (ancestral strain, Delta, Omicron). Last, source of immune status was defined based on SARS-CoV-2 vaccination status (self-reported) and SARS-CoV-2 infection, determined as seropositivity for anti-nucleocapsid IgG (MFI≥6), report of a positive polymerase chain reaction (PCR) or rapid antigen test or presence of anti-spike IgG antibodies in the absence of a SARS-CoV-2 vaccination. We categorized immune status as follows: immune naïve (i.e. no detectable antibodies and no reported infection or SARS-CoV-2 vaccination), vaccine-induced only, infection-induced only or hybrid immunity (SARS-CoV-2 vaccination and infection).
Statistical analysis
We used medians and interquartile ranges (numerical variables) or absolute numbers and percentages (categorical variables) for the descriptive analyses.
We calculated seroprevalence using a Bayesian logistic regression model adjusted for age group (16–29, 30–44, 45–64 and 65+) and sex with weak normal(0, 1) priors on beta coefficients per canton. We incorporated the uncertainty of sensitivity (hierarchical prior) and specificity [uniform(0, 1) prior] of the serological test as binomial models. We used a Markov chain Monte Carlo sampling approach with four chains (250 warm-up iterations and 1250 estimation iterations per chain, 5000 iterations in total with warm-up iterations not considered) using the probabilistic programming language stan and the rstan package to run the model in R. Model convergence has been assessed using R-hat and by inspecting traceplots. We applied post-stratification weights based on the target population’s demographic structure (population size per age group and sex) to obtain seroprevalence estimates by estimating weighted means of probability of seropositivity based on the posterior distribution. Reported estimates are medians and 95% confidence intervals 2.5- and 97.5-quantiles of the resulting probability distributions.2,25,26
Furthermore, we determined the percentage of individuals in whom anti-spike IgG antibodies remained negative or positive (i.e. unchanged) or changed from negative to positive or positive to negative. We conducted all analyses in R, version 4.2.1.27
Results
Participation rate in March 2022 (Phase 5 of the Corona Immunitas research programme) was 18.1% in Ticino (850/4687) and 21.4% in Zurich (1044/4875). All participants were invited to take part in the Phase 6 blood sampling in June/July 2022, of whom 86.9% (739/850) in Ticino and 92.3% (964/1044) in Zurich decided to participate. Participation rate in Vaud in Phase 6 was 12.2% (850/6963).
Between 30 May and 11 July 2022 (Phase 6), we assessed in total 2553 cohort participants. Median age of participants of the three cantons was 49 years in Ticino [interquartile range (IQR) 35–64], 55 in Vaud (IQR 39–69) and 52 in Zurich (IQR 35–66). The percentage of female participants was 58.1% in Ticino, 55.9% in Vaud and 54.4% in Zurich. Most participants had received at least one dose of a SARS-CoV-2 vaccine (89.9% in Ticino, 91.1% in Vaud and 93.6% in Zurich). Around half of the study sample reported to have been infected recently, likely in 2022 (53% in Ticino, 48.7% in Vaud, 51.8% in Zurich) [Table 1 (stratified by canton); Supplementary Table S1, available as Supplementary data at IJE online (stratified by canton and age group)].
Table 1.
Characteristics of the sample, Ticino, Vaud and Zurich, Switzerland, June–July 2022 (n = 2553), stratified by cantona
| Characteristic | Ticino | Vaud | Zurich | All |
|---|---|---|---|---|
| Sample size | 739 | 850 | 964 | 2553 |
| Median age, years (IQR) | 49 (35–64) | 55 (39–69) | 52 (35–66) | 52 (36–66) |
| Age group, years | ||||
| 16–29 | 128 (17.3%) | 107 (12.6%) | 156 (16.2%) | 391 (15.3%) |
| 30–44 | 186 (25.2%) | 183 (21.5%) | 235 (24.4%) | 604 (23.7%) |
| 45–64 | 241 (32.6%) | 275 (32.4%) | 306 (31.7%) | 822 (32.2%) |
| 65+ | 184 (24.9%) | 285 (33.5%) | 267 (27.7%) | 736 (28.8%) |
| Female sex | 429 (58.1%) | 475 (55.9%) | 524 (54.4%) | 1428 (55.9%) |
| Education | ||||
| Primary | 73 (9.9%) | 73 (8.6%) | 62 (6.4%) | 208 (8.1%) |
| Secondary | 409 (55.3%) | 337 (39.6%) | 394 (40.9%) | 1140 (44.7%) |
| Tertiary | 250 (33.8%) | 426 (50.1%) | 502 (52.1%) | 1178 (46.1%) |
| Missing data | 7 (0.9%) | 14 (1.6%) | 6 (0.6%) | 27 (1.1%) |
| Household income, CHF/monthb | ||||
| 0–6000 | 317 (42.9%) | 215 (25.3%) | 320 (33.2%) | 852 (33.4%) |
| 6000–12 000 | 275 (37.2%) | 349 (41.1%) | 365 (37.9%) | 989 (38.7%) |
| 12 000–18 000 | 49 (6.6%) | 159 (18.7%) | 163 (16.9%) | 371 (14.5%) |
| 18 000+ | 40 (5.4%) | 72 (8.5%) | 70 (7.3%) | 182 (7.1%) |
| Missing data | 58 (7.8%) | 55 (6.5%) | 46 (4.8%) | 159 (6.2%) |
| Working | 453 (61.3%) | 519 (61.1%) | 666 (69.1%) | 1638 (64.2%) |
| Missing data | 3 (0.4%) | 8 (0.9%) | 6 (0.6%) | 17 (0.7%) |
| Swiss citizen | 590 (79.8%) | 701 (82.5%) | 823 (85.4%) | 2114 (82.8%) |
| Missing data | 1 (0.1%) | 4 (0.5%) | 4 (0.4%) | 9 (0.4%) |
| Smoking | 140 (18.9%) | 159 (18.7%) | 174 (18%) | 473 (18.5%) |
| Missing data | 2 (0.3%) | 0 | 3 (0.3%) | 5 (0.2%) |
| Obese (BMI ≥30 kg/m2) | 85 (11.5%) | 108 (12.7%) | 122 (12.7%) | 315 (12.3%) |
| Missing data | 0 | 0 | 0 | 0 |
| Chronic diseasec | 165 (22.3%) | 242 (28.5%) | 268 (27.8%) | 675 (26.4%) |
| Missing data | 1 (0.1%) | 0 | 0 | 1 (0%) |
| Assessment period | ||||
| First blood sample | 2022-06-01 | 2022-05-30 | 2022-06-02 | 2022-05-30 |
| Last blood sample | 2022-06-25 | 2022-07-02 | 2022-07-11 | 2022-07-11 |
| Anti-SARS-CoV-2 antibodies | 723 (97.8%) | 835 (98.2%) | 954 (99%) | 2512 (98.4%) |
| Known to be infected recently (NuC positive or positive test 2022) | 392 (53%) | 414 (48.7%) | 499 (51.8%) | 1305 (51.1%) |
| Missing data | 0 | 3 (0.4%) | 0 | 3 (0.1%) |
| Vaccinated (≥1 dose) | 664 (89.9%) | 774 (91.1%) | 902 (93.6%) | 2340 (91.7%) |
| Missing data | 4 (0.5%) | 1 (0.1%) | 2 (0.2%) | 7 (0.3%) |
BMI, body mass index; CHF, Swiss francs; IQR, interquartile range; NuC, nucleocapsid; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Cantons (here Ticino, Vaud and Zurich) are federal states of the Swiss confederation.
