Abstract
Background
Evidence on body fat distribution shows opposing effects of waist circumference (WC) and hip circumference (HC) for coronary heart disease (CHD). We aimed to investigate the causality and the shape of such associations.
Methods
UK Biobank is a prospective cohort study of 0.5 million adults aged 40–69 years recruited between 2006 and 2010. Adjusted hazard ratios (HRs) for the associations of measured and genetically predicted body mass index (BMI), WC, HC and waist-to-hip ratio with incident CHD were obtained from Cox models. Mendelian randomization (MR) was used to assess causality. The analysis included 456 495 participants (26 225 first-ever CHD events) without prior CHD.
Results
All measures of adiposity demonstrated strong, positive and approximately log-linear associations with CHD risk over a median follow-up of 12.7 years. For HC, however, the association became inverse given the BMI and WC (HR per usual SD 0.95, 95% CI 0.93–0.97). Associations for BMI and WC remained independently positive after adjustment for other adiposity measures and were similar (1.14, 1.13–1.16 and 1.18, 1.15–1.20, respectively), with WC displaying stronger associations among women. Blood pressure, plasma lipids and dysglycaemia accounted for much of the observed excess risk. MR results were generally consistent with the observational, implying causality.
Conclusions
Body fat distribution measures displayed similar associations with CHD risk as BMI except for HC, which was inversely associated with CHD risk (given WC and BMI). These findings suggest that different measures of body fat distribution likely influence CHD risk through both overlapping and independent mechanisms.
Keywords: Obesity, epidemiology, genetics, coronary heart disease
Key Messages.
Measures of general adiposity and body fat distribution showed strong, positive and approximately log-linear associations with risk of coronary heart disease (CHD) independently of each other, apart from hip circumference, which was inversely associated.
Blood pressure, plasma lipids and dysglycaemia (or correlates thereof) explained much of the observed association between adiposity and CHD risk.
Mendelian randomization of adiposity on CHD showed overall consistent results with those from the observational analyses, implying potential causality.
Introduction
Excess adiposity is a leading risk factor for coronary heart disease (CHD) globally.1 The majority of evidence for adiposity-related CHD risk has been derived from epidemiological studies of body mass index (BMI) but additional evidence from clinical trials further supports cardiovascular benefits of weight loss.2 Although BMI reflects general adiposity, it does not indicate body fat distribution. There is growing evidence that distinct fat depots exert different effects on CHD risk compared with overall body fat such as the opposing effect of waist circumference (WC) and hip circumference (HC) on CHD risk beyond other indicators.3–5 Therefore, it is possible that other measures of body fat distribution, such as WC, HC or waist-to-hip ratio (WHR), could complement or supersede BMI when estimating adiposity-related CHD risk.6,7
The exact mechanisms underlying the relationship between adiposity and CHD remain unclear but it is thought to be chiefly mediated by blood pressure, cholesterol and insulin resistance.8 Prior evidence on the role of intermediate factors includes large-scale meta-analyses, but most included studies were unable to account for medication use.9,10
It has not been possible to assess the independent causal relevance of different adiposity measures to CHD risk using observational evidence alone due to reverse causality, confounding and the correlation between different adiposity measures. Mendelian randomization (MR) approaches can overcome some of these limitations by using genetic variants as proxies—or ‘instrumental variables’ (IVs)—for an exposure, which, given a number of assumptions, can provide unbiased causal estimates of exposure–outcome associations.11,12
The causal relevance of elevated adiposity to CHD risk has been studied previously using MR.13–19 However, due to a lack of genetic information on large longitudinal cohorts, the majority of MR studies have used summary-level data for the primary analysis. Only a handful of studies have included a subset of individual-level participant data, which allows the investigation of the shape of associations, and most of them have focused on general adiposity rather than body fat distribution.
With full genetic data available in a very large and contemporary prospective cohort study with sufficient follow-up time, UK Biobank (UKB) allows a direct comparison of the relevance of different measures of general adiposity as well as body fat distribution to CHD risk using both observational and genetic data in the same study population more reliably and in greater detail than has previously been done (e.g. by examining the shape of the association and assessing potential mediators).
Methods
Study population characteristics and baseline procedures
UKB is a large observational study of 502 678 UK adults aged 40–69 years. Eligible adults were invited to one of 22 assessment centres around the UK from 2006 to 2010. The study has been described in detail previously.20,21 Participants reported lifestyle exposures, medical history and medications before undergoing standardized assessments including body size and biomarker measurements. Anthropometric measurements included body weight (using a Tanita BC418MA body composition analyser or a standard scale if the participant did not undergo bioimpedance), standing height (Seca 240-cm height measure) and waist and HC (Seca 200-cm tape measure around the narrowest part of the trunk and the widest part of the hips, respectively).
Baseline exclusions
Participants were excluded from the analysis if they: (i) were aged <40 or ≥70 years at baseline; (ii) had withdrawn at the time of analysis; or (iii) had missing or outlying values for selected anthropometric measures at baseline (Supplementary Figure S1, available as Supplementary data at IJE online). To limit reverse causation, individuals who self-reported or whose hospital records indicated previous cardiovascular disease (CVD) were also excluded. Participants who were genetically related (Supplementary Methods, available as Supplementary data at IJE online), of self-reported non-European ancestry, with discordance between the genetic sex and the self-reported biological sex, with sex chromosome aneuploidy or who lacked genetic information were excluded from genetic analyses (Supplementary Figure S1, available as Supplementary data at IJE online).
Study outcome and follow-up procedures
Participants were followed up by linkage to electronic health databases including Hospital Episode Statistics, disease registries and Office for National Statistics cause-of-death data to obtain fatal and non-fatal incident CHD events. The main outcome in the present study was first incident CHD as recorded on a hospital admission (based on any reason for admission) (Supplementary Table S1, available as Supplementary data at IJE online). The censoring date was defined as the date of the first non-fatal CHD event or the date of death due to CHD if no non-fatal CHD event had been recorded. Otherwise participants were followed up until the end of the study period or date of death.
