Abstract
Proteins in intercellular washing fluid (IWF) from noninoculated and stem rust-affected wheat leaves were separated by isoelectric focusing and polyacrylamide gel electrophoresis under nondenaturing conditions, transferred to nitrocellulose membranes, and assayed in situ for peroxidase and glycosidase activity.
Two infection-related peroxidase isozymes were detected in addition to more than ten that were present only in noninoculated plants. One other peroxidase isozyme was present in much higher concentration in IWF from infected leaves than in IWF from noninoculated leaves.
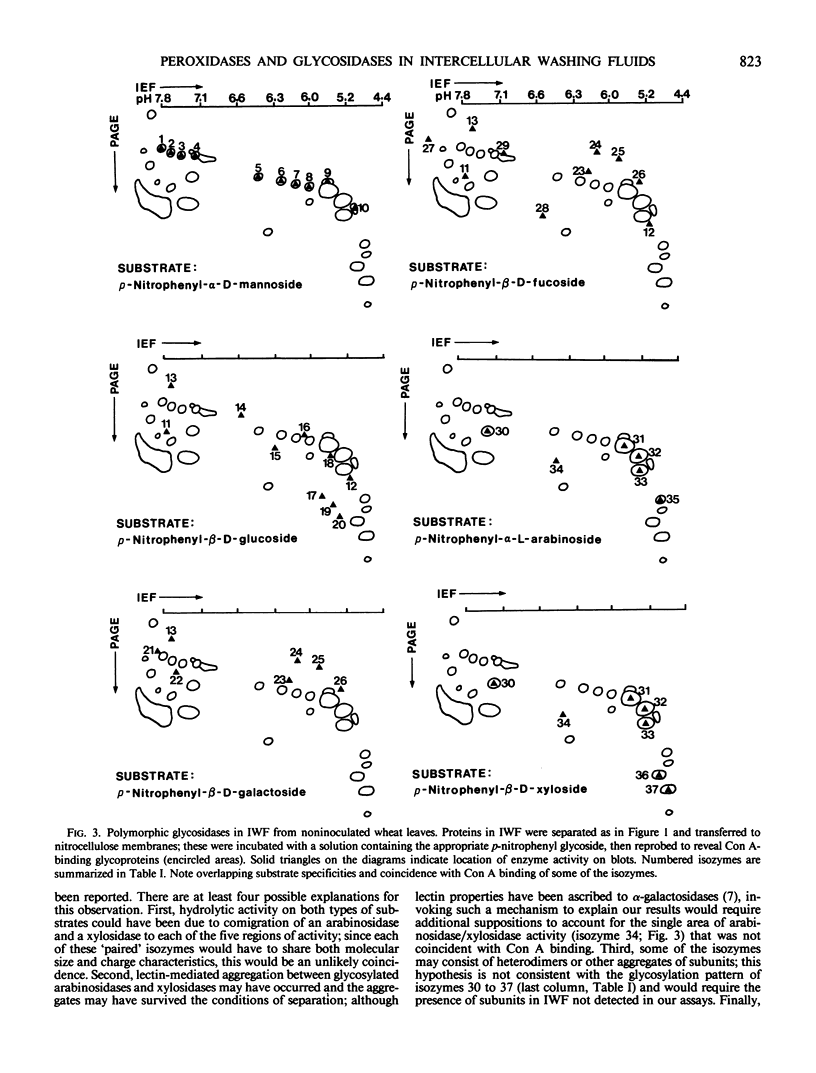
IWF contained many polymorphic glycosidases. A new method is described to localize the glycosidase isozymes accurately on nitrocellulose blots for evaluation of their substrate specificities: each blot was developed with the appropriate p-nitrophenyl glycoside to reveal glycosidase activity, then reprobed for concanavalin A-binding glycoproteins to serve as an internal reference frame for blot-to-blot comparisons. This procedure also provided information on glycosylation of the isozymes.
The locations of at least 15 (out of 37) isozymes were coincident with concanavalin A binding, including those of all 10 α-d-mannosidases, and of 6 β-d-xylosidases. On five areas of the blots there was coincidence of β-d-xylosidase and α-l-arabinosidase activity. Three of these areas corresponded to three of the most prominent Coomassie brilliant blue-stainable IWF proteins in gels. Isozymes that could convert p-nitrophenyl-β-d-glucoside, -β-d-galactoside, and/or -β-d-fucoside revealed a complex pattern of partially overlapping substrate specificities: two isozymes utilized both glucose and fucose derivatives, one utilized all three derivatives, and several others converted only one of the three substrates. No enzymes were detected with activity on p-nitrophenyl-β-d-galactosaminide, -β-l-fucoside, or -α-d-galactoside. No additional glycosidases were detected in IWF from stem rust-affected leaves.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowles D. J., Chaplin M. F., Marcus S. E. Interaction of concanavalin A with native and denatured forms of jackbean alpha-D-mannosidase. Eur J Biochem. 1983 Feb 15;130(3):613–618. doi: 10.1111/j.1432-1033.1983.tb07193.x. [DOI] [PubMed] [Google Scholar]
- Dey P. M., Del Campillo E. Biochemistry of the multiple forms of glycosidases in plants. Adv Enzymol Relat Areas Mol Biol. 1984;56:141–249. doi: 10.1002/9780470123027.ch3. [DOI] [PubMed] [Google Scholar]
- Ferrari T. E. Extraction and partial characterization of cellulases from expanding pea epicotyls. Plant Physiol. 1974 Oct;54(4):487–493. doi: 10.1104/pp.54.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve L. C., Ordin L. Isolation and Purification of an alpha-Mannosidase from Coleoptiles of Avena sativa. Plant Physiol. 1977 Oct;60(4):478–481. doi: 10.1104/pp.60.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R. Identification of concanavalin A-binding proteins after sodium dodecyl sulfate--gel electrophoresis and protein blotting. Anal Biochem. 1982 Jun;123(1):143–146. doi: 10.1016/0003-2697(82)90634-0. [DOI] [PubMed] [Google Scholar]
- Holden D. W., Rohringer R. Proteins in intercellular washing fluid from noninoculated and rust-affected leaves of wheat and barley. Plant Physiol. 1985 Aug;78(4):715–723. doi: 10.1104/pp.78.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell W. G., Sequeira L. Soluble peroxidase in fluid from the intercellular spaces of tobacco leaves. Plant Physiol. 1974 Feb;53(2):317–318. doi: 10.1104/pp.53.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohringer R., Holden D. W. Protein blotting: detection of proteins with colloidal gold, and of glycoproteins and lectins with biotin-conjugated and enzyme probes. Anal Biochem. 1985 Jan;144(1):118–127. doi: 10.1016/0003-2697(85)90092-2. [DOI] [PubMed] [Google Scholar]
- Seevers P. M., Daly J. M., Catedral F. F. The role of peroxidase isozymes in resistance to wheat stem rust disease. Plant Physiol. 1971 Sep;48(3):353–360. doi: 10.1104/pp.48.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford H. A., Bravinder-Bree S. Peroxidase isozymes of first internodes of sorghum: tissue and intracellular localization and multiple peaks of activity isolated by gel filtration chromatography. Plant Physiol. 1972 Jun;49(6):950–956. doi: 10.1104/pp.49.6.950. [DOI] [PMC free article] [PubMed] [Google Scholar]