Mean exchange rate in June 2022: 1 Swiss franc = 1.03 euro/0.95 US dollar.
Chronic disease includes reporting any of the following conditions: cancer, diabetes, diseases/treatments that weaken the immune system, physician-diagnosed high blood pressure, cardiovascular diseases and chronic respiratory diseases.
By June/July 2022, seroprevalence was estimated at 98.3% in Ticino [95% confidence interval (CI) 96.9–99.3%)], 98.4% in Vaud (95% CI 97.3–99.3%) and 98.9% in Zurich (95% CI 98–99.5%, Table 2). Anti-spike IgG antibodies were high across cantons and age groups (>90%). The percentage of individuals whose antibodies showed neutralization (ACE2r-blocking; functional capacity) was high against the ancestral strain (93.1% in Ticino, 93.9% in Vaud, 95.4% in Zurich) and Delta (90.7% in Ticino, 91.8% in Vaud, 90% in Zurich), and only slightly lower for the Omicron (84.3% in Ticino, 86.9% in Vaud, 83.6% in Zurich) variant of SARS-CoV-2, with no evident patterns across age groups and study sites.
Table 2.
Prevalence of SARS-CoV-2 IgG antibodies and ACE2r-blocking (neutralizing capacity) as measured by a virus-free assay, Ticino, Vaud and Zurich, Switzerland, June–July 2022, (n=2553), stratified by cantona and age group
| Ticino |
Vaud |
Zurich |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence | All | 16–29 years | 30–44 years | 45–64 years | 65+ years | All | 16–29 years | 30–44 years | 45–64 years | 65+ years | All | 16–29 years | 30–44 years | 45–64 years | 65+ years |
| Level of anti-spike IgG antibodiesb | |||||||||||||||
| Not detectable | 18 (2.4%) | 4 (3.1%) | 3 (1.6%) | 7 (2.9%) | 4 (2.2%) | 16 (1.9%) | NA | 3 (1.6%) | 9 (3.3%) | 4 (1.4%) | 12 (1.2%) | 2 (1.3%) | 5 (2.1%) | 2 (0.7%) | 3 (1.1%) |
| Low | 8 (1.1%) | 2 (1.6%) | 3 (1.6%) | 2 (0.8%) | 1 (0.5%) | 13 (1.5%) | 2 (1.9%) | 6 (3.3%) | 3 (1.1%) | 2 (0.7%) | 10 (1%) | 0 | 2 (0.9%) | 4 (1.3%) | 4 (1.5%) |
| Moderate | 20 (2.7%) | 6 (4.7%) | 6 (3.2%) | 4 (1.7%) | 4 (2.2%) | 24 (2.8%) | 4 (3.7%) | 6 (3.3%) | 4 (1.5%) | 10 (3.5%) | 19 (2%) | 3 (1.9%) | 5 (2.1%) | 9 (2.9%) | 2 (0.7%) |
| High | 693 (93.8%) | 116 (90.6%) | 174 (93.5%) | 228 (94.6%) | 175 (95.1%) | 797 (93.8%) | 101 (94.4%) | 168 (91.8%) | 259 (94.2%) | 269 (94.4%) | 923 (95.7%) | 151 (96.8%) | 223 (94.9%) | 291 (95.1%) | 258 (96.6%) |
| WHO U/mL, median (IQR)c | 4505 (3279–6198) | 4570 (3123–6588) | 4485 (3182–5728) | 4642 (3684–6298) | 4417 (3393–5589) | 4178 (3217–5334) | 4120 (3057–5458) | 4146 (3230–5140) | 4172 (3187–5264) | 4237 (3284–5558) | 4224 (3245–5649) | 4683 (3464–5587) | 4017 (3170–5680) | 4218 (3375–5491) | 4310 (3308–5749) |
| Seroprevalence, % (95% CI) | 98.3 (96.9–99.3) | 97.4 (94.5–99) | 99 (96.9–99.8) | 98.3 (95.9–99.6) | 98.6 (96.2–99.7) | 98.4 (97.3–99.3) | 98.2 (96.0–99.3) | 98.9 (96.7–99.8) | 98.1 (95.9–99.5) | 99.0 (97.4–99.8) | 98.9 (98.0–99.5) | 98.4 (96.4–99.4) | 98.8 (96.8–99.7) | 99.5 (98.3–99.9) | 99.1 (97.4–99.8) |
| Neutralization (≥50) | |||||||||||||||
| Ancestral strain | 688 (93.1%) | 114 (89.1%) | 173 (93.0%) | 227 (94.2%) | 174 (94.6%) | 798 (93.9%) | 101 (94.4%) | 169 (92.3%) | 259 (94.2%) | 269 (94.4%) | 920 (95.4%) | 150 (96.2%) | 222 (94.5%) | 292 (95.4%) | 256 (95.9%) |
| Delta | 670 (90.7%) | 110 (85.9%) | 169 (90.9%) | 221 (91.7%) | 170 (92.4%) | 780 (91.8%) | 99 (92.5%) | 163 (89.1%) | 254 (92.4%) | 264 (92.6%) | 868 (90.0%) | 148 (94.9%) | 207 (88.1%) | 278 (90.8%) | 235 (88.0%) |
| Omicron | 623 (84.3%) | 107 (83.6%) | 157 (84.4%) | 208 (86.3%) | 151 (82.1%) | 739 (86.9%) | 96 (89.7%) | 157 (85.8%) | 238 (86.5%) | 248 (87.0%) | 806 (83.6%) | 140 (89.7%) | 194 (82.6%) | 260 (85.0%) | 212 (79.4%) |
ACE2r, angiotensin-converting enzyme 2 receptor; CI, confidence interval; IgG, immunoglobulin G; IQR, interquartile range; NA, not applicable; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WHO U/mL, World Health Organization units per millilitre (according to Elecsys ® Anti-SARS-CoV-2 S).
Cantons (here Ticino, Vaud and Zurich) are federal states of the Swiss confederation.
Unit for levels of anti-spike IgG antibodies is the median fluorescence intensity (MFI) as measured by the Luminex binding assay SenASTrIS (Sensitive Anti-SARS-CoV-2 Spike Trimer Immunoglobulin Serological).20 Low: from threshold of test positivity to less than 3 standard deviations above this threshold (≥6 to <12); moderate: ≥3 standard deviations above positivity threshold but unlikely to provide neutralization (≥12 to <40); high: neutralizing capacity likely (≥40).