Selection and construction of genetic instruments
Details on the genotyping and quality control of the genetic data have been previously reported.20 The causal relevance of the four adiposity measures on CHD risk was estimated by using individual-level data from UKB to create a genetic risk score (GRS) for each measure. After exclusions of single-nucleotide polymorphisms (SNPs) based on quality control checks (Supplementary Methods, available as Supplementary data at IJE online), we identified from Genome Wide Association Study (GWAS) of the Genetic Investigation of Anthropometric Traits (GIANT) consortium (not including UKB) 75 independent SNPs to derive the GRS for BMI, 45 SNPs for WHR and WHR adjusted for BMI (WHRadjBMI), 69 SNPs for WC and WC adjusted for BMI (WCadjBMI) and 89 SNPs for HC and HC adjusted for BMI (HCadjBMI), respectively, using the sex-specific external betas from GIANT as weights due to observed heterogeneity in the betas by sex for WHR and WC (Supplementary Figure S2 and Supplementary Tables S2–S5, available as Supplementary data at IJE online).22–24 Each GRS was expressed per average allele effect (Supplementary Methods, available as Supplementary data at IJE online).25
Statistical analysis
Prospective associations between adiposity and CHD risk
Hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs) for the association between adiposity and risk of incident CHD were calculated using Cox proportional-hazards regression models. Where appropriate, models were stratified by age at risk in 5-year groups, region and sex, and adjusted for education, Townsend Deprivation Index, ethnicity, smoking and alcohol consumption. In a secondary analysis, we also adjusted for physical activity and family history of CVD. Supplementary Methods (available as Supplementary data at IJE online) provide more information on the derivation of the covariates.
To correct for regression dilution bias,26 the ‘usual SD’ of each adiposity measure was obtained by multiplying the baseline standard deviation (SD) by the square root of the regression dilution ratios calculated as partial correlation coefficients between baseline and resurvey measures.27 Likewise, to describe the shapes of associations between ‘usual’ levels of adiposity and CHD risk, estimates were plotted against the mean value at resurvey within each baseline group. Group-specific variances were used to calculate 95% CIs for all groups, including the reference group.28 Where associations were approximately log-linear, CHD risk was estimated per the usual SD higher adiposity level. The main analyses were performed separately for men and women.29 Joint effects and independence between adiposity measures were investigated by comparing HRs with increasing BMI (per SD usual levels) before and after adjusting for measures of body fat distribution (WC, HC and WHR) and vice versa.
The potential mediating effects of systolic blood pressure (SBP), lipids [Apolipoprotein A (Apo A), Apolipoprotein B (Apo B), low-density lipoprotein, high-density lipoprotein, triglycerides], glycated haemoglobin (HbA1c), self-reported type II diabetes, C-reactive protein (CRP), estimated glomerular filtration rate (eGFR) and liver enzymes [alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT) and gamma-glutamyl transferase (GGT)]30 on adiposity-related CHD risk were investigated. Proportional reductions in the log-HR and Wald χ2 following adjustment for potential mediators provided semi-quantitative estimates of mediation.
Genetically predicted adiposity and CHD risk
An IV ratio estimate method was used to scale results to represent the effect size per SD higher adiposity measure.31 Associations of each GRS with CHD risk were stratified by age at risk and UKB region, and adjusted for sex, baseline age, baseline age squared, the first 10 principal components, batch number and array type in Cox models. Non-linear associations of BMI with CVD have been previously reported in observational studies.10 Therefore, in order to assess the shape and strength of associations, we employed a non-linear MR approach in addition to standard linear MR (Supplementary Methods, available as Supplementary data at IJE online).32,33
To assess the MR assumptions: (i) the strength of association between each GRS and respective adiposity measure was quantified by estimating the F-statistic (F > 10 suggesting lower probability of weak instrument bias); (ii) the association of covariates with each GRS was assessed separately to investigate whether GRSs were independent of confounders; and (iii) MR–Egger regression using effect sizes on associations of SNPs with adiposity traits from GIANT (not including UKB) and effect sizes on associations of SNPs with CHD from UKB was performed to assess whether genetic variants are only associated with the exposure of interest and to provide an indication of directional pleiotropy.34 The I2GX statistic was used to assess the no measurement error assumption, which provides an assessment of the expected dilution of the MR–Egger estimate due to uncertainty in the SNP–exposure associations and has been suggested as a measure of instrument strength for the MR–Egger method.34 The simulation extrapolation (SIMEX) method was used in combination with MR–Egger to get more robust estimates when the I2GX statistic was lower.34 Weighted median MR, which assumes that at least half of the information comes from valid instruments, and MR Pleiotropy Residual Sum and Outlier (MR-PRESSO), which detects and corrects for horizontal pleiotropy by removing outliers,35 were also employed for additional sensitivity analyses. Multivariable MR was also performed using the GRSs that were not adjusted for BMI.
Analyses were conducted using SAS (version 9.4) and R (version 3.6.2). R package ‘MendelianRandomization’ was used for MR–Egger. Plots were created using R package ‘Jasper' (https://github.com/arnhew99/Jasper). Reporting of analyses and results followed the recommendations of the STROBE36 and STROBE-MR37 statements.
Results
Baseline characteristics
The adiposity measures were higher among men than women (except HC) and within each sex among those who developed CHD (Table 1). Men were also more likely than women to be smokers, daily drinkers, highly active, have diabetes and have higher SBP and liver enzyme levels and lower ApoA levels. Those who developed CHD were older, of lower educational level and higher deprivation index, and more likely to smoke and have diabetes. Further, those who developed CHD had higher baseline levels of SBP, HbA1c and GGT, but lower levels of ApoA than those who did not develop CHD. For each measure of adiposity, there was a strong correlation between baseline and resurvey values (r2 > 0.8 apart from WHR with ∼0.65; Supplementary Table S6, available as Supplementary data at IJE online). The adiposity measures were generally highly correlated with each other (r2 = 0.61–0.89; Supplementary Table S7, available as Supplementary data at IJE online) with the exception of BMI and WHR among women (r2 = 0.46) and WHR and HC in both men (r2 = 0.33) and women (r2 = 0.25,). The genetic analysis included 368 613 participants with similar characteristics at baseline compared with the main study population (Supplementary Table S8, available as Supplementary data at IJE online).
