Abstract
Background
Alcohol consumption is linked to decreased platelet function. Whether this link is dependent on sex or type of beverage remains unclear.
Methods
Cross-sectional data were obtained from the Framingham Heart Study (N = 3427). Alcohol consumption was assessed by using standardized medical history and Harvard semi-quantitative food frequency questionnaires. Five bioassays measured 120 platelet reactivity traits across agonists in whole-blood and platelet-rich plasma samples. Linear mixed-effects models adjusted for age, sex and aspirin use, hypertension, body mass index, cholesterol, high-density lipoprotein, triglycerides, smoking and diabetes evaluated associations between platelet reactivity and alcohol consumption. Beta effects, the regression coefficients that estimate the amount of change in each unit of the predictor variable whereas all other predictor variables remain fixed, for heavy alcohol consumption were compared with effects of aspirin use.
Results
Alcohol consumption was associated with decreased platelet reactivity, with more associations among wine and liquor compared with beer. Many platelet–alcohol associations in the full sample (86%, P < 0.01) had larger effect sizes in females. Lower light transmission aggregometry adenosine diphosphate (1.82 µM) maximum aggregation (P = 2.6E-3, 95% CI = –0.07, –0.02, β = –0.042) and area under the curve (P = 7.7E-3, 95% CI = –0.07, –0.01, β = –0.039) were associated with white wine consumption; however, red wine had no associations with platelet reactivity. The effect of aspirin use was on average 11.3 (±4.0) times greater than that of heavy drinking in our full sample.
Conclusions
We confirm associations between alcohol consumption and decreased platelet reactivity. Effects appeared larger for liquor and wine intake and in our female cohort. Red wine consumption is not associated with lower platelet function, contrasting with prior population studies. Although we report an inhibitory relationship between alcohol intake and platelet function, these effects appear much smaller than that of aspirin use.
Keywords: Platelets, alcohol, ethanol, wine, beer, liquor
Key Messages.
Heavy drinking (≥8 drinks per week for females and ≥15 drinks per week for males) is associated with decreased platelet reactivity in both females and males across a wide range of platelet traits.
Habitual wine and liquor consumption showed the most associations with the largest effects on decreased platelet reactivity, particularly in our female sample.
Alcohol consumption is unlikely to be of great utility in thrombosis prevention since the effect of aspirin associations are greater on the same platelet reactivity traits compared with heavy alcohol consumption.
Our research does not suggest that increased alcohol consumption is a holistic approach to cardiovascular risk prevention, especially when considering health risks related to heavy alcohol consumption.
Introduction
The relationship between cardiovascular disease (CVD) and alcohol consumption is intricate and multifactorial. Both epidemiological and experimental data seem to suggest that light-to-moderate alcohol consumption is associated with cardioprotective benefits for individuals who are at risk of atherothrombotic-related diseases such as myocardial infarction (MI), peripheral artery disease and cardiac death.1–5 Comparably, studies have demonstrated a U- or J-shaped correlation between cardiovascular health and alcohol intake where heavy or binge drinking has been noted to increase the risk of ischaemic strokes, fatal coronary heart disease and related sudden deaths.6–10
Whereas some of the physiological details underlying this relationship have been elucidated, others remain inadequately understood. For example, increases in high-density lipoprotein (HDL) levels, concentrations of total fatty acids and blood pressure, along with decreases in low-density lipoprotein levels in response to alcohol consumption, have been common explanations used to describe subsequent reductions in CVD risk. Yet, the question remains as to whether these biomarkers are causally related to the observed cardioprotective outcomes.2,11,12,13 Furthermore, it is speculated that the beneficial effects of alcohol consumption are attributable to the phenolic antioxidant compounds found in certain alcoholic beverages that biologically neutralize reactive oxygen species.14 The regular consumption of red wine has been central to these hypotheses; however, these results are unresolved due to the speculated bioavailability of wine flavonoids and polyphenols.15,16
To understand how these hemostatic associations are mediated, prior studies have investigated the relationship between alcohol consumption and platelet aggregate formation, and other measurements of platelet reactivity. Hyperreactive platelet function is an undesirable health condition that increases the risk of thrombotic morbidity and mortality, particularly in older adults.17 Results suggest that generally alcohol consumption decreases platelet reactivity and alters platelet function.11,18,19,20,21,22,23 In in vivo experiments, moderate concentrations of ethanol act as inhibitors of thromboxane (Tx) A2 formation and secondary platelet aggregation to agonists such as adenosine diphosphate (ADP), epinephrine and collagen.24,25 In cases of binge drinking and alcohol withdrawals, Hillbom et al.26 showed increased TxA2 levels and hyperaggregability that was speculated to cause increases in ischaemic stroke. Previous epidemiological and experimental studies have generally reported congruent findings for the association between alcohol consumption and platelet reactivity.19,27,28 However, these studies did not investigate the possibility that associations are dependent on sex and alcoholic beverage type (wine, liquor or beer) in large well-powered samples, which further limits the conclusions that can be drawn from the results.
To further explore the relationship between alcohol consumption and platelet reactivity, we investigated five separate platelet function assays and three alcoholic beverage types (wine, liquor and beer) within both males and females enrolled in the Framingham Heart Study (FHS). The effect sizes of our attenuated platelet function results were then compared with the effect of aspirin in hopes of elucidating the broader clinical relevance of alcohol consumption as a prevention strategy for CVD through platelet-mediated mechanisms.
Methods
Study sample
The FHS is a longitudinal cardiovascular cohort study based on community members from Framingham, MA. Cross-sectional data were collected from participants enrolled in the Generation 3 (Gen 3), New Offspring Spouse (NOS) and ethnically diverse OMNI 2 cohorts during the third examination cycle (2016–19). Only two individuals were excluded from the analysis due to missing alcohol consumption information, resulting in 3427 participants in our study (Table 1). Recruitment and epidemiological details of the FHS cohorts have been previously outlined.29
Table 1.
Demographic and health characteristics of participants in the Framingham Heart Study Exam 3
| Characteristic | Drinking category |
|||||
|---|---|---|---|---|---|---|
| 0 | >0 to <2 vs 0 | ≥2 to <8 vs 0 | ≥8 to <14 vs 0 | ≥15 vs 0 | P | |
| Number of participants | 803 | 800 | 907 | 657 | 260 | – |
| Male | 42.2% | 33.6% | 43.2% | 59.1% | 76.2% | 3.12E-41 |
| Age (years) | 56.5 ± 9.5 | 54.0 ± 9.1 | 53.0 ± 9.0 | 54.1 ± 8.9 | 55.0 ± 9.9 | 3.25E-7 |
| Average drinks/week | 0 | 1.0 ± 0.6 | 4.3 ± 1.3 | 9.6 ± 2.5 | 23.4 ± 10.7 | <5E-324 |
| Current smoker | 7.8% | 4.1% | 5.0% | 7.8% | 13.8% | 3.92E-7 |
| BMI (kg/m2) | 29.7 ± 6.5 | 28.9 ± 6.2 | 27.8 ± 5.5 | 27.9 ± 5.1 | 28.3 ± 4.7 | 3.96E-11 |
| Diabetes | 5.7% | 2.5% | 1.0% | 2.1% | 1.5% | 8.4E-14 |
| Hypertension | 56.1% | 48.8% | 47.5% | 62.7% | 65.0% | 6.29E-6 |
| Total cholesterol level (mg/dL) | 182.4 ± 37.3 | 185.7 ± 34.2 | 191.9 ± 37.6 | 195.2 ± 35.5 | 197.5 ± 35.5 | 5.93E-15 |
| HDL-C level (mg/dL) | 53.5 ± 16.7 | 58.6 ± 18.1 | 62.0 ± 19.8 | 63.3 ± 21.6 | 62.3 ± 19.0 | 4.53E-25 |
| Triglyceride level (mg/dL) | 119.0 ± 73.2 | 109.1 ± 76.0 | 105.0 ± 70.3 | 115.7 ± 90.2 | 126.9 ± 104.6 | 6.38E-4 |
BMI, body mass index; HDL-C, high-density lipoprotein cholesterol.