The MFI values have additionally been translated to the U/mL scale as measured by the Elecsys Anti-SARS-CoV-2 immunoassay by Roche and are presented as population median and IQR.
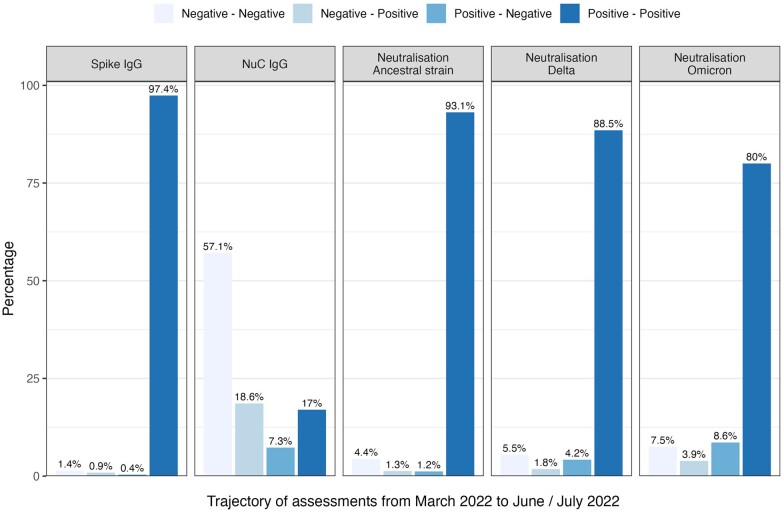
From March 2022 to June/July 2022, the percentage of participants from Ticino and Zurich with detectable anti-spike IgG antibodies remained stable (>96% across age groups); only in seven participants the anti-spike IgG decreased below the threshold; all of these were unvaccinated and had become infected in 2022. In contrast, anti-nucleocapsid IgG antibodies fluctuated more and changed from positive to negative in 7.3% of the participants and from negative to positive in 18.6%. The neutralization capacity against the variants remained more stable (from positive to positive: 93.1% for the ancestral strain, 88.5% for Delta, and 80% for Omicron), with little variation across age groups [Figure 1 (overall trajectories), Supplementary Table S2, available as Supplementary data at IJE online (trajectories stratified by age group)]. There was a higher loss of neutralization capacity (from positive to negative) observed for Omicron with 8.6% (ancestral strain 1.2%, Delta 4.2%), whereas on the population level only little changed with respect to newly obtained neutralization capacity (from negative to positive: ancestral strain 1.3%, Delta 1.8%, Omicron 3.9%).
Figure 1.
Trajectories of SARS-CoV-2 IgG antibodies and ACE2r-blocking (neutralizing activity) as measured by a virus-free assay, from March 2022 to June/July 2022. NuC, nucleocapsid; IgG, immunglobulin G. Seropositivity is defined based on the presence of anti-spike IgG antibodies according to the threshold of SenASTrIS test positivity with median fluorescence intensity (MFI) ≥6. Neutralization capacity based on virus-free assay with cut-off value of ≥50. Participants of Corona Immunitas from Ticino and Zurich, Switzerland (n = 1702)
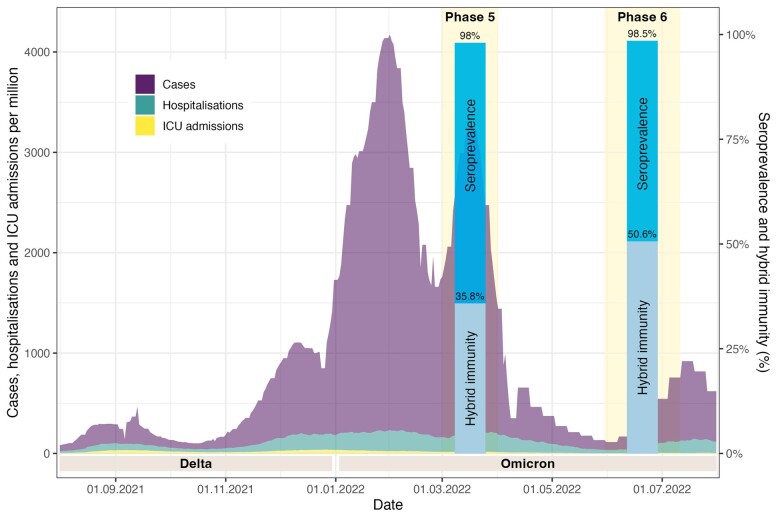
In June/July 2022, 1.0% (n = 25) of all participants were immune naïve (i.e. no detectable antibodies and no reported infection or SARS-CoV-2 vaccination), 41.1% (n = 1050) had vaccination-induced immunity only, 7.1% (n = 181) infection-induced immunity only, and 50.6% (n = 1289) hybrid immunity (vaccination and infection). For eight participants, relevant data to determine immune status were missing. Seroprevalence and hybrid immunity in Phases 5 and 6 of Corona Immunitas, in relation to the evolution of the pandemic in Switzerland, are illustrated in Figure 2. The percentage with high levels of anti-spike IgG antibodies was more than double in persons with a hybrid immunity (99.8%) and vaccinated-only individuals (99%) compared with individuals with an infection only (45.9%) [Table 3 (pooled results); and Supplementary Table S3, available as Supplementary data at IJE online (results stratified by canton)]. Such large differences were also observed for neutralization capacity. Neutralization against Delta and Omicron was highest in participants with hybrid immunity, followed by those who had only been vaccinated and much lower in those with infection only (ancestral strain: hybrid 99.8%, vaccinated 98%, infected 47.5%; Delta: hybrid 99%, vaccinated 92.2%, infected 38.7%; Omicron: hybrid 96.4%, vaccinated 79.5%, infected 47.5%). Compared with March 2022 (Phase 5), hybrid immunity in participants from Ticino and Zurich increased from 35.8% to 50.6% by June/July 2022 (Phase 6), reflecting the high incidence with Omicron infections since spring 2022.
Figure 2.
Seroprevalence and hybrid immunity in Phases 5 and 6 of Corona Immunitas, Switzerland, in relation to the evolution of the pandemic, August 2021–August 2022. ICU, intensive care unit. The evolution of the pandemic is visualized by the number of laboratory-confirmed SARS-CoV-2 cases (in purple), hospitalizations (turquoise) and intensive care unit admissions (in yellow) in Switzerland between August 2021 and August 2022, retrieved from: [https://ourworldindata.org/coronavirus].37 The time frame of Phases 5 (March 2022, n =1894) and 6 (June/July 2022, n = 2553) of Corona Immunitas, when the blood sampling and questionnaire assessments took place, are highlighted in light yellow. The results regarding seroprevalence and percentage of participants with hybrid immunity are visualized in dark and light blue bars, respectively
Table 3.