Table 1.
Baseline characteristics by first-ever CHD event status over follow-upa and sex
| Men |
Women |
|||
|---|---|---|---|---|
| CHD (n=17 010) | No CHD (n=183 652) | CHD (n=9215) | No CHD (n=246 618) | |
| Body mass index (kg/m²) | 28.5 (4.3) | 27.6 (4.0) | 28.6 (5.5) | 26.9 (5.0) |
| Hip circumference (cm) | 104.0 (7.8) | 103.2 (7.2) | 105.5 (11.1) | 103.0 (10.0) |
| Waist circumference (cm) | 99.2 (11.5) | 96.2 (10.9) | 89.2 (13.1) | 84.2 (12.2) |
| Waist-to-hip ratio | 0.95 (0.06) | 0.93 (0.06) | 0.84 (0.07) | 0.82 (0.07) |
| Socio-demographic and lifestyle factors | ||||
| Age (years) | 59.7 (7.0) | 55.8 (8.2) | 60.6 (6.6) | 55.9 (8.0) |
| Achieved higher education | 9723 (57%) | 119 727 (65%) | 4319 (47%) | 142 795 (58%) |
| Townsend deprivation index | –1.1 (3.2) | –1.3 (3.1) | –1.0 (3.2) | –1.4 (3.0) |
| Current smoker | 2641 (16%) | 22 144 (12%) | 1286 (14%) | 21 079 (9%) |
| Daily drinker | 4306 (25%) | 46 956 (26%) | 1356 (15%) | 40 339 (16%) |
| Very physically active (METs ≥50 h/week) | 3311 (19%) | 37 075 (20%) | 1412 (15%) | 38 843 (16%) |
| Family history of CVD | 10 306 (61%) | 94 118 (51%) | 6469 (70%) | 141 045 (57%) |
| Potential mediators | ||||
| Systolic blood pressure (mmHg) | 145.8 (18.1) | 140.6 (17.2) | 142.8 (20.2) | 134.7 (19.2) |
| Apolipoprotein A (g/L) | 1.40 (0.23) | 1.44 (0.23) | 1.60 (0.27) | 1.64 (0.27) |
| Apolipoprotein B (g/L) | 1.07 (0.25) | 1.04 (0.23) | 1.09 (0.26) | 1.04 (0.24) |
| Low-density lipoprotein cholesterol (mmol/L) | 3.6 (0.9) | 3.6 (0.8) | 3.8 (1.0) | 3.6 (0.9) |
| High-density lipoprotein cholesterol (mmol/L) | 1.2 (0.3) | 1.3 (0.3) | 1.5 (0.4) | 1.6 (0.4) |
| Triglycerides (mmol/L) | 2.1 (1.2) | 2.0 (1.1) | 1.9 (1.0) | 1.5 (0.8) |
| HbA1c (mmol/mol) | 38.1 (9.1) | 35.9 (6.8) | 38.3 (8.8) | 35.5 (5.5) |
| Diabetes | 1895 (11%) | 9436 (5%) | 886 (10%) | 7595 (3%) |
| C-reactive protein (mg/L) | 3.0 (4.9) | 2.3 (4.1) | 3.7 (5.3) | 2.6 (4.2) |
| Estimated glomerular filtration rate | 92.3 (13.9) | 95.7 (12.5) | 90.9 (14.5) | 95.1 (12.9) |
| Alkaline phosphatase (U/L) | 84.7 (26.6) | 81.5 (24.4) | 92.5 (29.8) | 84.1 (27.1) |
| Aspartate aminotransferase (U/L) | 28.4 (12.0) | 28.1 (11.3) | 25.6 (11.0) | 24.4 (9.5) |
| Alanine aminotransferase (U/L) | 27.5 (16.1) | 27.5 (15.3) | 21.9 (13.2) | 20.1 (12.1) |
| Gamma-glutamyl transferase (U/L) | 48.8 (51.9) | 44.6 (46.4) | 36.7 (41.6) | 29.5 (32.4) |
CHD, coronary heart disease; CVD, cardiovascular disease; HbA1c, glycated haemoglobin; METs, metabolic equivalents of task.
First-ever CHD event defined as documentation of a CHD event upon hospital admission in medical records over the study period among persons without CHD at baseline.
Genetic risk scores
Most variants included in each GRS were positively associated with their respective adiposity measure in UKB, with no evidence of heterogeneity between the UKB and GIANT for BMI or WHRadjBMI and with a small underestimation of associations for WCadjBMI and HCadjBMI. (Supplementary Figure S3, available as Supplementary data at IJE online). The scores were robustly associated with each adiposity measure that they were derived for at baseline (Q5–Q1: BMI = 1.8 kg/m2, WC = 7.2 cm and HC = 1.9 cm) and, whereas the BMI GRS was positively associated with all adiposity measures, all other GRSs were weakly and inversely associated with BMI (Supplementary Tables S9 and S10, available as Supplementary data at IJE online). Potential confounders and mediators did not materially differ across quintiles of the GRSs, whereas more favourable markers were observed among those in the bottom quintiles of the measured adiposity markers. Together the GRSs explained 2.2%, 2.4%, 4.8% and 0.7% of variance for BMI, WHR, WC and HC, respectively, in UKB (corresponding F-statistics: 6883, 1544, 14 577 and 2367).
Observed associations between adiposity measures and CHD risk
During a median of 12.7 years, 26 225 (17 010 in men, 9215 in women) first-ever CHD events occurred. After adjusting for potential confounders, all four adiposity measures demonstrated strong, positive and approximately log-linear associations with incident CHD in both men and women (Figures 1 and 2). Associations for WC and WHR were slightly stronger for women than for men [HR per SD increasing WC: 1.27 (1.25–1.29) vs 1.21 (1.19–1.23) and WHR: 1.25 (1.23–1.27) vs 1.22 (1.21–1.24) for women vs men: Supplementary Figure S4, available as Supplementary data at IJE online, PHet<0.05]. HC displayed weaker positive associations with CHD risk in both men (1.13, 1.12–1.15) and women (1.16, 1.14–1.17). Results did not materially differ when further adjusted for physical activity and family history of CVD (Supplementary Figure S5, available as Supplementary data at IJE online).