Blood collection and platelet assays
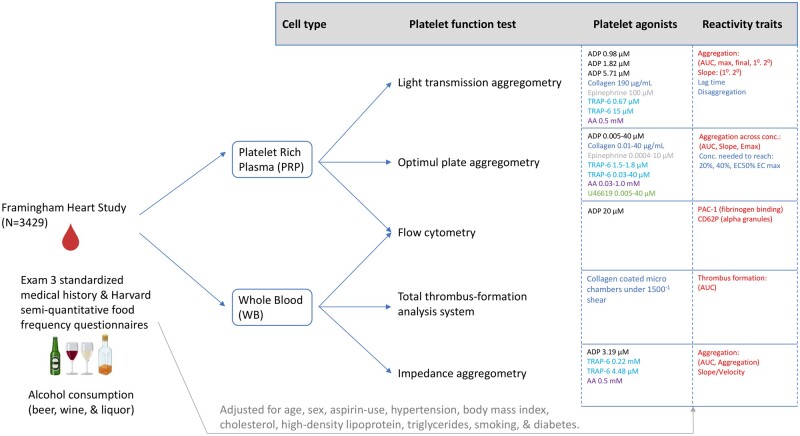
Participant blood was drawn in resting supine position the morning subsequent to an overnight fasting period. Five bioassays including light transmission aggregometry (LTA) using an eight-channel aggregometer (Bio/Data, Horsham, PA), Optimul 96-well aggregometry (Chan 2011; Chan 2012), flow cytometry using an Accuri C6 (BD Biosciences, Santa Cruz, CA), Multiplate® whole-blood impedance aggregometry (MP) (Roche Diagnostics) and Total-Thrombus Formation Assay System (T-TAS) (Zacros, Tokyo, Japan) were used to measure platelet reactivity traits across several agonists in both platelet-rich plasma isolated from 4.5-mL sodium citrate (1:9) tubes (BD; San Jose, CA) and whole-blood samples from 3.0-mL hirudin anti-coagulated tubes (Diapharma; West Chester, OH). Detailed platelet assay parameters are previously described30 and are shown in Figure 1. Additional assay-specific information, including platelet reactivity positive beta interpretations, can be found in Supplementary Table S1 (available as Supplementary data at IJE online).
Figure 1.
Framingham Heart Study Exam 3 overview and design adapted from Grech et al.30 Primary platelet agonists and concentrations are organized for each of the five separate platelet assays conducted. Platelet reactivity traits measure increased reactivity in terms of increased aggregation, PAC-1 (activated GP IIb/IIIa) and CD62P (platelet surface P-selectin) stimulation and thrombus formation, as well as decreased reactivity in terms of lag time, disaggregation and concentration needed to meet % aggregation
Alcohol intake measurement
Quantitative consumption of alcohol was measured through a standardized medical history questionnaire administered by a FHS provider at the time of blood collection. Exam questions assessed quantitative weekly and monthly alcohol consumption by alcohol type for wine (4 oz), liquor (1 oz) and beer (12 oz). Drink units measured in the FHS questionnaire differed in grams of alcohol per drink; therefore, we standardized grams of alcohol per drink to ∼14 g. Total alcohol consumption, measured in drinks per week, was categorized into five drinking categories for males and females (0 drinks, 0 to <2 drinks, ≥2 to <8 drinks, ≥8 to <15 drinks, ≥15 drinks) similarly to prior studies2,19 and in accordance to the Centers for Disease Control and Prevention (CDC), which defines heavy alcohol use as ≥8 drinks per week for a female or ≥15 drinks per week for a male.31 Individual white and red wine consumption was measured using the Harvard semi-quantitative food frequency questionnaire (FFQ). Prior to the exam, FFQs were mailed to participants with instructions on how to accurately self-fill the questionnaire. Missing or incomplete questionnaires were not included (n = 621), resulting in a smaller sample size of 2806 FFQs for analysis. The FFQ contains a checklist of foods and beverages with a standard serving size and a response section for participants to report how often each item was consumed annually.32 Alcoholic beverage serving sizes in the FFQ were defined as a 5-oz glass of wine, 12-oz bottle of beer and 1.5-oz shot of liquor. Participants were able to answer their individual drinking pattern from nine frequency categories (Never or less than once per month, 1–3 drinks per month, 1 drink per week, 2–4 drinks per week, 5–6 drinks per week, 1 drink per day, 2–3 drinks per day, 4–5 drinks per day, >6 drinks per day). FFQ drinking range categories were rounded into narrow weekly categories for analyses: 0.47 (1–3 drinks per month), 3 (2–4 drinks per week), 5.5 (5–6 drinks per week), 7 (1 drink per day), 17.5 (2–3 drinks per day), 31.5 (4–5 drinks per day) and 42 (>6 drinks per day) drinks per week.
Statistical analyses
Five main analyses were conducted. The first and second assessed categorical and quantitative alcohol consumption, respectively. In our categorical alcohol consumption analysis, a global P-value based on the Wald chi-square statistic was reported for each trait where 0 drinks per week or no alcohol consumption was used as the reference group. Sex interactions among quantitative alcohol consumption were investigated in our third analysis. Our fourth analysis focused on alcohol intake in individuals who only consumed either red or white wine. Lastly, we performed an analysis to infer whether the effect size of aspirin use was larger than the effect size of the heavy drinking indicator (≥8 and ≥15 drinks per week for females and males, respectively), where the drinking categories were further dichotomized. One-sided statistical testing was used to derive P-values for the aspirin use versus heavy drinking analysis whereas two-sided statistical tests were used to derive all other P-values. Beta regression coefficients estimate the amount of change in each unit of the predictor variable whereas all other predictor variables remain fixed. All analyses were conducted using the R language and environment.33P-values of <5E-324 were reported as the 5E-324, as this is the minimum value computed by R. We performed inverse normal transformations to all platelet reactivity traits. Transformed traits were then analysed using a linear mixed-effects model implemented in the GMMAT (Generalized Linear Mixed Model Association Tests) R package (https://cran.r-project.org/web/packages/GMMAT/index.html) that accounted for familial correlation and was adjusted for age, sex, aspirin use, hypertension (HTN), body mass index (BMI), total cholesterol, HDL levels, triglycerides, smoking and diabetes. We found that the LTA area under the curve (AUC) calculations were not comparable with instrument built-in software (PAP-8E, BioData, Inc.) for uncommon occurrences in which data collection varied from the standard 6-min duration. This was addressed by recomputing the AUC from the raw data aggregation percentages (measured every 0.5 s), rescaling between 0 and 1, and dividing the output by the total duration of the assay after agonist injection, in seconds, resulting in a standardized AUC with values ranging from 0 to 1.
flowAI
To minimize the impact of abnormal events due mainly to pressure fluctuations during data acquisition and to exclude events affected by technical artefacts, we performed quality control on all compensated Flow Cytometric Standard (fcs) files using the flowAI package (version1.12.7)34 on FlowJo (version 10.7.1).35 The flowAI package allows the screening, selection and exclusion of bad-quality events, based on the evaluation of flow rate abnormalities, parameter instability and omission of fluorescence signals or events that fall out of the specific dynamic range of each fluorescence channel.34
Results
Study sample
Overall characteristics of health for participants in the FHS cohort were categorized by sex and categorical weekly drinking quantity (Table 1). Our population sample had a mean age of 54.5 years and was 53.4% female. Of the 3427 participants in our study, 1247 male participants (78.6%) and 1377 female participants (74.8%) reported weekly alcohol consumption (Table 2). Sample sizes varied between platelet assays due to differences in blood collection availability throughout Exam 3 and can be found in Supplementary Tables S2–S8 (available as Supplementary data at IJE online).
Table 2.
Sample sizes and quantitative consumption (mean ± standard deviation) for each alcoholic beverage type consumed by participants in the Framingham Heart Study Generation 3/New Offspring Spouse/OMNI 2 Exam 3a
| All alcohol consumption |
Any wine drinkers |
Only wine drinkers |
Any beer drinkers |
Only beer drinkers |
Any liquor drinkers |
Only liquor drinkers |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | ||
| Sample (n) | 1247 | 1377 | 739 | 1125 | 58 | 393 | 1052 | 610 | 247 | 91 | 706 | 667 | 63 | 101 | |
| Age (years) | 54.6 ± 9.3 | 54.2 ± 9.3 | 54.6 ± 9.8 | 53.7 ± 9.1 | 57.5 ± 8.3 | 55.8 ± 9.2 | 53.7 ± 9.2 | 51.9 ± 8.8 | 54.0 ± 8.4 | 51.2 ± 8.8 | 53.8 ± 9.5 | 53.1 ± 8.6 | 53.7 ± 9.1 | 54.7 ± 9.2 | |
| Drinks/week | 8.6 ± 9.1 | 4.8 ± 5.0 | 9.1 ± 8.2 | 5.1 ± 4.8 | 3.3 ± 3.7 | 3.7 ± 4.4 | 9.1 ± 9.1 | 5.8 ± 5.4 | 7.6 ± 11.2 | 3.7 ± 6.0 | 10.0 ± 9.1 | 5.4 ± 5.0 | 5.1 ± 5.8 | 2.0 ± 3.4 | |
| Beers/week | 4.5 ± 6.9 | 1.0 ± 2.7 | 3.5 ± 4.5 | 0.7 ± 1.8 | 0 | 0 | 5.3 ± 7.2 | 2.2 ± 3.7 | 7.6 ± 11.2 | 3.7 ± 6.0 | 3.9 ± 5.2 | 0.9 ± 2.5 | 0 | 0 | |
| Wine/week | 2.2 ± 4.0 | 3.0 ± 4.0 | 3.7 ± 4.6 | 3.7 ± 4.1 | 3.3 ± 3.7 | 3.7 ± 4.4 | 2.0 ± 3.7 | 2.9 ± 3.9 | 0 | 0 | 2.6 ± 4.5 | 2.8 ± 3.7 | 0 | 0 | |
| Red wine/weekb | 1.5 ± 1.6 | 1.5 ± 1.6 | 2.3 ± 1.6 | 1.8 ± 1.6 | 2.2 ± 1.7 | 1.5 ± 1.7 | 1.5 ± 1.6 | 1.6 ± 1.6 | 0 | 0 | 1.6 ± 1.7 | 1.5 ± 1.6 | 0 | 0 | |
| White wine/weekb | 0.8 ± 1.4 | 1.5 ± 1.7 | 1.2 ± 1.6 | 1.8 ± 1.7 | 1.1 ± 1.7 | 1.9 ± 1.8 | 0.7 ± 1.3 | 1.4 ± 1.6 | 0 | 0 | 1.0 ± 1.6 | 1.5 ± 1.6 | 0 | 0 | |
| Liquor/week | 2.0 ± 4.4 | 0.8 ± 1.8 | 2.0 ± 4.5 | 0.7 ± 1.5 | 0 | 0 | 1.7 ± 3.7 | 0.8 ± 1.7 | 0 | 0 | 3.4 ± 5.4 | 1.6 ± 2.3 | 5.1 ± 5.8 | 2.0 ± 3.4 | |
Generation 3 represents the third generation of the Framingham Heart Study. The New Offspring Spouse cohort represents the spouses of offspring participants who were not otherwise enrolled and had at least two biological children in the Generation 3 cohort. OMNI 2 represents a second generation of the ethnically diverse cohort at the Framingham Heart Study. All three cohorts were seen during the third exam period (2016–19).