Prevalence of SARS-CoV-2 IgG antibodies and ACE2r-blocking (neutralizing capacity) as measured by a virus-free assay, Ticino, Vaud and Zurich, Switzerland, pooled, June–July 2022, (n=2520a), stratified by vaccination and infection status of participants
| Prevalence | Only vaccinated | Only infected | Vaccinated and infected |
|---|---|---|---|
| Sample size | 1050 | 181 | 1289 |
| Anti-spike IgG antibodiesb | |||
| Not detectable | 0 | 17 (9.4%) | 1 (0.1%) |
| Low | 1 (0.1%) | 30 (16.6%) | 0 |
| Moderate | 10 (1%) | 51 (28.2%) | 1 (0.1%) |
| High | 1039 (99%) | 83 (45.9%) | 1287 (99.8%) |
| WHO U/mL, median (IQR)c | 4304 (3303–5716) | 269 (34–1820) | 4518 (3733–6005) |
| Anti-NuC IgG antibodies | |||
| Not detectable | 1050 (100%) | 59 (32.6%) | 522 (40.5%) |
| Low (≥6 to <12) | 0 | 38 (21%) | 407 (31.6%) |
| Moderate (≥12 to <40) | 0 | 84 (46.4%) | 360 (28%) |
| High (≥40) | 0 | 0 | 0 |
| Neutralization (≥50) | |||
| Ancestral strain | 1029 (98%) | 86 (47.5%) | 1287 (99.8%) |
| Delta | 968 (92.2%) | 70 (38.7%) | 1276 (99%) |
| Omicron | 835 (79.5%) | 86 (47.5%) | 1243 (96.4%) |
ACE2r, angiotensin-converting enzyme 2 receptor; IgG, immunoglobulin G; IQR, interquartile range; NuC, nucleocapsid; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WHO U/mL, World Health Organization units per millilitre (according to Elecsys ® Anti-SARS-CoV-2 S).
Participants who were immunologically naïve (n = 25) or were missing relevant data to determine their immune status (n = 8) have been excluded.
Unit for levels of anti-spike IgG antibodies is the median fluorescence intensity (MFI) as measured by the Luminex binding assay SenASTrIS (Sensitive Anti-SARS-CoV-2 Spike Trimer Immunoglobulin Serological).20 Low: from threshold of test positivity to less than 3 standard deviations above this threshold (≥6 to <12); moderate: ≥3 standard deviations above positivity threshold but unlikely to provide neutralization (≥12 to <40); high: neutralizing capacity likely (≥40).
The MFI values have additionally been translated to the U/mL scale as measured by the Elecsys Anti-SARS-CoV-2 immunoassay by Roche and are presented as population median and IQR.
Discussion
This population-based cohort study showed that not only SARS-CoV-2 seroprevalence but also antibody titres were very high in the Swiss general population by June/July 2022, without notable differences across cantons, age or sex strata. At least 51% of participants developed hybrid immunity, and among those more than 96% had neutralizing antibodies against the ancestral strain, Delta and Omicron variants. In participants who received vaccination but were not infected previously, the percentage with neutralizing antibodies was lower, in particular against Omicron. The 7% of participants with only infection-induced immunity had about 15 times lower antibody titres and less than 50% of them showed neutralizing antibodies.
Trajectories of anti-spike IgG antibodies from March 2022 to June/July 2022 remained remarkably stable in participants from Ticino and Zurich. The fluctuation of anti-nucleocapsid IgG antibodies in contrast reflected quick waning of anti-nucleocapsid antibodies as well as substantial infection activity with the Omicron variant in spring 2022 in Switzerland. Thus, we observed stable seroprevalence and high levels of antibodies in the general population. Since the summer 2022 up to March 2023, the number of SARS-CoV-2 infections was still moderately high in Switzerland but fluctuated less than before and with very few hospital admissions due to COVID-19. Our results of stable and high population immunity together with the rather stable epidemiological situation imply that the transition from the pandemic to an endemic situation is taking place.
Our findings are in line with previous studies, mainly conducted in non-representative, convenience and relatively small samples, and/or in sub-populations (e.g. health care workers28 and children),29 showing that hybrid immunity confers higher immune protection and exhibits better neutralizing capacity compared with vaccine- and infection-induced immunity.28–34 However, to our knowledge, this study is the first to demonstrate the extent of hybrid immunity and neutralization capacity in the general population in 2022.
A large study from Israel in 2021 showed that hybrid immunity provided stronger protection than vaccination and infection alone.15 Although the proportion of persons with hybrid immunity was not reported, the observation time for persons with infection and vaccination up to (re-) infection or censoring was shorter compared with those only vaccinated or only infected, implying a very low prevalence of hybrid immunity back in 2021.
Strengths of our study include the prospective, population-based cohort study design, coverage of the three main language and cultural regions of a country, the well-established methods of the Corona Immunitas research programme, the large sample size and the use of previously validated serological tests and neutralizing antibodies.21,24 In addition, retention of participants since March 2022 was high. Limitations include the modest participation rate, as is commonly the case in population-based studies; however, this may have introduced self-selection bias. We observed that in general, individuals with higher health literacy and trust in public health authorities in dealing with the pandemic were more likely to participate. Overall, this may have led to an overrepresentation of vaccinated persons and, consequently, of the seroprevalence. Another limitation is the lack of measures of cellular immunity, which is not feasible to test in large population-based studies. In addition, we may have underestimated hybrid immunity, as anti-nucleocapsid antibodies wane quickly and we likely missed some infections that occurred before 2022. Self-reports of infections compensate only to some extent for the low to moderate sensitivity of anti-nucleocapsid assays beyond 6 months of infection, because many infections are mild or asymptomatic. We assessed self-reported SARS-CoV-2 vaccination status and did not check vaccine certificates for feasibility reasons. Although we do not expect that many participants answered this question dishonestly or that recall bias occurred regarding vaccination, we cannot exclude this possibility. It is difficult to estimate how such a potential bias may have affected the results.