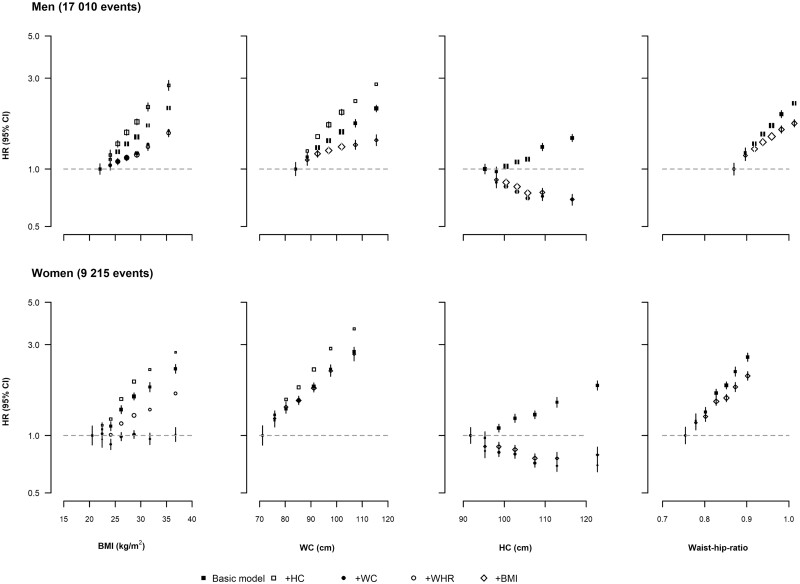
Figure 1.
Sex-specific hazard ratios for coronary heart disease with increasing levels of adiposity among men and women before and after mutual adjustment. Hazard ratios (HRs) were stratified by age at risk and UK region, and adjusted for education, deprivation index, ethnicity, smoking and alcohol consumption among (a) men and (b) women. HRs were calculated for tenths defined by the overall baseline adiposity measurements, with the middle six tenths combined in pairs, thus plotting seven groups corresponding to the top and bottom two tenths and the middle three fifths of each distribution. The area of each box is proportional to the amount of statistical information (i.e. it is inversely proportional to the variance of the log-HR). Error bars indicate the 95% CI. Closed squares represent HRs not adjusted for other adiposity measures. n=456 495 after exclusion of participants as described in Supplementary Figure S1 (available as Supplementary data at IJE online). BMI, body mass index; HC, hip circumference; WHR, waist-to-hip ratio; WC, waist circumference
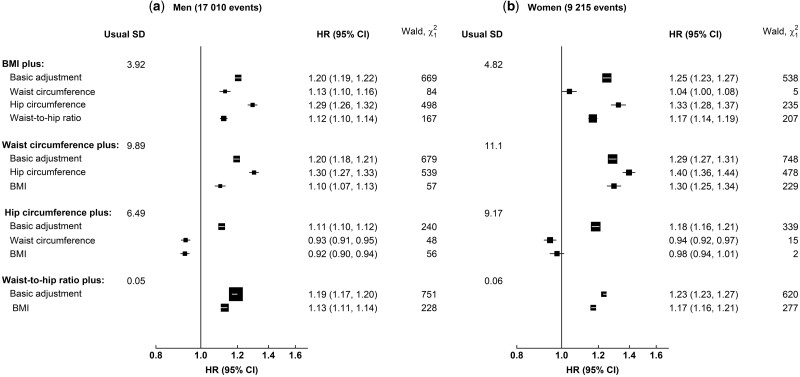
Figure 2.
Adjusted hazard ratios (95% confidence intervals) for coronary heart disease per standard deviation higher adiposity measure before and after mutual adjustments in (a) men and (b) women. Hazard ratios (HRs) were stratified by age at risk and UK region, and adjusted for education, deprivation index, ethnicity, smoking and alcohol consumption. The area of each box is proportional to the amount of statistical information (i.e. it is inversely proportional to the variance of the log-HR). Error bars indicate the 95% CIs. BMI, body mass index
For a given HC, the associations for both BMI and WC became stronger (Figure 2). However, after accounting for BMI and WC, HC displayed an inverse association with CHD risk. Associations with CHD risk for both BMI and WHR remained after mutual adjustment.
Intermediate factors
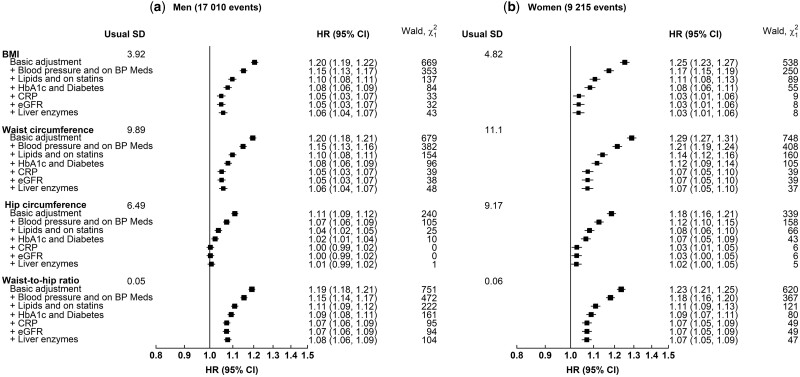
The associations of CHD risk with all four adiposity measures were largely accounted for by blood pressure, lipids, HbA1c levels and diabetes diagnosis (log-HR decrease by a range of 50% for WHR to 81% for HC among men and 55% for WC to 66% for BMI in women and the corresponding decreases in χ2 statistic were 79% and 96% for men and 86% and 90% for women, respectively) (Figure 3). Any remaining associations were further attenuated by additional adjustment for liver enzymes, glomerular filtration rate and inflammatory markers (Supplementary Figure S6, available as Supplementary data at IJE online).