All reported values in the table are based on questions at the time of the exam, except for red and white wine consumption, which were assessed by using mail-out food frequency questionnaires and reflect varying time periods from the platelet data collection.
Alcohol consumption in FHS
In the Exam 3 FHS sample, female participants generally consumed less alcohol compared with male participants, resulting in higher percentages of male drinkers in the top drinking categories (≥8 to <15 drinks per week: 59.1%, ≥15 drinks: 76.2%). A more quantitative look at the drinking patterns in the FHS sample stratified by sex and type of alcoholic beverage agreed with the drinking patterns of the categorical drinking groups and revealed that after excluding non-drinkers, male participants consumed an average of 8.5 drinks per week whereas female participants consumed an average of 4.8 drinks per week (P = 1.69E-41). Generally, males consumed more beer (4.5 drinks per week) compared with wine (2.2 drinks per week) and liquor (2.0 drinks per week) beverages and females consumed more wine (3.0 drinks per week) compared with beer (1.0 drinks per week) and liquor (0.8 drinks per week) beverages. Preferences in beverage type between our male and female cohorts were most distinct in individuals who only consumed beer or wine from the three beverage types. Of the individuals who only drank beer (n = 338), 73% were male; moreover, of the individuals who only drank wine (n = 451), 87% were female. Sample sizes and quantitative intake details for each alcoholic beverage type consumed by participants can be found in Table 2.
Alcohol consumption and platelet reactivity traits
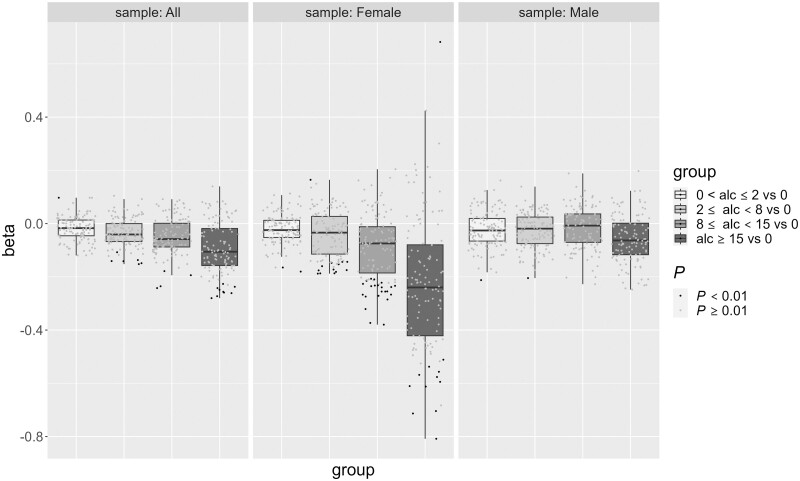
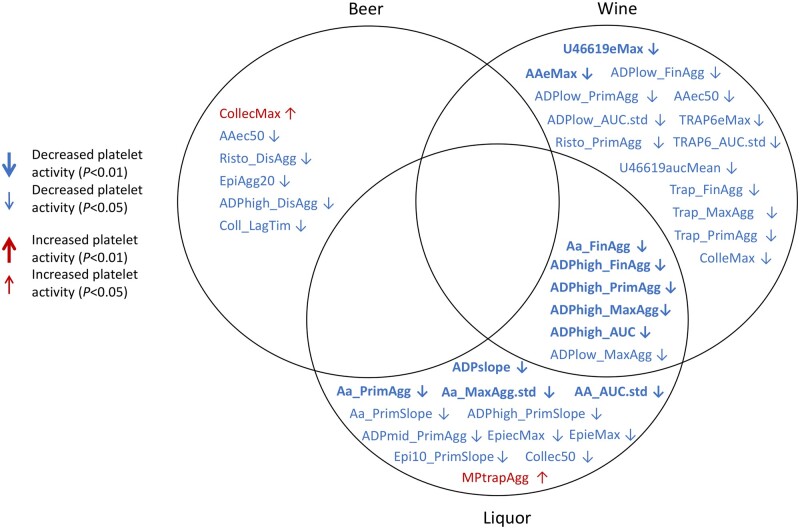
Our categorical alcohol consumption analysis revealed 20 platelet reactivity traits associated with alcohol consumption (P < 0.01). The mild drinkers (0 to <2 drinks per week) category revealed a single increased platelet reactivity association for ADP (middle concentration, 1.82 µM) secondary wave % aggregation in LTA (P = 8.27E-03, 95% CI = 0.03, 0.17, β = 0.097) compared with the reference group (0 drinks per week of alcohol consumption). We observed six, four and nine decreased platelet reactivity traits associated with the ≥2 to <8, ≥8 to <15 and ≥15 drinks per week alcohol consumption categories, respectively (Supplementary Table S2, available as Supplementary data at IJE online). Optimul trait U46619 (TxA2 receptor mimetic) maximal % aggregation was the only trait that showed associations with the top three drinking categories with a P-value of <0.01. The heavy drinking indicator primarily showed associations with decreased LTA AUC (P = 1.1E-3, 95% CI = –0.44, –0.11, β = –0.273), maximal (P = 8.7E-4, 95% CI = –0.44, –0.12, β = –0.280), final (P = 2.5E-3, 95% CI = –0.41, –0.09, β = –0.249) and primary wave (P = 7.5E-3, 95% CI = –0.40, –0.08, β = –0.227) % aggregations in response to ADP stimulation (0.95 µM), as well as Optimul traits U46619 concentration needed to reach 20% aggregation (P = 6.0E-3, 95% CI = –0.44, –0.07, β = –0.256), 40% aggregation (P = 8.2E-3, 95% CI = –0.42, –0.06, β = –0.242), maximal % aggregation (P = 4.5E-3, 95% CI = –0.44, –0.08, β = –0.259) and AUC (P = 4.1E-3, 95% CI = –0.44, –0.08, β = –0.261) across the concentration range (0.005, 0.02, 0.10, 0.44, 1.98, 8.89 and 40 µM). Patterns of associations between platelet reactivity traits and the heavier drinking categories suggests an inhibitory dose–response relationship between alcohol consumption and platelets. For the same traits showing a P < 0.01 in both ≥8 to <15 vs 0, and ≥15 vs 0 drinks per week analyses, the magnitude of the effect on the platelet trait was on average greater in the heaviest drinking group (Figure 2). Correspondingly, our quantitative analysis for total alcohol consumption revealed seven traits with decreased platelet reactivity in response to all concentrations of ADP (low: 0.95 µM, mid: 1.82 µM and high: 5.71 µM) and U46619 agonists associated with total alcohol consumption amount (P < 0.01 in all samples). The top 10 strongest platelet reactivity trait associations for quantitative analysis of each beverage consumption type are summarized in Table 3, together with sex-stratified and sex-interaction results from separate analyses. Notably, of the three beverage types, we observed that a majority of the platelet reactivity traits with a P-value of <0.01 were associated with wine and liquor consumption and not beer in the full sample, and especially in our female sample (Figure 3 and Supplementary Table S3, available as Supplementary data at IJE online). In addition, we show that the largest negative platelet effects are among liquor consumption (seven traits P < 0.01, mean β = –0.022 ± 0.002) compared with wine (six traits P < 0.01, mean β = –0.015 ± 0.002). The majority of associations (86% at P < 0.01) tended to have larger effect sizes in our female sample. Sex–total alcohol consumption interaction tests revealed 25 platelet traits associated with the interaction (6 traits P < 0.001, 19 traits P < 0.01) (Supplementary Table S4, available as Supplementary data at IJE online). Of these 25 platelet traits, only two were associated (P < 0.01) with total alcohol consumption in the overall sample. When looking at distinguishing between the three alcohol types, sex-interaction tests showed similar associations among all three alcohol types indicating effect size differences between males and females. Of the platelet traits associated with the specific beverage type of either beer (0 traits P < 0.01), liquor (7 traits P < 0.01) or wine (5 traits P < 0.01) consumption, we found no interactions with sex. It is possible that the overall pattern of decreased platelet reactivity traits with a P-value of <0.01 among our female sample may partially reflect the observed preference for wine consumption.