Our results have implications for vaccination strategies. Recommendations for primary series and booster vaccination need to consider the effectiveness and safety of vaccines as well as the epidemiological and societal context.35 Seroprevalence is only a rough proxy marker of immunity in the population, since seropositive persons have a wide range of antibody titres and neutralizing capacity against SARS-CoV-2 VOCs as a consequence of infection only, vaccination only or both infection and vaccination, as this study and other studies showed.13,14 Therefore, information on the proportion of persons in the general population with neutralizing capacity and hybrid immunity provides more solid guidance. The Swiss Federal Vaccination Commission recently released finely granulated recommendations for booster and primary series vaccinations, based on the best available international evidence on the effectiveness and safety of bivalent or other booster vaccines and based on the results of Corona Immunitas presented here. Whereas the Commission issued a strong recommendation for a second booster for people above 64 years of age, for those with chronic conditions and for pregnant women, the recommendation was moderately strong for health care staff and formal and informal caregivers, and only weak for the general population between 16 and 64 years of age. In addition, they recommended only one primary series dose for unvaccinated persons since most of them have had a SARS-CoV-2 infection (>90% according to the results presented here). These recommendations considered the high seroprevalence in Switzerland and the high proportion of persons with hybrid immunity and neutralizing capacity and include considerations on the optimal timing for the next booster campaign in autumn/winter 2022. The Canadian authorities issued similar recommendations for booster vaccines but population-based data on immunity in the population were not available to the extent and level of detail presented here.36
Conclusion
This prospective population-based cohort study with 2553 participants showed that seroprevalence remained very high in Switzerland in 2022, without differences across cantons and age groups. Antibody titres increased, and the majority of participants developed hybrid immunity with very high levels of neutralization against the ancestral strain, Delta and Omicron variants of SARS-CoV-2. Individuals with immunity only from infection had 15 times lower antibody titres, and less than half of them showed neutralization. Our results support the emerging recommendations of some countries where booster vaccinations are still strongly recommended for vulnerable persons but less strongly recommended for individuals in the general population.
Corona Immunitas Research Group
Emiliano Albanese (Institute of Public Health, Università della Svizzera italiana, Lugano, Switzerland); Rebecca Amati (Institute of Public Health, Università della Svizzera italiana, Lugano, Switzerland); Antonio Amendola (Department of Business Economics, Health and Social Care, University of Applied Sciences & Arts of Southern Switzerland, Switzerland); Alexia Anagnostopoulos (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Daniela Anker (Population Health Laboratory, University of Fribourg, Switzerland; Institute of Primary Health Care, University of Bern, Switzerland); Anna Maria Annoni (Institute of Public Health, Università della Svizzera italiana, Lugano, Switzerland); Hélène Aschmann (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Andrew Azman (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Institute of Global Health, Faculty of Medicine, University of Geneva, Geneva, Switzerland); Antoine Bal (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Tala Ballouz (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Hélène Baysson (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland; Department of Health and Community Medicine, Faculty of Medicine, University of Geneva, Geneva, Switzerland); Kleona Bezani (Institute of Public Health, Università della Svizzera italiana, Lugano, Switzerland); Annette Blattmann (Cantonal Hospital St. Gallen, Clinic for Infectious Diseases and Hospital Epidemiology, St. Gallen, Switzerland); Patrick Bleich (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Murielle Bochud (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Patrick Bodenmann (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Gaëlle Bryand Rumley (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Peter Buttaroni (Institute of Public Health, Università della Svizzera italiana, Lugano, Switzerland); Audrey Butty Dettwiler (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Anne Linda Camerini (Institute of Public Health, Università della Svizzera italiana, Lugano, Switzerland); Arnaud Chiolero (Population Health Laboratory, University of Fribourg, Switzerland; Institute of Primary Health Care, University of Bern, Switzerland; Department of Epidemiology, Biostatistics and Occupational Health, McGill University, Montréal, Canada); Patricia Orializ Chocano-Bedoya (Institute of Primary Health Care, University of Bern; Population Health Laboratory, University of Fribourg, Switzerland); Prune Collombet (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland; Department of Health and Community Medicine, Faculty of Medicine, University of Geneva, Geneva, Switzerland); Laurie Corna (Department of Business Economics, Health and Social Care, University of Applied Sciences & Arts of Southern Switzerland, Switzerland); Luca Crivelli (Department of Business Economics, Health and Social Care, University of Applied Sciences & Arts of Southern Switzerland, Switzerland; Institute of Public Health, Università della Svizzera italiana, Lugano, Switzerland); Stéphane Cullati (Population Health Laboratory, University of Fribourg, Switzerland; Department of Readaptation and Geriatrics, University of Geneva, Switzerland); Valérie D'Acremont (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland; Swiss Tropical and Public Health Institute, Basel, Switzerland); Diana Sofia Da Costa Santos (Institute of Public Health, Università della Svizzera italiana, Lugano, Switzerland); Agathe Deschamps (Cantonal Medical Service Neuchâtel); Paola D’Ippolito (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Anja Domenghino (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Richard Dubos (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Roxane Dumont (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Olivier Duperrex (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Julien Dupraz (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Malik Egger (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Emna El-May (Population Health Laboratory, University of Fribourg, Switzerland); Nacira El Merjani (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Nathalie Engler (Cantonal Hospital St. Gallen, Clinic for Infectious Diseases and Hospital Epidemiology, St. Gallen, Switzerland); Adina Mihaela Epure (Population Health Laboratory, University of Fribourg, Switzerland); Lukas Erksam (Institute of Primary Health Care, University of Bern, Department of General Internal Medicine, Inselspital, Bern University Hospital, University of Bern); Sandrine Estoppey (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Marta Fadda (Institute of Public Health, Università della Svizzera italiana, Lugano, Switzerland); Vincent Faivre (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Jan Fehr (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Andrea Felappi (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Maddalena Fiordelli (Institute of Public Health, Università della Svizzera italiana, Lugano, Switzerland); Antoine Flahault (Institute of Global Health, Faculty of Medicine, University of Geneva, Geneva, Switzerland; Division of Tropical and Humanitarian Medicine, Geneva University Hospitals, Geneva, Switzerland; Department of Health and Community Medicine, Faculty of Medicine, University of Geneva, Geneva, Switzerland); Luc Fornerod (Observatoire valaisan de la santé, Sion, Switzerland); Cristina Fragoso Corti (Department of environment construction and design, University of Applied Sciences & Arts of Southern Switzerland, Switzerland); Natalie Francioli (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Marion Frangville (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Irène Frank (Luzerner Kantonsspital, Spitalstrasse, 6000 Luzern 16); Giovanni Franscella (Institute of Public Health, Università della Svizzera italiana, Lugano, Switzerland); Anja Frei (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Marco Geigges (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Semira Gonseth Nusslé (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Clément Graindorge (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Idris Guessous (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland; Department of Health and Community Medicine, Faculty of Medicine, University of Geneva, Geneva, Switzerland); Erika Harju (Department of Health Sciences and