Figure 3.
Hazard ratios (95% confidence intervals) for coronary heart disease risk per standard deviation higher adiposity measures with progressive adjustment for baseline risk factors. Hazard ratios (HRs) were stratified by age at risk and UK region, and adjusted for education, Townsend deprivation index, ethnicity, smoking and alcohol consumption. The area of each box is proportional to the amount of statistical information (i.e. it is inversely proportional to the variance of the log-HR). Error bars indicate the 95% CIs. Participants were excluded as shown in Supplementary Figure S1 (available as Supplementary data at IJE online). BP, blood pressure; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin
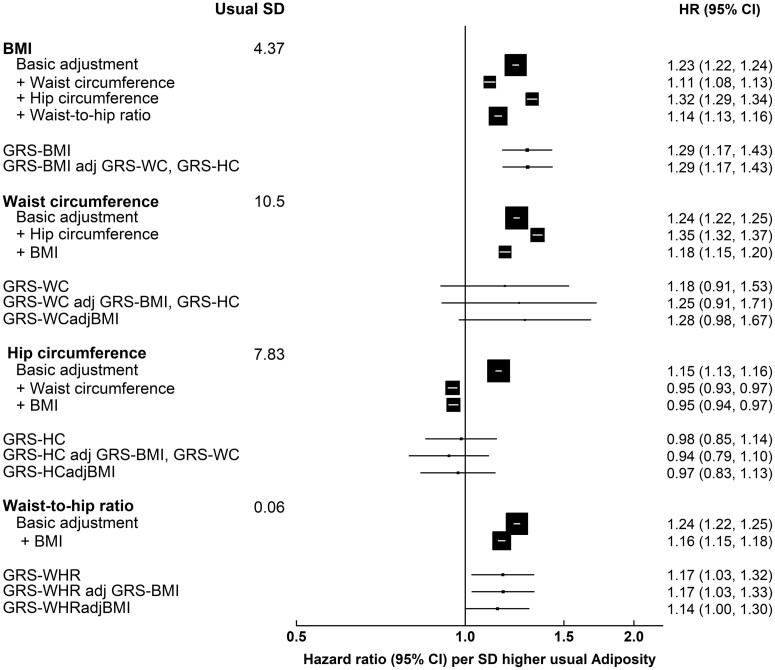
Associations between genetically predicted adiposity and CHD risk
Non-linear MR estimates from piecewise linear plots, as well as the doubly ranked method, showed linear trends similar to the observational associations (Supplementary Figures S7 and S8, available as Supplementary data at IJE online). Although less precise and weaker, estimated CHD risk per SD genetically predicted elevated adiposity from linear MR showed similar trends to the observed risk of CHD across all measures, but less for HC (Figure 4). Multivariable MR showed similar results. No heterogeneity by sex was observed (Supplementary Figure S4, available as Supplementary data at IJE online). With the exception of HC, each GRS was associated with at least one socio-economic or lifestyle confounder implicating potential pleiotropic effects (Supplementary Table S10, available as Supplementary data at IJE online). MR–Egger regression revealed that there was little evidence for directional pleiotropy for BMI among both men and women, WCadjBMI among women (Supplementary Tables S11A and B, available as Supplementary data at IJE online). After accounting for some evidence of pleiotropy for BMI, BMI was not associated with CHD risk whereas WC displayed strong positive associations with CHD among women [MR–Egger OR (95% CI): 2.89 (1.71, 4.90)]. However, MR–Egger has been shown to only work well when I2GX is large (>90%)34 and, whilst the I2GX statistic was relatively large for BMI, WC and WHR (women only) (>80%), it was lower for WHRadjBMI among men (63.0%) and HCadjBMI (∼62%) (Supplementary Tables S11A and B, available as Supplementary data at IJE online). In these cases, SIMEX did not show any evidence of regression dilution bias or any substantial associations. Results from weighted median MR only showed an association for WHRadjBMI among women [weighted median MR OR (95% CI): 1.32 (1.02, 1.70)] and results from MR-PRESSO did not show any evidence of a causal association between adiposity measures and CHD risk, apart from BMI and WHR among women, which were positively associated after removal of one outlier, which was considered to cause horizontal pleiotropy.
Figure 4.
Observed and genetic coronary heart disease risk (95% confidence intervals) per usual standard deviation higher adiposity measure among men and women combined. Observational analyses were restricted to 456 495 participants without outlying/missing adiposity variables or a history of cardiovascular disease at baseline. Genetic analyses were restricted to unrelated 369 225 participants of European ancestry, with complete genetic information, and without outlying/missing adiposity variables or a history of cardiovascular disease at baseline. Observational estimates were adjusted for age at baseline, ethnicity, education, region, smoking and alcohol. Genetic risk scores were composed of single-nucleotide polymorphisms (SNPs) discovered in European populations by using the GIANT consortia at genome-wide significance (P=5×10–8). All SNPs were weighted by the effect size as estimated in the GIANT consortia. Genetic analyses include also multivariable Mendelian randomization and were stratified by age at risk and region, and adjusted for age at baseline (5-year groups), age,2 sex, the first 10 genetic principle components, batch number and array type. The area of each box is proportional to the amount of statistical information (i.e. it is inversely proportional to the variance of the log-HR). GRS, genetic risk score; GRS-BMI, genetic risk score for body mass index; GRS-HC, genetic risk score for hip circumference; GRS-WHR, genetic risk score for waist-to-hip ratio; GRS-WC, genetic risk score for waist circumference; HR, hazard ratio
Discussion
This study demonstrates the independent relevance of body fat distribution and general adiposity to CHD risk in a large contemporary UK adult population. Apart from HC, adiposity measures remained independently associated with CHD over and above their associations with established risk factors, which together explained much of the associations with adiposity measures. Importantly, we found an independent inverse association between HC and CHD risk. This is the largest single study to investigate the observational and genetic associations of four commonly used adiposity measures with CHD risk in the same population. The standardized exposure measurements, together with the availability of repeat measurements, biomarkers and genetic data have allowed the assessment of adiposity-related CHD risk with greater detail and precision than has been possible previously.