Figure 2.
Box plot with jittered data points assessing categorical alcohol consumption in the full, female and male samples. 120 distinct platelet traits are plotted by alcohol consumption group (x-axis) and effect size (y-axis). Positive beta effects that indicate decreased platelet reactivity (e.g. Aa_LagTim, AAagg20, AAec50, etc.; full table in Supplementary Table S1, available as Supplementary data at IJE online) are flipped to represent the correct effect of the trait. Traits are adjusted for age, sex, aspirin use, hypertension, body mass index, cholesterol, high-density lipoprotein, triglycerides, smoking and diabetes. Jittered data points representing a platelet trait with a P-value of <0.01 are coloured in black, whereas platelet traits with a P-value of >0.01 are represented by a grey-coloured data point
Table 3.
Top 10 platelet reactivity traits associated with any beer, liquor and wine consumption along with their sex-stratified and sex-interaction results (separate analyses)
| Total |
Male |
Female |
Sex interaction |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Trait | Assaya | Beta | SE | P | Beta | SE | P | Beta | SE | P | Beta | SE | P |
| Alcohol | ADPlow_MaxAgg | LTA | –0.0088 | 0.0028 | 0.0019 | –0.0071 | 0.0033 | 0.0327 | –0.0145 | 0.0059 | 0.0140 | –0.0882 | 0.0375 | 0.0185 |
| Alcohol | ADPlow_AUC.std | LTA | –0.0087 | 0.0028 | 0.0021 | –0.0071 | 0.0031 | 0.0234 | –0.0140 | 0.0062 | 0.0236 | –0.1198 | 0.0374 | 0.0014 |
| Alcohol | ADPlow_FinAgg | LTA | –0.0084 | 0.0028 | 0.0025 | –0.0063 | 0.0030 | 0.0359 | –0.0144 | 0.0061 | 0.0183 | –0.1190 | 0.0366 | 0.0011 |
| Alcohol | ADPmid_PrimAgg | LTA | –0.0077 | 0.0028 | 0.0052 | –0.0072 | 0.0033 | 0.0265 | –0.0088 | 0.0057 | 0.1195 | –0.0568 | 0.0361 | 0.1150 |
| Alcohol | U46619eMax | Optimul | –0.0086 | 0.0031 | 0.0059 | –0.0044 | 0.0038 | 0.2574 | –0.0216 | 0.0059 | 0.0003 | –0.0631 | 0.0403 | 0.1176 |
| Alcohol | ADPhigh_AUC.std | LTA | –0.0072 | 0.0026 | 0.0062 | –0.0030 | 0.0032 | 0.3511 | –0.0209 | 0.0053 | 0.0001 | –0.0444 | 0.0342 | 0.1946 |
| Alcohol | ADPmid_MaxAgg | LTA | –0.0074 | 0.0027 | 0.0066 | –0.0074 | 0.0033 | 0.0272 | –0.0065 | 0.0053 | 0.2240 | –0.0304 | 0.0355 | 0.3909 |
| Alcohol | ADPmid_AUC.std | LTA | –0.0067 | 0.0027 | 0.0124 | –0.0065 | 0.0032 | 0.0396 | –0.0064 | 0.0055 | 0.2482 | –0.0547 | 0.0351 | 0.1189 |
| Alcohol | ADPhigh_MaxAgg | LTA | –0.0065 | 0.0026 | 0.0126 | –0.0023 | 0.0031 | 0.4577 | –0.0204 | 0.0052 | 0.0001 | –0.0425 | 0.0335 | 0.2044 |
| Alcohol | ADPhigh_FinAgg | LTA | –0.0060 | 0.0025 | 0.0163 | –0.0019 | 0.0030 | 0.5214 | –0.0195 | 0.0050 | 0.0001 | –0.0414 | 0.0323 | 0.1991 |
| Beer | CollecMax | Optimul | –0.0064 | 0.0027 | 0.0180 | –0.0054 | 0.0029 | 0.0623 | –0.0098 | 0.0071 | 0.1664 | –0.0013 | 0.0275 | 0.9632 |
| Beer | Risto_DisAgg | LTA | 0.0067 | 0.0030 | 0.0245 | 0.0066 | 0.0033 | 0.0433 | 0.0043 | 0.0080 | 0.5904 | 0.0760 | 0.0310 | 0.0141 |
| Beer | AAec50 | Optimul | 0.0084 | 0.0039 | 0.0297 | 0.0015 | 0.0041 | 0.7187 | 0.0417 | 0.0100 | 0.0000 | 0.0236 | 0.0384 | 0.5389 |
| Beer | ADPhigh_DisAgg | LTA | 0.0041 | 0.0019 | 0.0309 | 0.0024 | 0.0022 | 0.2930 | 0.0124 | 0.0048 | 0.0090 | 0.0338 | 0.0199 | 0.0894 |
| Beer | EpiAgg20 | Optimul | 0.0085 | 0.0040 | 0.0322 | 0.0051 | 0.0044 | 0.2457 | 0.0297 | 0.0099 | 0.0027 | 0.0721 | 0.0402 | 0.0727 |
| Beer | Coll_LagTim | LTA | 0.0062 | 0.0032 | 0.0497 | 0.0030 | 0.0036 | 0.4047 | 0.0248 | 0.0082 | 0.0024 | 0.0247 | 0.0328 | 0.4518 |
| Beer | ADPlow_AUC.std | LTA | –0.0067 | 0.0035 | 0.0531 | –0.0047 | 0.0037 | 0.1972 | –0.0132 | 0.0098 | 0.1791 | –0.1235 | 0.0373 | 0.0009 |
| Beer | ADPlow_FinAgg | LTA | –0.0065 | 0.0034 | 0.0571 | –0.0041 | 0.0035 | 0.2442 | –0.0127 | 0.0097 | 0.1899 | –0.1221 | 0.0365 | 0.0008 |
| Beer | U46619Agg20 | Optimul | 0.0079 | 0.0042 | 0.0579 | 0.0064 | 0.0047 | 0.1705 | 0.0178 | 0.0101 | 0.0782 | 0.1382 | 0.0410 | 0.0008 |
| Beer | ADPmid_MaxAgg | LTA | –0.0064 | 0.0034 | 0.0579 | –0.0061 | 0.0039 | 0.1189 | –0.0015 | 0.0086 | 0.8639 | –0.0331 | 0.0354 | 0.3509 |
| Liquor | Aa_FinAgg | LTA | –0.0213 | 0.0063 | 0.0008 | –0.0190 | 0.0066 | 0.0039 | –0.0459 | 0.0185 | 0.0130 | –0.0102 | 0.0269 | 0.7035 |
| Liquor | Aa_MaxAgg | LTA | –0.0203 | 0.0064 | 0.0014 | –0.0178 | 0.0067 | 0.0077 | –0.0466 | 0.0186 | 0.0121 | –0.0161 | 0.0271 | 0.5530 |
| Liquor | Aa_PrimAgg | LTA | –0.0203 | 0.0064 | 0.0014 | –0.0178 | 0.0067 | 0.0077 | –0.0466 | 0.0186 | 0.0121 | –0.0161 | 0.0271 | 0.5530 |
| Liquor | AA_AUC.std | LTA | –0.0197 | 0.0064 | 0.0022 | –0.0183 | 0.0067 | 0.0063 | –0.0409 | 0.0188 | 0.0294 | –0.0115 | 0.0274 | 0.6742 |
| Liquor | ADPhigh_AUC.std | LTA | –0.0234 | 0.0080 | 0.0035 | –0.0227 | 0.0090 | 0.0114 | –0.0367 | 0.0222 | 0.0987 | –0.0452 | 0.0342 | 0.1862 |
| Liquor | ADPslope | Optimul | –0.0268 | 0.0094 | 0.0044 | –0.0279 | 0.0099 | 0.0049 | –0.0147 | 0.0282 | 0.6012 | 0.0459 | 0.0411 | 0.2635 |
| Liquor | ADPhigh_MaxAgg | LTA | –0.0222 | 0.0079 | 0.0048 | –0.0217 | 0.0088 | 0.0137 | –0.0354 | 0.0218 | 0.1042 | –0.0430 | 0.0335 | 0.1995 |
| Liquor | ADPmid_PrimAgg | LTA | –0.0215 | 0.0084 | 0.0106 | –0.0248 | 0.0093 | 0.0075 | –0.0130 | 0.0233 | 0.5760 | –0.0587 | 0.0360 | 0.1034 |
| Liquor | ADPhigh_PrimSlope | LTA | –0.0225 | 0.0089 | 0.0110 | –0.0215 | 0.0099 | 0.0307 | –0.0376 | 0.0245 | 0.1251 | –0.0324 | 0.0377 | 0.3908 |
| Liquor | ADPhigh_FinAgg | LTA | –0.0190 | 0.0076 | 0.0120 | –0.0179 | 0.0084 | 0.0325 | –0.0351 | 0.0212 | 0.0981 | –0.0419 | 0.0323 | 0.1939 |
| Wine | U46619eMax | Optimul | –0.0182 | 0.0062 | 0.0032 | –0.0166 | 0.0099 | 0.0925 | –0.0187 | 0.0079 | 0.0179 | –0.0674 | 0.0403 | 0.0942 |
| Wine | ADPhigh_MaxAgg | LTA | –0.0153 | 0.0052 | 0.0036 | –0.0128 | 0.0082 | 0.1175 | –0.0172 | 0.0068 | 0.0118 | –0.0466 | 0.0335 | 0.1633 |