Medicine, University of Lucerne, Frohburgstrasse 3, 6002 Lucerne); Séverine Harnal (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Medea Imboden (Swiss Tropical and Public Health Institute, Department of Epidemiology and Public Health, Basel, Switzerland; University of Basel, Basel, Switzerland); Emilie Jendly (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Ayoung Jeong (Swiss Tropical and Public Health Institute, Department of Epidemiology and Public Health, Basel, Switzerland; University of Basel, Basel, Switzerland); Christian R Kahlert (Cantonal Hospital St. Gallen, Clinic for Infectious Diseases and Hospital Epidemiology, St. Gallen, Switzerland; Children's Hospital of Eastern Switzerland, Infectious Diseases and Hospital Epidemiology, St. Gallen, Switzerland); Laurent Kaiser (Geneva Center for Emerging Viral Diseases and Laboratory of Virology, Geneva University Hospitals, Geneva, Switzerland; Division of Infectious Diseases, Geneva University Hospitals, Geneva, Switzerland; Department of Medicine, Faculty of Medicine, University of Geneva, Geneva, Switzerland); Laurent Kaufmann (Service de La Santé Publique, Canton de Neuchâtel, Neuchâtel, Switzerland); Marco Kaufmann (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Dirk Keidel (Swiss Tropical and Public Health Institute, Department of Epidemiology and Public Health, Basel, Switzerland; University of Basel, Basel, Switzerland); Simone Kessler (Cantonal Hospital St. Gallen, Clinic for Infectious Diseases and Hospital Epidemiology, St. Gallen, Switzerland); Philipp Kohler (Cantonal Hospital St. Gallen, Clinic for Infectious Diseases and Hospital Epidemiology, St. Gallen, Switzerland); Christine Krähenbühl (Luzerner Kantonsspital, Spitalstrasse, 6000 Luzern 16); Susi Kriemler (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Julien Lamour (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Sara Levati (Department of Business Economics, Health and Social Care, University of Applied Sciences & Arts of Southern Switzerland, Switzerland); Pierre Lescuyer (Division of Laboratory Medicine, Geneva University Hospitals, Geneva, Switzerland); Andrea Loizeau (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Elsa Lorthe (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Chantal Luedi (Department Health Sciences and Medicine, University of Lucerne, Frohburgstrasse 3, 6002 Lucerne); Jean-Luc Magnin (Laboratory, HFR-Fribourg, Fribourg, Switzerland); Chantal Martinez (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Eric Masserey (Cantonal Medical Office, General Health Department, Canton of Vaud, Switzerland); Dominik Menges (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Gisela Michel (Department of Health Sciences and Medicine, University of Lucerne, Frohburgstrasse 3, 6002 Lucerne); Rosalba Morese (Faculty of Communication, Culture and Society, Università della Svizzera italiana, Lugano, Switzerland; Faculty of Biomedical Sciences, Università della Svizzera italiana, Lugano, Switzerland); Nicolai Mösli (Swiss TPH, Basel, Switzerland; University of Basel, Basel, Switzerland); Natacha Noël (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Daniel Henry Paris (Swiss TPH, Basel, Switzerland; University of Basel, Basel, Switzerland); Jérôme Pasquier (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Francesco Pennacchio (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Stefan Pfister (Laboratory, HFR-Fribourg, Fribourg, Switzerland); Giovanni Piumatti (Fondazione Agnelli, Turin, Italy); Géraldine Poulain (Division of Laboratory Medicine, Geneva University Hospitals, Geneva, Switzerland); Nicole Probst-Hensch (Swiss Tropical and Public Health Institute, Department of Epidemiology and Public Health, Basel, Switzerland; University of Basel, Basel, Switzerland); Caroline Pugin (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Milo Puhan (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Nick Pullen (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Thomas Radtke (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Manuela Rasi (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Aude Richard (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland; Institute of Global Health, University of Geneva, Switzerland); Viviane Richard (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Claude-François Robert (Cantonal Medical Service Neuchâtel); Pierre-Yves Rodondi (Institute of Family Medicine, University of Fribourg, Fribourg, Switzerland); Nicolas Rodondi (Institute of Primary Health Care, University of Bern; Department of General Internal Medicine, Inselspital, Bern University Hospital, University of Bern); Serena Sabatini (Institute of Public Health, Università della Svizzera italiana, Lugano, Switzerland); Khadija Samir (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Javier Sanchis Zozaya (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Virginie Schlüter (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Alexia Schmid (Institute of Family Medicine, University of Fribourg, Fribourg, Switzerland); Valentine Schneider (Cantonal Medical Service Neuchâtel); Maria Schüpbach (Institute of Primary Health Care, University of Bern, Department of General Internal Medicine, Inselspital, Bern University Hospital, University of Bern); Nathalie Schwab (Institute of Primary Health Care, University of Bern, Department of General Internal Medicine, Inselspital, Bern University Hospital, University of Bern); Claire Semaani (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Alexandre Speierer (Institute of Primary Health Care, University of Bern; Department of General Internal Medicine, Inselspital, Bern University Hospital, University of Bern); Amélie Steiner-Dubuis (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Silvia Stringhini (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland; Department of Health and Community Medicine, Faculty of Medicine, University of Geneva, Geneva, Switzerland); Stefano Tancredi (Population Health Laboratory, University of Fribourg, Switzerland); Stéphanie Testini (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Julien Thabard (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Mauro Tonolla (Department of environment construction and design, University of Applied Sciences & Arts of Southern Switzerland, Switzerland); Nicolas Troillet (Office du médecin cantonal, Sion, Switzerland); Agne Ulyte (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Sophie Vassaux (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Thomas Vermes (Swiss Tropical and Public Health Institute, Department of Epidemiology and Public Health, Basel, Switzerland; University of Basel, Basel, Switzerland); Jennifer Villers (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Viktor von Wyl (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Cornelia Wagner (Population Health Laboratory, University of Fribourg, Switzerland); Rylana Wenger (Institute of Primary Health Care, University of Bern, Department of General Internal Medicine, Inselspital, Bern University Hospital, University of Bern); Erin West (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Ania Wisniak (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland; Institute of Global Health, Faculty of Medicine, University of Geneva, Geneva, Switzerland); Melissa Witzig (Swiss Tropical and Public Health Institute, Department of Epidemiology and Public Health, Basel, Switzerland; University of Basel, Basel, Switzerland); María-Eugenia Zaballa (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Kyra Zens (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Claire Zuppinger (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland)
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the responsible ethics committees of the cantons of Zurich (first approval 28 May 2020, BASEC Registration No 2020–01247), Ticino (first approval 23 June 2020, BASEC Registration No 2020–01514) and Vaud (first approval 23 April 2020, BASEC No 2020–00887), Switzerland. Written informed consent was obtained from all individual participants included in this study.
Supplementary Material
Acknowledgements
The authors thank the study administration teams in Ticino, Vaud and Zurich for their dedicated support of the study, and the study participants for their valuable contribution to this project.
Contributor Information
Anja Frei, Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland.
Marco Kaufmann, Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland.
Rebecca Amati, Institute of Public Health, Faculty of Biomedical Sciences, Università della Svizzera Italiana, Lugano, Switzerland.
Audrey Butty Dettwiler, Department of Epidemiology and Health Systems, Center for Primary Care and Public Health (Unisanté), Lausanne University, Lausanne, Switzerland.
Viktor von Wyl, Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland; Institute for Implementation Science in Health Care, University Zurich, Zurich, Switzerland.
Anna Maria Annoni, Institute of Public Health, Faculty of Biomedical Sciences, Università della Svizzera Italiana, Lugano, Switzerland.