For all the adiposity measures, we observed strong, positive log-linear associations with CHD risk throughout the range studied with no evidence of thresholds above or below which higher levels of adiposity were no longer associated with risk. In contrast, most previous studies have observed a J-shaped association, despite similarly strong, positive associations above a threshold.9,10,29,38,39 However, most large prior observational studies have investigated fatal CHD events only, primarily in relation to BMI and WHR. Although the greater relevance of body fat distribution measures to CHD and CVD risk compared with general adiposity has also been observed previously,40–42 large-scale data on HC are limited.5 Consistently with the findings presented in the current study, a number of previous MR studies have identified positive associations with genetically elevated adiposity and CHD risk.13–19,43 However, of the previous MR studies assessing the genetic associations of body fat distribution (as measured via WHRadjBMI or WC or HCadjBMI) with CHD risk,13–15,19 the majority used summary-level statistics from the CARDIoGRAMplusC4D Consortium,13,15,19 limiting the ability to investigate the shape of associations.
The biological mechanisms through which excess adiposity contributes to CHD risk are not fully understood, although elevated blood pressure, dysglycaemia and dyslipidaemia are thought to play key roles.8,9,17 The results from the current study support this hypothesis, as evidenced by the observed attenuation of adiposity-related CHD risk after adjusting observational associations for multiple potential intermediate factors, although CHD risk remained for WHR that was unexplained by these risk factors.
The opposing associations with CHD risk observed between HC and central adiposity (WC and WHR) support the theory that upper- and lower-body fat depots exert fundamental functional differences, although this has not been fully elucidated.44 Comparing the adipose tissue function between lower- and upper-body fat, in particular visceral fat, the gluteofemoral tissue may exert protective cardiometabolic effects by acting as a metabolic ‘sink’, thereby reducing ectopic fat storage. Several potential underlying differences in mechanisms of action between adipose tissues include lower triglyceride turnover and resistance to stress hormone-induced lipolysis45 or upregulated leptin or palmitoleic acid pathways in the lower-body adipose tissue.44
Importantly, the findings from the current study have important implications for population health. Principally, by demonstrating an independent relevance of body fat distribution on CHD risk, our findings suggest that HC, WC and WHR could provide additional information beyond BMI for improved CHD risk prediction at the population level.40,41 Many risk tools have been developed, most of which estimate an individual’s 10-year absolute risk of CHD or CVD from measures of varying applicability to general practice settings.46 Pragmatic risk scores that utilize simple measures such as body size instead of lipid levels may be of greater value in low-resource settings. This is of particular importance given the rising incidence of CVD in developing countries relative to developed countries.47 Despite several population studies describing independent effects of WHR and WC on CVD outcomes beyond BMI,40,41 there is a lack of evidence for the contribution of these measures to CVD risk tools.
The current study has potential limitations. First, participants were primarily White and middle-aged, potentially limiting the generalizability of these findings. In contemporary Western populations exposed to ‘obesogenic’ environments for decades, the distribution of adiposity has shifted towards higher levels. Therefore, the current study lacks statistical power to investigate CHD risk at low levels of adiposity commonly seen in lower-income settings. Second, WC and HC measures are prone to measurement error. However, this was somewhat counteracted by the availability of repeat measures, which allowed us to calculate ‘usual’ levels.26 Fifth, the GRSs were associated with a number of socio-economic and lifestyle factors as noted previously in UKB48 but GRSs did not display evidence of directional pleiotropy using MR–Egger, apart from WCadjBMI. After accounting for this, there was evidence of a causal association, supporting the primary results. Finally, the GRSs for WC and HC explained only a small proportion of variance of the corresponding adiposity measures, resulting in weak instrument bias. Future discovery efforts in diverse populations will be important to address this limitation.
Conclusions
In summary, BMI, WC and WHR adiposity measures demonstrated strong, positive and approximately log-linear associations with CHD risk, with no evidence of threshold effects within the ranges studied. Blood pressure, lipids and dysglycaemia largely accounted for the observational prospective associations. In contrast, HC demonstrated an independent protective effect against CHD risk. Genetically predicted elevated adiposity measures were directionally concordant with observational estimates. Therefore, body fat distribution and general adiposity should be considered separately when estimating CHD risk and investigating the aetiology of CHD.
Ethics approval
Ethics approval for UKB was granted by the North West Multi-Centre Research Ethics Committee in May 2006. All participants provided informed written consent.
Supplementary Material
Acknowledgements
This research has been conducted using the UKB resource under application number 31461. This work used data provided by patients and collected by the NHS as part of their care and support. Copyright © (2023), NHS England. Re-used with the permission of the NHS England and UKB. All rights reserved. This research also used data assets made available by National Safe Haven as part of the Data and Connectivity National Core Study, led by Health Data Research UK in partnership with the Office for National Statistics and funded by UK Research and Innovation (research that commenced between 1 October 2020–31 March 2021, grant ref. MC_PC_20029, and 1 April 2021–30 September 2022, grant ref. MC_PC_20058).
Contributor Information
Eirini Trichia, Clinical Trial Service Unit & Epidemiological Studies Unit (CTSU), Nuffield Department of Population Health, University of Oxford, Oxford, UK; MRC Population Health Research Unit, Clinical Trial Service Unit & Epidemiological Studies Unit (CTSU), Nuffield Department of Population Health, University of Oxford, Oxford, UK.
Debbie E Malden, Clinical Trial Service Unit & Epidemiological Studies Unit (CTSU), Nuffield Department of Population Health, University of Oxford, Oxford, UK.
Danyao Jin, Clinical Trial Service Unit & Epidemiological Studies Unit (CTSU), Nuffield Department of Population Health, University of Oxford, Oxford, UK.