| Wine | AAeMax | Optimul | –0.0141 | 0.0051 | 0.0059 | –0.0125 | 0.0082 | 0.1290 | –0.0168 | 0.0065 | 0.0101 | 0.0014 | 0.0333 | 0.9658 |
| Wine | ADPhigh_FinAgg | LTA | –0.0138 | 0.0051 | 0.0065 | –0.0120 | 0.0077 | 0.1201 | –0.0154 | 0.0067 | 0.0209 | –0.0455 | 0.0322 | 0.1579 |
| Wine | ADPhigh_AUC.std | LTA | –0.0145 | 0.0054 | 0.0066 | –0.0119 | 0.0083 | 0.1523 | –0.0169 | 0.0070 | 0.0153 | –0.0492 | 0.0342 | 0.1499 |
| Wine | ADPhigh_PrimAgg | LTA | –0.0139 | 0.0054 | 0.0099 | –0.0124 | 0.0084 | 0.1391 | –0.0154 | 0.0070 | 0.0287 | –0.0507 | 0.0344 | 0.1410 |
| Wine | TRAP6eMax | Optimul | –0.0170 | 0.0067 | 0.0110 | –0.0037 | 0.0101 | 0.7153 | –0.0293 | 0.0090 | 0.0011 | –0.0571 | 0.0432 | 0.1860 |
| Wine | Trap_MaxAgg | LTA | –0.0145 | 0.0060 | 0.0156 | –0.0063 | 0.0094 | 0.5019 | –0.0201 | 0.0078 | 0.0098 | –0.0178 | 0.0382 | 0.6413 |
| Wine | Risto_PrimAgg | LTA | –0.0141 | 0.0059 | 0.0181 | –0.0083 | 0.0093 | 0.3725 | –0.0189 | 0.0077 | 0.0140 | –0.0252 | 0.0380 | 0.5068 |
| Wine | ADPlow_MaxAgg | LTA | –0.0132 | 0.0058 | 0.0231 | –0.0133 | 0.0087 | 0.1275 | –0.0159 | 0.0079 | 0.0442 | –0.0940 | 0.0374 | 0.0119 |
CollecMax, AAec50, EpiAgg20, Coll_LagTim and U46619Agg20 positive beta values indicate decreased platelet reactivity (ST1).
LTA stands for light transmission aggregometry, the gold-standard platelet function test that determines platelet aggregation percentages in platelet-rich plasma through light transmittance in response to various agonists. Optimul is a custom 96-well plate-based absorbance assay that measures platelet aggregation across various agonists and concentrations in platelet-rich plasma.
Figure 3.
Associations of quantitative alcohol consumption (drinks per week) and platelet reactivity traits (P < 0.05) adjusted for age, sex, aspirin use, hypertension, body mass index, cholesterol, high-density lipoprotein, triglycerides, smoking and diabetes. Platelet reactivity traits are organized by alcoholic beverage type (wine/liquor/beer) consumption among the total sample of participants enrolled in the Framingham Heart Study with a complete standardized medical history questionnaire (N = 3427). Bolded traits had alcohol-platelet associations of P < 0.01, whereas non-bolded traits had associations of P < 0.05. Traits with a pointed-down arrow (↓) or a pointed-up arrow (↑) indicate decreased or increased platelet reactivity associated with the beverage type consumed, respectively. Platelet trait abbreviations reflect the following assays: (i) Optimul platelet-rich plasma absorbance assays reflect values across a range of agonist concentrations and are labelled with their corresponding agonist and either ec50, Agg20, aucMean, slope (and eMax, which reflects maximal aggregation at any agonist concentration; and ecMax, the concentration where eMax was reached); (ii) MP (Multiplate Impedance aggregometry in hirudin anti-coagulated blood)—AUC, Agg or Velocity/Slope for ADP (3.19 uM), TRAP-6 (670 uM); (iii) Total-Thrombus Formation Analysis System (T-TAS) collagen-coated shear stress platelet thrombus formation: occlusion start time; (iv) light transmission aggregometry (LTA) with a Bio/Data 8 channel aggregometer—all derived traits from curves reflected with ‘_’ in the trait name including lag time, primary and secondary slope, final and maximal % aggregation, % disaggregation and total AUC. Agonist concentrations in LTA include arachidonic acid (500 µM/mL), collagen (190 µM/mL), epinephrine (100 µM), ristocetin (1.5 mg/mL), TRAP-6 (216 µM) and ADP_low (0.98 µM), ADP_mid (1.82 µM) and ADP_high (5.71 µM)
Red wine vs white wine drinking
Total (n = 1663), red (n = 1377) and white (n = 1147) wine consumption data were utilized from the FFQ data sets resulting in smaller overall sample sizes for analysis. To assess a Pearson correlation, wine consumption data collected from the FFQs were fit to wine consumption data collected from the FHS exam questions, resulting in an R-value of 0.8142 (P < 1E-5) (Supplementary Table S5, available as Supplementary data at IJE online). Among individuals who consumed red wine only (n = 72) in this subsample analysis, we observed no associations with platelet reactivity (Supplementary Table S6, available as Supplementary data at IJE online). On the other hand, our analysis revealed two platelet reactivity traits (P < 0.01) associated with white-wine-only consumption (n = 108) (Supplementary Table S7, available as Supplementary data at IJE online). White wine consumption was primarily associated with lower LTA ADP (1.82 µM) maximum aggregation (P = 2.6E-3, 95% CI = –0.07, –0.02, β = –0.042) and AUC (P = 7.7E-3, 95% CI = –0.07, –0.01, β = –0.039).
Effect of aspirin vs alcohol on platelet inhibition
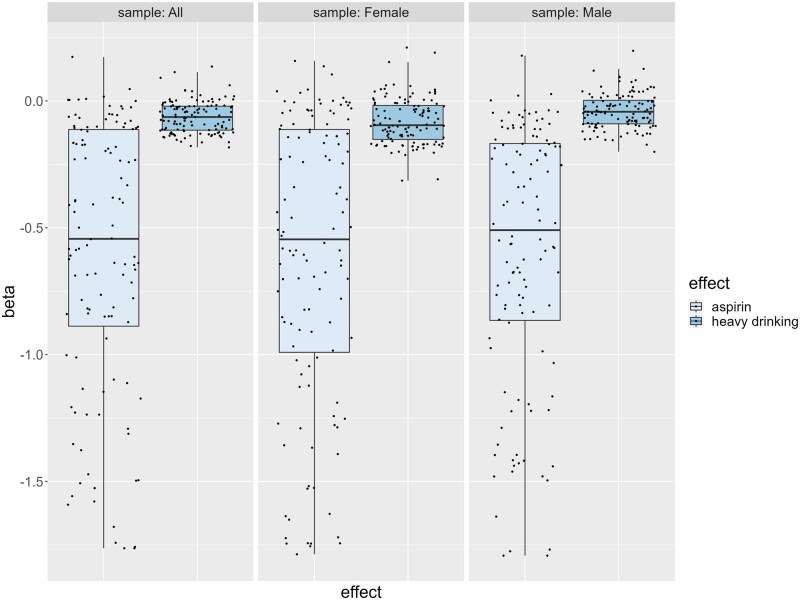
In order to give a broader insight into the effect size of the antiplatelet effect of alcohol, we conducted an analysis that evaluated whether the effect size of aspirin inhibition was greater than that of heavy alcohol consumption (≥8 and ≥15 drinks/week for females and males, respectively). In the full sample, results showed that of the 11 platelet traits associated with heavy drinking, 9 (82%) had negative effect sizes smaller than that of aspirin use (P < 1.83E-22), where on average, the effect of aspirin use was 11.3 (±4.0) times greater than that of heavy drinking (Supplementary Table S8, available as Supplementary data at IJE online). Box plots displaying a comparison of the effects for all platelet traits in either the heavy alcohol consumption analyses and/or the aspirin analyses broadly illustrate the larger effect size of aspirin compared with heavy drinking (Figure 4).