Julia Vincentini, Department of Epidemiology and Health Systems, Center for Primary Care and Public Health (Unisanté), Lausanne University, Lausanne, Switzerland.
Céline Pellaton, Service of Immunology and Allergy, Lausanne University Hospital, University of Lausanne, Lausanne, Switzerland.
Giuseppe Pantaleo, Service of Immunology and Allergy, Lausanne University Hospital, University of Lausanne, Lausanne, Switzerland.
Jan S Fehr, Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland.
Valérie D'Acremont, Department of Research and Innovation, Center for Primary Care and Public Health (Unisanté), Lausanne University, Lausanne, Switzerland.
Murielle Bochud, Department of Epidemiology and Health Systems, Center for Primary Care and Public Health (Unisanté), Lausanne University, Lausanne, Switzerland.
Emiliano Albanese, Institute of Public Health, Faculty of Biomedical Sciences, Università della Svizzera Italiana, Lugano, Switzerland.
Milo A Puhan, Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland.
Corona Immunitas Research Group:
Emiliano Albanese, Rebecca Amati, Antonio Amendola, Alexia Anagnostopoulos, Daniela Anker, Anna Maria Annoni, Hélène Aschmann, Andrew Azman, Antoine Bal, Tala Ballouz, Hélène Baysson, Kleona Bezani, Annette Blattmann, Patrick Bleich, Murielle Bochud, Patrick Bodenmann, Gaëlle Bryand Rumley, Peter Buttaroni, Audrey Butty Dettwiler, Anne Linda Camerini, Arnaud Chiolero, Patricia Orializ Chocano-Bedoya, Prune Collombet, Laurie Corna, Luca Crivelli, Stéphane Cullati, Valérie D'Acremont, Diana Sofia Da Costa Santos, Agathe Deschamps, Paola D’Ippolito, Anja Domenghino, Richard Dubos, Roxane Dumont, Olivier Duperrex, Julien Dupraz, Malik Egger, Emna El-May, Nacira El Merjani, Nathalie Engler, Adina Mihaela Epure, Lukas Erksam, Sandrine Estoppey, Marta Fadda, Vincent Faivre, Jan Fehr, Andrea Felappi, Maddalena Fiordelli, Antoine Flahault, Luc Fornerod, Cristina Fragoso Corti, Natalie Francioli, Marion Frangville, Irène Frank, Giovanni Franscella, Anja Frei, Marco Geigges, Semira Gonseth Nusslé, Clément Graindorge, Idris Guessous, Erika Harju, Séverine Harnal, Medea Imboden, Emilie Jendly, Ayoung Jeong, Christian R Kahlert, Laurent Kaiser, Laurent Kaufmann, Marco Kaufmann, Dirk Keidel, Simone Kessler, Philipp Kohler, Christine Krähenbühl, Susi Kriemler, Julien Lamour, Sara Levati, Pierre Lescuyer, Andrea Loizeau, Elsa Lorthe, Chantal Luedi, Jean-Luc Magnin, Chantal Martinez, Eric Masserey, Dominik Menges, Gisela Michel, Rosalba Morese, Nicolai Mösli, Natacha Noël, Daniel Henry Paris, Jérôme Pasquier, Francesco Pennacchio, Stefan Pfister, Giovanni Piumatti, Géraldine Poulain, Nicole Probst-Hensch, Caroline Pugin, Milo Puhan, Nick Pullen, Thomas Radtke, Manuela Rasi, Aude Richard, Viviane Richard, Claude-François Robert, Pierre-Yves Rodondi, Nicolas Rodondi, Serena Sabatini, Khadija Samir, Javier Sanchis Zozaya, Virginie Schlüter, Alexia Schmid, Valentine Schneider, Maria Schüpbach, Nathalie Schwab, Claire Semaani, Alexandre Speierer, Amélie Steiner-Dubuis, Silvia Stringhini, Stefano Tancredi, Stéphanie Testini, Julien Thabard, Mauro Tonolla, Nicolas Troillet, Agne Ulyte, Sophie Vassaux, Thomas Vermes, Jennifer Villers, Viktor von Wyl, Cornelia Wagner, Rylana Wenger, Erin West, Ania Wisniak, Melissa Witzig, María-Eugenia Zaballa, Kyra Zens, and Claire Zuppinger
Data availability
De-identified individual participant data underlying the findings and the used codes of this study will be available for researchers. Requests can be made to the Executive Committee of Corona Immunitas [https://zenodo.org/record/7520125] or to the corresponding author.
Supplementary data
Supplementary data are available at IJE online.
Authors contributions
M.A.P., E.A., J.S.F., A.F., M.K., M.B., V.D.A. and R.A. conceptualized and designed this study. A.F., R.A., A.B.D., J.V., M.K., V.V.W. and A.M.A. contributed to the acquisition of the data. M.K. prepared the analytical datasets, conducted the statistical analyses and drafted the figures. C.P. and G.P. contributed to the laboratory analyses. R.A., A.M.A. and .EA. conducted a systematic literature search. M.A.P. and J.S.F. obtained funding. All authors had full access to the data and contributed to the interpretation of the findings. M.A.P., A.F., M.K. and R.A. drafted the first version of the manuscript. All authors critically revised the manuscript for important intellectual content. All authors accept full responsibility for the content of the paper and have seen and approved the final version. A.F., M.K. and R.A. contributed equally to this study. M.A.P. is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding
This study is part of Corona Immunitas research network, coordinated by the Swiss School of Public Health (SSPH+), and funded by fundraising of SSPH+ including funds of the Swiss Federal Office of Public Health and private funders (ethical guidelines for funding stated by SSPH+ were respected), by funds of the Cantons of Switzerland (Vaud, Zurich and Basel) and by institutional funds of the universities. The funding bodies had no influence on the design, conduct, analysis or interpretation of the study, nor on the decision to publish, preparation or revisions of the manuscript.
Conflict of interest
None declared.
References
- 1. World Health Organization. Interim Statement on Hybrid Immunity and Increasing Population Seroprevalence Rates. 2022. https://www.who.int/news/item/01-06-2022-interim-statement-on-hybrid-immunity-and-increasing-population-seroprevalence-rates (3 October 2022, date last accessed).
- 2. Stringhini S, Wisniak A, Piumatti G. et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet 2020;396:313–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vaselli NM, Hungerford D, Shenton B, Khashkhusha A, Cunliffe NA, French N.. The seroprevalence of SARS-CoV-2 during the first wave in Europe 2020: a systematic review. PLoS One. 2021;16:e0250541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pathela P, Crawley A, Weiss D. et al. ; NYC Serosurvey Team. Seroprevalence of Severe Acute Respiratory Syndrome Coronavirus 2 Following the Largest Initial Epidemic Wave in the United States: Findings From New York City, 13 May to 21 July 2020. J Infect Dis 2021;224:196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kalish H, Klumpp-Thomas C, Hunsberger S. et al. Mapping a pandemic: SARS-CoV-2 seropositivity in the United States. medRxiv, doi: 10.1101/2021.01.27.21250570, 31 January 2021, preprint: not-peer reviewed.