Neil Wright, Clinical Trial Service Unit & Epidemiological Studies Unit (CTSU), Nuffield Department of Population Health, University of Oxford, Oxford, UK.
Hannah Taylor, Clinical Trial Service Unit & Epidemiological Studies Unit (CTSU), Nuffield Department of Population Health, University of Oxford, Oxford, UK; NIHR Oxford Biomedical Research Centre, Oxford University Hospitals (OUH) Foundation Trust, Oxford, UK.
Fredrik Karpe, NIHR Oxford Biomedical Research Centre, Oxford University Hospitals (OUH) Foundation Trust, Oxford, UK; The Oxford Centre for Diabetes, Endocrinology and Metabolism (OCDEM), University of Oxford, Oxford, UK.
Paul Sherliker, Clinical Trial Service Unit & Epidemiological Studies Unit (CTSU), Nuffield Department of Population Health, University of Oxford, Oxford, UK; MRC Population Health Research Unit, Clinical Trial Service Unit & Epidemiological Studies Unit (CTSU), Nuffield Department of Population Health, University of Oxford, Oxford, UK.
Federico Murgia, Clinical Trial Service Unit & Epidemiological Studies Unit (CTSU), Nuffield Department of Population Health, University of Oxford, Oxford, UK.
Jemma C Hopewell, Clinical Trial Service Unit & Epidemiological Studies Unit (CTSU), Nuffield Department of Population Health, University of Oxford, Oxford, UK.
Ben Lacey, Clinical Trial Service Unit & Epidemiological Studies Unit (CTSU), Nuffield Department of Population Health, University of Oxford, Oxford, UK.
Jonathan Emberson, Clinical Trial Service Unit & Epidemiological Studies Unit (CTSU), Nuffield Department of Population Health, University of Oxford, Oxford, UK; MRC Population Health Research Unit, Clinical Trial Service Unit & Epidemiological Studies Unit (CTSU), Nuffield Department of Population Health, University of Oxford, Oxford, UK.
Derrick Bennett, Clinical Trial Service Unit & Epidemiological Studies Unit (CTSU), Nuffield Department of Population Health, University of Oxford, Oxford, UK; MRC Population Health Research Unit, Clinical Trial Service Unit & Epidemiological Studies Unit (CTSU), Nuffield Department of Population Health, University of Oxford, Oxford, UK; NIHR Oxford Biomedical Research Centre, Oxford University Hospitals (OUH) Foundation Trust, Oxford, UK.
Sarah Lewington, Clinical Trial Service Unit & Epidemiological Studies Unit (CTSU), Nuffield Department of Population Health, University of Oxford, Oxford, UK; MRC Population Health Research Unit, Clinical Trial Service Unit & Epidemiological Studies Unit (CTSU), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Health Data Research UK Oxford, University of Oxford, Oxford, UK.
Data availability
The data underlying this article were provided by UKB under application number 31461. Data can be accessed under the UKB data access process (https://www.ukbiobank.ac.uk/enable-your-research).
Supplementary data
Supplementary data are available at IJE online.
Author contributions
E.T. performed the analysis and completed the first draft of the manuscript. D.M. designed the project, acquired the data, performed part of the analysis and started the first draft of the manuscript. D.J. processed the outcome variable. H.T., N.W. and P.S. performed part of the analysis. F.D. processed and provided the genetic data. J.H. supervised the processing of the genetic data. B.L., J.E., D.B. and S.L. supervised the project and contributed to the project design. All the authors revised the manuscript and gave critical input.
Funding
This work was supported by the National Institute for Health Research Oxford Biomedical Research Centre to F.K., H.T., D.B., B.L. and J.C.H.; the British Heart Foundation Centre of Research Excellence, Oxford to B.L. and J.C.H.; British Heart Foundation (grant numbers RG/17/1/32663, FS/14/55/30806) to F.K. and J.C.H., respectively; the Medical Research Council (NEWTON-MRC/2020/004) to S.L.; the US Centers for Disease Control and Prevention Foundation (with support from Amgen) to S.L.; and the World Health Organization to S.L.
Conflict of interest
The Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU) receives research grants from industry that are governed by University of Oxford contracts that protect its independence and has a staff policy of not taking personal payments from industry; further details can be found at https://www.ndph.ox.ac.uk/files/about/ndph-independence-of-research-policy-jun-20.pdf. The authors declare no other conflict of interest.