Figure 4.
Three box plots with jittered data points illustrate beta effects of all 120 platelet traits associated with aspirin use vs heavy drinking (≥8 drinks for females and ≥15 drinks for males) in the full, female and male samples. Each jittered data point represents the beta effect of an individual platelet trait associated with aspirin use (light shaded box) or heavy alcohol consumption (dark shaded box). Associations between platelet reactivity traits and heavy drinking are adjusted for age, sex, aspirin use, hypertension, body mass index, cholesterol, high-density lipoprotein, triglycerides, smoking and diabetes. Positive beta effects that indicate decreased platelet reactivity (e.g. Aa_LagTim, AAagg20, AAec50, etc.; full table in Supplementary Table S1, available as Supplementary data at IJE online) are flipped to represent the correct effect of the trait
Discussion
In this cross-sectional analysis of FHS participants, we show numerous associations between heavy alcohol consumption (≥8 drinks per week for females and ≥15 drinks per week for males) and attenuated platelet reactivity traits among both male and female participants. Wine and liquor beverages were observed to have a stronger relationship with reduced platelet aggregation with more associated platelet traits and larger effect sizes compared with beer when differentiating between alcoholic beverage type. Differences are observed within the alcohol consumption–platelet reactivity relationship for males and females, where sex-interaction tests revealed moderate associations between sex and decreased platelet reactivity with respect to alcohol consumption. Collectively, the results from our study illustrate habitual alcohol consumption as one socio-environmental determinant of platelet function and can be used to inform broader public health schemes for cardiovascular bleeding risks on a population scale.
In agreement with previous literature, our results strongly suggest that heavy alcohol consumption inhibits platelet function and aggregation to various agonists.21,24,25 We observed notable decreased platelet aggregation to ADP, epinephrine and ristocetin, as well as decreased activation to stimulation with U46619 and arachidonic acid (AA) consistent overall for both Optimul and LTA assays. Our categorical analysis shows the greatest number of associations and largest effect sizes to attenuated platelet reactivity when comparing the highest consumption group of ≥15 drinks per week with the reference group of 0 drinks per week. Our results indicate that attenuated effect sizes increase and level out towards a maximum from the lowest drinking category to the highest. One example of this is shown by the Optimul trait U46619 maximal % aggregation that has associations (P < 0.01) with the top three drinking categories where the negative effect size of the platelet trait increases with the next highest drinking indicator, then levels off (≥2 to <8 drinks/week: β = –0.0.141; ≥8 to <15 drinks/week: β = –0.242; ≥15 drinks/week: β = –0.260).The results from our categorical alcohol consumption analysis suggest that alcohol intake inhibits platelet reactivity in a direct dose–response manner, which is not entirely consistent to the complex U- or J-shaped phenomenon for mortality and CVD risk seen in individuals who binge-drink. However, this phenomenon may be modulated by non-platelet-driven effects of heavy drinking such as increased hypertension, ventricular and atrial dysfunction and arrythmia.9
Mechanisms for ethanol-induced platelet inhibition are thought to act on the initial responses of platelet activation.36 Fundamentally, ethanol disrupts various activation agonists including collagen, AA, ADP released by activated platelets and thrombin produced from the coagulation cascade. Ethanol initially prevents platelet activation by enhancing prostacyclin and nitric oxide bioavailability within endothelial cells.37,38 Moreover, signalling pathways from these agonists are disrupted and lead to a decreased entry of intracellular Ca2+.39 Intracellular Ca2+ concentration changes are crucial for platelet aggregation and activation of phospholipase A2, an AA release mediator, followed by TxA2, which accelerates further platelet activation and secondary aggregation.40
Our quantitative alcohol consumption analysis was consistent with our categorical results and again revealed notable lower platelet activation to ADP, U46619 and AA for both Optimul and LTA. We observed more relationships of reduced platelet functioning with wine and liquor consumption compared with beer. Reasons for this observation remain unclear and could be modulated by several different social-habitual or biochemical factors. Theoretically, a standard drink in the USA is defined as ∼14 g of pure alcohol, which is found in 12 oz of regular beer (5% alcohol), 5 oz of wine (12% alcohol) or 1.5 oz of distilled spirits (40% alcohol). In most cases beer is packaged in a 12-oz serving but that is not usually the case for wine or liquor beverages, which are more typically packaged in 750-mL (∼25.4-oz) bottles and are not often standardly measured upon consumption. In the case of wine, which has even greater associations with platelet function compared with liquor beverages, studies have suggested that high polyphenol and antioxidant content are the primary modulators of platelet activity, even more so than that of ethanol concentrations.14,40,41
Polyphenol compounds are mainly hypothesized to increase the production of nitric oxide by endothelial-mediated vasodilation that results in inhibition of platelet aggregation and adhesion.41 Upon activation, platelets produce reactive oxygen species and, in this way, further stimulate platelet activation. Antioxidants are the main regulators of reactive oxygen species in the body and exert antithrombotic effects through the neutralization of free radicals into more stable compound forms.42 When looking at the antiplatelet effects of wine based on their polyphenol and antioxidant makeup, it is important to note that the fermentation process of red wine is performed with the inclusion of the grape skins, whereas for white wine fermentation the grape skin is not included, resulting in an ∼10-fold higher concentration of polyphenols in red wine compared with white wine.43 Some studies have concluded that greater platelet inhibition is attributable to red wines that contain greater resveratrol concentrations—a common polyphenol found in grape skins with a high free radical scavenging ability, though results of this have varied.14,44,45 Our results, in a comparatively larger cohort study, show no platelet reactivity associations in individuals who reported only consuming red wine and rather suggest some notable inhibition of ADP-driven platelet activation among white-wine-only drinkers. Previous studies that have compared antioxidant and polyphenol contents with antithrombotic activity among red and white wines observed that different types of extracts found in both red and white wines demonstrated comparative activity against oxidative stress and platelet reactivity.43,46 Evidence from our results, as well as previous literature, demonstrate that the overall antithrombotic effect of wine cannot be assumed solely based on its red or white colour, but likely varies by the specific grape composition of the wine and potentially other factors such as consumption patterns.
When looking at the drinking demographics of the FHS cohort, we observed sex differences in alcohol-drinking behaviour that were consistent with previous FHS studies on alcohol consumption.2,19 Our sex-stratified results show that attenuated platelet effects are greater among females compared with males. Male participants tended to consume a higher volume of alcoholic drinks per week compared with female participants; however, the differences in beer- and wine-drinking preferences could postulate the distinction in platelet reactivity responses observed between male and female participants. Studies that investigated sex as a modulator for alcohol-induced platelet inhibition are mostly inconclusive. It may be appropriate to consider biological components such as increased blood alcohol concentrations, which persist for longer durations in females than in males, and may result in larger differences in platelet activation.47
More broadly, there have been several observational studies that have similarly described the cardioprotective effects of light-to-moderate alcohol consumption in females, unlike males, with respect to thrombotic-related incidents such as ischaemic stroke,48 PAD49 and hypertension.50,51 However, the inconsistent relationships between alcohol and cardiovascular disease morbidity and mortality52 confuse the ideal recommendations for safe alcohol consumption, particularly when considering sex-specific recommendations. Our results that report a greater attenuation of platelet reactivity associated with alcohol consumption, specifically wine and liquor consumption, among females may have theoretical and clinical implications in the discussions of the aforementioned cardioprotective relationships as well as current guidelines which recommend that females consume less alcohol than males in order to achieve the same beneficial cardiovascular outcomes.53
The relationship between alcohol consumption and cardiovascular health is multifactorial but the cardioprotective associations suggested by platelet inhibition are limited to only one cell type in the cardiovascular system. Our results support previous findings that established an inhibitory relationship between moderate to heavy alcohol consumption and platelet activation and aggregation. It is important to note that causation in our observational study cannot be clearly identified from the associations we determined between alcohol and platelet reactivity. Social and environmental determinants of health that are also related to patterns of alcohol consumption such as exposure to mental stress, diet and levels of physical activity all may have additional modulatory effects on platelet function.54–56 From a public health standpoint, our research does not suggest that increased alcohol consumption is holistically cardioprotective since associations varied by beverage type and sex, and may not attenuate the incidence of stroke, MI and mortality, as previously observed.57,58 In some studies, the modulatory effects of alcohol have been compared with aspirin based on inhibition mechanisms of similar coagulation pathways. However, we found that for a large proportion of the platelet reactivity traits tested, the effect of aspirin associations is greater on the same platelet reactivity traits compared with heavy alcohol consumption. The use of aspirin itself in primary and secondary thrombosis remains conflicting when balanced against increased bleeding risks.59 Thus, given the much larger effect size of aspirin on platelet inhibition, alcohol consumption is unlikely to be of great utility in thrombosis prevention, at least via a platelet-specific mechanism. This may be particularly valid considering the additional risks carried by heavy alcohol use.9
It is fundamental to consider the limitations of the results within our study. Our research made use of self-reported alcohol consumption, which may be susceptible to inaccuracies on account of recall, temporal and self-desirability biases. In addition, the FHS exam questionnaire did not ask about the participant’s specific drinking pattern such as weekends vs specific weekdays or the most recent acute alcohol exposure in order to capture recency, which may mediate the effects on platelet reactivity; however, participants were examined consistently after an overnight fasting period of ≥10 h (mean fasting: 12.7 ± 2.1 h). Given the nature of our cross-sectional study, we are able to highlight the effects of habitual alcohol consumption on platelet function since most acute effects of alcohol intake can be presumed to have subsided by the time of platelet activation assays. Lastly, the FHS cohort studied in Exam 3 comprised mostly individuals with European ancestry and cannot be generalized to all US populations. On the other hand, the FHS cohort has a large sample size that consists of participants who are mostly healthy and middle-aged, which gives a broader insight into the antiplatelet effects of alcohol consumption.