- 6. Bergeri I, Whelan M, Ware H. et al. Global epidemiology of SARS-CoV-2 infection: a systematic review and meta-analysis of standardized population-based seroprevalence studies. medRxiv, doi: 10.1101/2021.12.14.21267791, 14 February 2022, preprint: not-peer reviewed.
- 7. Stringhini S, Zaballa M-E, Perez-Saez J. et al. ; Specchio-COVID19 Study Group. Seroprevalence of anti-SARS-CoV-2 antibodies after the second pandemic peak. Lancet Infect Dis 2021;21:600–01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stringhini S, Zaballa M-E, Pullen N. et al. Seroprevalence of anti-SARS-CoV-2 antibodies 6 months into the vaccination campaign in Geneva, Switzerland, 1 June to 7 July 2021. Euro Surveill. 2021;26:2100830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tartof SY, Slezak JM, Puzniak L. et al. Effectiveness and durability of BNT162b2 vaccine against hospital and emergency department admissions due to SARS-CoV-2 omicron sub-lineages BA.1 and BA.2 in a large health system in the USA: a test-negative, case-control study. Lancet Respir Med 2023;11:176–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andrews N, Stowe J, Kirsebom F. et al. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med. 2022;28:831–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Callaway E. Will there be a COVID winter wave? What scientists say. Nature2022;610:239–41. [DOI] [PubMed] [Google Scholar]
- 12. Amati R, Frei A, Kaufmann M. et al. Functional immunity against SARS-CoV-2 in the general population after a booster campaign and the Delta and Omicron waves, Switzerland, March 2022. Euro Surveill 2022;27:2200561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suryawanshi R, Ott M.. SARS-CoV-2 hybrid immunity: silver bullet or silver lining? Nat Rev Immunol 2022;22:591–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hall V, Foulkes S, Insalata F. et al. ; SIREN Study Group. Protection against SARS-CoV-2 after covid-19 vaccination and previous infection. N Engl J Med 2022;386:1207–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goldberg Y, Mandel M, Bar-On YM. et al. Protection and waning of natural and hybrid immunity to SARS-CoV-2. N Engl J Med 2022;386:2201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. West EA, Anker D, Amati R. et al. ; Corona Immunitas Research Group. Corona Immunitas: study protocol of a nationwide program of SARS-CoV-2 seroprevalence and seroepidemiologic studies in Switzerland. Int J Public Health 2020;65:1529–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Speierer A, Chocano-Bedoya PO, Anker D. et al. The corona immunitas digital follow-up ecohort to monitor impacts of the SARS-CoV-2 pandemic in switzerland: study protocol and first results. Int J Public Health 2022;67:1604506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choi Y-W, Tuel A, Eltahir EAB.. On the environmental determinants of COVID-19 seasonality. Geohealth 2021;5:e2021GH000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Viswanath K, Bekalu M, Dhawan D, Pinnamaneni R, Lang J, McLoud R.. Individual and social determinants of COVID-19 vaccine uptake. BMC Public Health 2021;21:818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Larremore DB, Fosdick BK, Bubar KM. et al. Estimating SARS-CoV-2 seroprevalence and epidemiological parameters with uncertainty from serological surveys. eLife 2021;10:e64206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fenwick C, Croxatto A, Coste AT. et al. Changes in SARS-CoV-2 spike versus nucleoprotein antibody responses impact the estimates of infections in population-based seroprevalence studies. J Virol 2021;95:e01828-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG.. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harris PA, Taylor R, Minor BL. et al. ; REDCap Consortium. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fenwick C, Turelli P, Pellaton C. et al. A high-throughput cell- and virus-free assay shows reduced neutralization of SARS-CoV-2 variants by COVID-19 convalescent plasma. Sci Transl Med 2021;13:eabi8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stan Development Team. RStan: the R interface to Stan. R Package Version 2.21.2. 2020. http://mc-stan.org/ (3 October 2022, date last accessed).
- 26. Gelman A, Carpenter B.. Bayesian analysis of tests with unknown specificity and sensitivity. J R Stat Soc Ser C Appl Stat 2020;69:1269–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. R Core Team. R: A Language and Environment for Statistical Computing. 2022. https://www.r-project.org/ (15 March 2023, date last accessed).
- 28. Decru B, Van Elslande J, Steels S. et al. IgG anti-spike antibodies and surrogate neutralizing antibody levels decline faster 3 to 10 months after BNT162b2 vaccination than after SARS-CoV-2 infection in healthcare workers. Front Immunol 2022;13:909910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seery V, Raiden S, Russo C. et al. Antibody response against SARS-CoV-2 variants of concern in children infected with pre-Omicron variants: an observational cohort study. eBioMedicine 2022;83:104230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jahrsdörfer B, Proffen M, Scholz J. et al. BNT162b2 booster vaccination elicits cross-reactive immunity against SARS-CoV-2 variants B.1.1.529 and B.1.617.2 in convalescents of all ages. Front Immunol 2022;13:920210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lavezzo E, Pacenti M, Manuto L. et al. Neutralizing reactivity against SARS-CoV-2 Delta and Omicron variants by vaccination and infection history. Genome Med 2022;14:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wratil PR, Stern M, Priller A. et al. Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat Med 2022;28:496–503. [DOI] [PubMed] [Google Scholar]
- 33. Li J, Wu J, Long Q. et al. Comprehensive humoral and cellular immune responses to SARS-CoV-2 variants in diverse Chinese population. Research (Wash DC) 2022;2022:9873831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gruell H, Vanshylla K, Tober-Lau P. et al. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Nat Med 2022;28:477–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. WHO SAGE. Roadmap for Prioritizing Uses of COVID-19 vaccines: An Approach to Optimize the Global Impact of COVID-19 Vaccines, Based on Public Health Goals, Global and National Equity, and Vaccine Access and Coverage Scenarios. 2022. https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-Vaccines-SAGE-Prioritization-2022.1 (3 October 2022, date last accessed).
- 36. Public Health Authority of Canada. Recommendations on the Use of Bivalent Omicron-containing mRNA COVID-19 Vaccines: NACI Statement. 2022. https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-bivalent-omicron-containing-mrna-covid-19-vaccines.html (3 October 2022, date last accessed).
- 37. Ritchie H, Mathieu E, Rodés-Guirao L. et al. Coronavirus Pandemic (COVID-19). Our World Data. 2020. https://ourworldindata.org/coronavirus (11 October 2022, date last accessed).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified individual participant data underlying the findings and the used codes of this study will be available for researchers. Requests can be made to the Executive Committee of Corona Immunitas [https://zenodo.org/record/7520125] or to the corresponding author.