The overall findings from this study strongly indicate associations between alcohol intake and decreased platelet reactivity, which may largely modulate the cardioprotective effects observed in light-to-moderate alcohol consumers, particularly for individuals who habitually consume wine. Although our study suggests an inhibitory dose–response relationship between alcohol consumption and platelets, increased health risks related to heavy drinking observed in the literature should be taken into consideration when considering recommendations for the social-habitual consumption of alcohol.
Ethics approval
Ethical approval to conduct this study was obtained from the Boston University Medical Center Institutional Review Board (IRB) and all participants provided written informed consent.
Supplementary Material
Acknowledgements
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the US Department of Health and Human Services. Figure 1 was adapted with BioRender software: https://www.biorender.com/ (July 2023, date last accessed).
Contributor Information
Robin E Pashek, National Heart, Lung and Blood Institute’s, The Framingham Heart Study, Framingham, Framingham, MA, USA; National Heart, Lung and Blood Institute, Population Sciences Branch, Framingham, MA, USA.
Bongani B Nkambule, National Heart, Lung and Blood Institute’s, The Framingham Heart Study, Framingham, Framingham, MA, USA; National Heart, Lung and Blood Institute, Population Sciences Branch, Framingham, MA, USA.
Melissa V Chan, National Heart, Lung and Blood Institute’s, The Framingham Heart Study, Framingham, Framingham, MA, USA; National Heart, Lung and Blood Institute, Population Sciences Branch, Framingham, MA, USA.
Florian Thibord, National Heart, Lung and Blood Institute’s, The Framingham Heart Study, Framingham, Framingham, MA, USA; National Heart, Lung and Blood Institute, Population Sciences Branch, Framingham, MA, USA.
Amber R Lachapelle, National Heart, Lung and Blood Institute’s, The Framingham Heart Study, Framingham, Framingham, MA, USA; National Heart, Lung and Blood Institute, Population Sciences Branch, Framingham, MA, USA.
Jason Cunha, National Heart, Lung and Blood Institute’s, The Framingham Heart Study, Framingham, Framingham, MA, USA; National Heart, Lung and Blood Institute, Population Sciences Branch, Framingham, MA, USA.
Ming-Huei Chen, National Heart, Lung and Blood Institute’s, The Framingham Heart Study, Framingham, Framingham, MA, USA; National Heart, Lung and Blood Institute, Population Sciences Branch, Framingham, MA, USA.
Andrew D Johnson, National Heart, Lung and Blood Institute’s, The Framingham Heart Study, Framingham, Framingham, MA, USA; National Heart, Lung and Blood Institute, Population Sciences Branch, Framingham, MA, USA.
Data availability
The data underlying this article are available in the NCBI database of Genotypes and Phenotypes (dbGaP) and can be accessed with accession number phs000007.
Supplementary data
Supplementary data are available at IJE online.
Author contributions
R.E.P. and A.D.J. wrote the manuscript. R.E.P., M-H.C. and A.D.J. designed the project. M.V.C., A.L., M-H.C. and A.D.J. designed the original platelet data collection. A.D.J. and A.L. collected the data. R.E.P., B.B.N., M.V.C., F.T., M-H.C. and A.D.J. analysed the data or contributed to the analysis approach. All authors critically revised and approved the manuscript content.
Funding
This research was primarily supported by a special Population Sciences funding award to A.D.J. from the National Heart, Lung and Blood Institute (NHBLI) Intramural Research programme. The FHS acknowledges the support of Contracts NO1-HC-25195, HHSN268201500001I and 75N92019D00031 from the NHBLI and grants HL107385, HL126136, HL93328, HL 142983, HL143227 and HL 131532 for this research. M.V.C. was also supported by the British Heart Foundation (RG/19/8/34500).
Conflict of interest
None declared.
References
- 1. Cahill PA, Redmond EM.. Alcohol and cardiovascular disease: modulation of vascular cell function. Nutrients 2012;4:297–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mukamal KJ, Jadhav PP, D'Agostino RB. et al. Alcohol consumption and hemostatic factors: analysis of the Framingham Offspring cohort. Circulation 2001;104:1367–73. [DOI] [PubMed] [Google Scholar]
- 3. Tousoulis D, Ntarladimas I, Antoniades C. et al. Acute effects of different alcoholic beverages on vascular endothelium, inflammatory markers and thrombosis fibrinolysis system. Clin Nutr 2008;27:594–600. [DOI] [PubMed] [Google Scholar]
- 4. Larsson SC, Wallin A, Wolk A.. Contrasting association between alcohol consumption and risk of myocardial infarction and heart failure: two prospective cohorts. Int J Cardiol 2017;231:207–10. [DOI] [PubMed] [Google Scholar]
- 5. Larsson SC, Burgess S, Mason AM, Michaelsson K.. Alcohol consumption and cardiovascular disease: a Mendelian randomization study. Circ Genom Precis Med 2020;13:e002814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fuchs CS, Stampfer MJ, Colditz GA. et al. Alcohol consumption and mortality among women. N Engl J Med 1995;332:1245–50. [DOI] [PubMed] [Google Scholar]
- 7. Gaziano JM, Gaziano TA, Glynn RJ. et al. Light-to-moderate alcohol consumption and mortality in the Physicians' Health Study enrollment cohort. J Am Coll Cardiol 2000;35:96–105. [DOI] [PubMed] [Google Scholar]
- 8. O'Keefe JH, Bhatti SK, Bajwa A, DiNicolantonio JJ, Lavie CJ.. Alcohol and cardiovascular health: the dose makes the poison…or the remedy. Mayo Clin Proc 2014;89:382–93. [DOI] [PubMed] [Google Scholar]
- 9. Day E, Rudd JHF.. Alcohol use disorders and the heart. Addiction 2019;114:1670–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ricci C, Wood A, Muller D. et al. Alcohol intake in relation to non-fatal and fatal coronary heart disease and stroke: EPIC-CVD case-cohort study. BMJ 2018;361:k934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee KW, Lip GY.. Effects of lifestyle on hemostasis, fibrinolysis, and platelet reactivity: a systematic review. Arch Intern Med 2003;163:2368–92. [DOI] [PubMed] [Google Scholar]
- 12. Zhao J, Stockwell T, Roemer A, Naimi T, Chikritzhs T.. Alcohol consumption and mortality from coronary heart disease: an updated meta-analysis of cohort studies. J Stud Alcohol Drugs 2017;78:375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wurtz P, Cook S, Wang Q. et al. Metabolic profiling of alcohol consumption in 9778 young adults. Int J Epidemiol 2016;45:1493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Snopek L, Mlcek J, Sochorova L. et al. Contribution of red wine consumption to human health protection. Molecules 2018;23:1684–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frankel EN, Kanner J, German JB, Parks E, Kinsella JE.. Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. Lancet 1993;341:454–57. [DOI] [PubMed] [Google Scholar]
- 16. Haseeb S, Alexander B, Baranchuk A.. Wine and cardiovascular health: a comprehensive review. Circulation 2017;136:1434–48. [DOI] [PubMed] [Google Scholar]
- 17. Fuentes E, Palomo I.. Role of oxidative stress on platelet hyperreactivity during aging. Life Sci 2016;148:17–23. [DOI] [PubMed] [Google Scholar]
- 18. Stach K, Kälsch A-I, Weiß C, Elmas E, Borggrefe M, Kälsch T.. Effects of ethanol on the properties of platelets and endothelial cells in model experiments. World J Cardiol 2012;4:201–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mukamal KJ, Massaro JM, Ault KA. et al. Alcohol consumption and platelet activation and aggregation among women and men: the Framingham Offspring Study. Alcohol Clin Exp Res 2005;29:1906–12. [DOI] [PubMed] [Google Scholar]
- 20. Smith S, Fair K, Goodman A, Watson J, Dodgion C, Schreiber M.. Consumption of alcohol leads to platelet inhibition in men. Am J Surg 2019;217:868–72. [DOI] [PubMed] [Google Scholar]
- 21. Salem RO, Laposata M.. Effects of alcohol on hemostasis. Am J Clin Pathol 2005;123(Suppl_1):S96–105. [DOI] [PubMed] [Google Scholar]
- 22. Ehrlich D, Humpel C.. Effects of ethanol on aggregation, serotonin release, and amyloid precursor protein processing in rat and human platelets. Platelets 2014;25:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ballard HS. The hematological complications of alcoholism. Alcohol Health Res World 1997;21:42–52. [PMC free article] [PubMed] [Google Scholar]
- 24. Renaud SC, Ruf JC.. Effects of alcohol on platelet functions. Clin Chim Acta 1996;246:77–89. [DOI] [PubMed] [Google Scholar]
- 25. Rubin R. Effect of ethanol on platelet function. Alcohol Clin Exp Res 1999;23:1114–18. [PubMed] [Google Scholar]
- 26. Hillbom M, Kangasaho M, Lowbeer C, Kaste M, Muuronen A, Numminen H.. Effects of ethanol on platelet function. Alcohol 1985;2:429–32. [DOI] [PubMed] [Google Scholar]
- 27. Gaziano JM, Hennekens CH, Godfried SL. et al. Type of alcoholic beverage and risk of myocardial infarction. Am J Cardiol 1999;83:52–57. [DOI] [PubMed] [Google Scholar]
- 28. Renaud SC, Beswick AD, Fehily AM, Sharp DS, Elwood PC.. Alcohol and platelet aggregation: the Caerphilly Prospective Heart Disease Study. Am J Clin Nutr 1992;55:1012–17. [DOI] [PubMed] [Google Scholar]
- 29. Andersson C, Johnson AD, Benjamin EJ, Levy D, Vasan RS.. 70-year legacy of the Framingham Heart Study. Nat Rev Cardiol 2019;16:687–98. [DOI] [PubMed] [Google Scholar]
- 30. Grech J, Chan MV, Ochin C. et al. Serotonin-affecting antidepressant use in relation to platelet reactivity. Clin Pharmacol Ther 2022;111:909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. (CDC) Centers for Disease Control and Prevention. Excessive Alcohol Use. 2023. https://www.cdc.gov/chronicdisease/resources/publications/factsheets/alcohol.htm#:~:text=Excessive%20alcohol%20use%20includes%3A,per%20week%20for%20a%20man (February 2023, date last accessed).
- 32. Al-Shaar L, Yuan C, Rosner B. et al. Reproducibility and validity of a semiquantitative food frequency questionnaire in men assessed by multiple methods. Am J Epidemiol 2021;190:1122–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2021. https://www.r-project.org (August 2022, date last accessed).
- 34. Monaco G, Chen H, Poidinger M, Chen J, de Magalhães JP, Larbi A.. flowAI: automatic and interactive anomaly discerning tools for flow cytometry data. Bioinformatics 2016;32:2473–80. [DOI] [PubMed] [Google Scholar]
- 35. Beckton Dac. FlowJoTM Software (for Mac) Version 10.7.1. 2021. https://www.flowjo.com/solutions/flowjo/downloads (August 2022, date last accessed).
- 36. Ekawa K, Marumo M, Wakabayashi I.. Inhibition by ethanol of shear stress-induced formation of platelet thrombi in whole blood. Alcohol Alcohol 2019;54:13–18. [DOI] [PubMed] [Google Scholar]
- 37. Huang P-H, Chen Y-H, Tsai H-Y. et al. Intake of red wine increases the number and functional capacity of circulating endothelial progenitor cells by enhancing nitric oxide bioavailability. Arterioscler Thromb Vasc Biol 2010;30:869–77. [DOI] [PubMed] [Google Scholar]
- 38. Landolfi R, Steiner M.. Ethanol raises prostacyclin in vivo and in vitro. Blood 1984;64:679–82. [PubMed] [Google Scholar]
- 39. Varga-Szabo D, Braun A, Nieswandt B.. Calcium signaling in platelets. J Thromb Haemost 2009;7:1057–66. [DOI] [PubMed] [Google Scholar]
- 40. Ruf JC. Alcohol, wine and platelet function. Biol Res 2004;37:209–15. [DOI] [PubMed] [Google Scholar]
- 41. Mann LB, Folts JD.. Effects of ethanol and other constituents of alcoholic beverages on coronary heart disease: a review. Pathophysiology 2004;10:105–12. [DOI] [PubMed] [Google Scholar]
- 42. Masselli E, Pozzi G, Vaccarezza M. et al. ROS in platelet biology: functional aspects and methodological insights. IJMS 2020;21:4866–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fragopoulou E, Petsini F, Choleva M. et al. Evaluation of anti-inflammatory, anti-platelet and anti-oxidant activity of wine extracts prepared from ten different grape varieties. Molecules 2020;25:5054–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Galiniak S, Aebisher D, Bartusik-Aebisher D.. Health benefits of resveratrol administration. Acta Biochim Pol 2019;66:13–21. [DOI] [PubMed] [Google Scholar]
- 45. Tozzi Ciancarelli MG, Di Massimo C, De Amicis D, Ciancarelli I, Carolei A.. Moderate consumption of red wine and human platelet responsiveness. Thromb Res 2011;128:124–29. [DOI] [PubMed] [Google Scholar]
- 46. Iwasaki M, Murakami M, Ijiri Y, Shimizu M, Yamamoto J.. Are all wines made from various grape varieties beneficial in the prevention of myocardial infarction and stroke? Future Sci OA 2020;7:FSO649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Erol A, Karpyak VM.. Sex and gender-related differences in alcohol use and its consequences: contemporary knowledge and future research considerations. Drug Alcohol Depend 2015;156:1–13. [DOI] [PubMed] [Google Scholar]
- 48. Malarcher AM, Giles WH, Croft JB. et al. Alcohol intake, type of beverage, and the risk of cerebral infarction in young women. Stroke 2001;32:77–83. [DOI] [PubMed] [Google Scholar]
- 49. Vliegenthart R, Geleijnse JM, Hofman A. et al. Alcohol consumption and risk of peripheral arterial disease: the Rotterdam study. Am J Epidemiol 2002;155:332–38. [DOI] [PubMed] [Google Scholar]
- 50. Sesso HD, Cook NR, Buring JE, Manson JE, Gaziano JM.. Alcohol consumption and the risk of hypertension in women and men. Hypertension 2008;51:1080–87. [DOI] [PubMed] [Google Scholar]
- 51. Roerecke M, Tobe SW, Kaczorowski J. et al. Sex-specific associations between alcohol consumption and incidence of hypertension: a systematic review and meta-analysis of cohort studies. J Am Heart Assoc 2018;7:e008202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Piano MR, Thur LA, Hwang CL, Phillips SA.. Effects of alcohol on the cardiovascular system in women. Alcohol Res 2020;40:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang CN, Liblik K, Haseeb S, Lopez-Santi R, Baranchuk A.. Women and alcohol: limitations in the cardiovascular guidelines. Curr Probl Cardiol 2023;48:101200. [DOI] [PubMed] [Google Scholar]
- 54. Heber S, Volf I.. Effects of physical (in)activity on platelet function. Biomed Res Int 2015;2015:165078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Koudouovoh-Tripp P, Hufner K, Egeter J. et al. Stress enhances proinflammatory platelet activity: the impact of acute and chronic mental stress. J Neuroimmune Pharmacol 2021;16:500–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McEwen BJ. The influence of diet and nutrients on platelet function. Semin Thromb Hemost 2014;40:214–26. [DOI] [PubMed] [Google Scholar]
- 57. Chiva-Blanch G, Badimon L.. Benefits and risks of moderate alcohol consumption on cardiovascular disease: current findings and controversies. Nutrients 2019;12:108–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Biddinger KJ, Emdin CA, Haas ME. et al. Association of habitual alcohol intake with risk of cardiovascular disease. JAMA Netw Open 2022;5:e223849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zheng SL, Roddick AJ.. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA 2019;321:277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the NCBI database of Genotypes and Phenotypes (dbGaP) and can be accessed with accession number phs000007.