References
- 1. Bennett DA, Krishnamurthi RV, Barker-Collo S. et al. ; Global Burden of Diseases, Injuries, and Risk Factors 2010 Study Stroke Expert Group. The global burden of ischemic stroke: findings of the GBD 2010 study. Glob Heart 2014;9:107–12. [DOI] [PubMed] [Google Scholar]
- 2. Neeland IJ, McGuire DK, Sattar N.. Cardiovascular outcomes trials for weight loss interventions: another tool for cardiovascular prevention? Circulation 2021;144:1359–61. [DOI] [PubMed] [Google Scholar]
- 3. Hajer GR, Van Haeften TW, Visseren FLJ.. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 2008;29:2959–71. [DOI] [PubMed] [Google Scholar]
- 4. Canoy D. Coronary heart disease and body fat distribution. Curr Atheroscler Rep 2010;12:125–33. [DOI] [PubMed] [Google Scholar]
- 5. Gnatiuc L, Tapia-Conyer R, Wade R. et al. Abdominal and gluteo-femoral markers of adiposity and risk of vascular-metabolic mortality in a prospective study of 150 000 Mexican adults. Eur J Prev Cardiol 2022;29:730–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee CMY, Huxley RR, Wildman RP, Woodward M.. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol 2008;61:646–53. [DOI] [PubMed] [Google Scholar]
- 7. Swainson MG, Batterham AM, Tsakirides C, Rutherford ZH, Hind K.. Prediction of whole-body fat percentage and visceral adipose tissue mass from five anthropometric variables. PLoS One 2017;12:e0177175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Gaal LF, Mertens IL, Christophe E.. Mechanisms linking obesity with cardiovascular disease. Nature 2006;444:875–80. [DOI] [PubMed] [Google Scholar]
- 9. Lu Y, Hajifathalian K, Ezzati M. et al. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet 2014;383:970–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wormser D, Kaptoge S, Di Angelantonio E. et al. ; Emerging Risk Factors Collaboration. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet 2011;377:1085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burgess S, Thompson SG.. Use of allele scores as instrumental variables for Mendelian randomization. Int J Epidemiol 2013;42:1134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smith GD, Ebrahim S.. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 2003;32:1–22. [DOI] [PubMed] [Google Scholar]
- 13. Dale CE, Fatemifar G, Palmer TM. et al. ; UCLEB Consortium; METASTROKE Consortium. Causal associations of adiposity and body fat distribution with coronary heart disease, stroke subtypes, and type 2 diabetes mellitus: a Mendelian randomization analysis. Circulation 2017;135:2373–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elosua R, Lluis-Ganella C, Subirana I. et al. Cardiovascular risk factors and ischemic heart disease. Circ Cardiovasc Genet 2016;9:279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Emdin CA, Khera AV, Natarajan P. et al. Genetic association of waist-to-hip ratio with cardiometabolic traits, type 2 diabetes, and coronary heart disease. JAMA 2017;317:626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hagg S, Fall T, Ploner A. et al. ; European Network for Genetic and Genomic Epidemiology Consortium. Adiposity as a cause of cardiovascular disease: A Mendelian randomization study. Int J Epidemiol 2015;44:578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holmes MV, Lange LA, Palmer T. et al. Causal effects of body mass index on cardiometabolic traits and events: a Mendelian randomization analysis. Am J Hum Genet 2014;94:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nordestgaard BG, Palmer TM, Benn M. et al. The effect of elevated body mass index on ischemic heart disease risk: causal estimates from a Mendelian randomisation approach. PLoS Med 2012;9:e1001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang X, Lv WQ, Qiu B. et al. Assessing causal estimates of the association of obesity-related traits with coronary artery disease using a Mendelian randomization approach. Sci Rep 2018;8:7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bycroft C, Freeman C, Petkova D. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018;562:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sudlow C, Gallacher J, Allen N. et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Locke AE, Kahali B, Berndt SI. et al. ; International Endogene Consortium. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015;518:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heid IM, Jackson AU, Randall JC, MAGIC et al. Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat Genet 2010;42:949–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shungin D, Winkler T, Croteau-Chonka DC. et al. ; ReproGen Consortium. New genetic loci link adipose and insulin biology to body fat distribution. Nature 2015;518:187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Palmer TM, Lawlor DA, Harbord RM. et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res 2012;21:223–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clarke R, Shipley M, Lewington S. et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol 1999;150:341–53. [DOI] [PubMed] [Google Scholar]
- 27. Clarke R, Emberson JR, Breeze E. et al. Biomarkers of inflammation predict both vascular and non-vascular mortality in older men. Eur Heart J 2008;29:800–809. [DOI] [PubMed] [Google Scholar]
- 28. Easton DF, Peto J, Babiker AGAG.. Floating absolute risk: an alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Stat Med 1991;10:1025–35. [DOI] [PubMed] [Google Scholar]
- 29. Peters SAE, Bots SH, Woodward M.. Sex differences in the association between measures of general and central adiposity and the risk of myocardial infarction: results from the UK Biobank. J Am Heart Assoc 2018;7:e008507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu J, Au Yeung SL, Lin SL, Leung GM, Schooling CM.. Liver enzymes and risk of ischemic heart disease and type 2 diabetes mellitus: a Mendelian randomization study. Sci Rep 2016;6:38813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wade KH, Carslake D, Sattar N, Davey Smith G, Timpson NJ.. BMI and mortality in UK Biobank: revised estimates using Mendelian randomization. Obesity (Silver Spring) 2018;26:1796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burgess S, Davies NM, Thompson SG. , EPIC-InterAct Consortium. Instrumental variable analysis with a nonlinear exposure–outcome relationship. Epidemiology 2014;25:877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Staley JR, Burgess S.. Semiparametric methods for estimation of a nonlinear exposure‐outcome relationship using instrumental variables with application to Mendelian randomization. Genet Epidemiol 2017;41:341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bowden J, Del Greco M F, Minelli C, Davey Smith G, Sheehan NA, Thompson JR.. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol 2016;45:1961–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Verbanck M, Chen C-Y, Neale B, Do R.. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 2018;50:693–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vandenbroucke JP, Von Elm E, Altman DG. et al. ; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Medicine 2007;4:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Skrivankova VW, Richmond RC, Woolf BA. et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA 2021;326:1614–21. [DOI] [PubMed] [Google Scholar]
- 38. Wormser D, White IR, Thompson SG, Wood AM.. Methodology within-person variability in calculated risk factors: Comparing the aetiological association of adiposity ratios with risk of coronary heart disease. Int J Epidemiol 2013;42:849–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Whitlock G, Lewington S, Sherliker P. et al. ; Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Canoy D, Cairns BJ, Balkwill A. et al. ; Million Women Study Collaborators. Coronary heart disease incidence in women by waist circumference within categories of body mass index. Eur J Prev Cardiol 2013;20:759–62. [DOI] [PubMed] [Google Scholar]
- 41. Egeland GM, Igland J, Vollset SE, Sulo G, Eide GE, Tell GS.. High population attributable fractions of myocardial infarction associated with waist-hip ratio. Obesity (Silver Spring) 2016;24:1162–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Leitzmann MF, Moore SC, Koster A. et al. Waist circumference as compared with body-mass index in predicting mortality from specific causes. PLoS One 2011;6:e18582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Larsson SC, Bäck M, Rees JMB, Mason AM, Burgess S.. Body mass index and body composition in relation to 14 cardiovascular conditions in UK Biobank: a Mendelian randomization study. Eur Heart J 2020;41:221–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Karpe F, Pinnick KE.. Biology of upper-body and lower-body adipose tissue - Link to whole-body phenotypes. Nat Rev Endocrinol 2015;11:90–100. [DOI] [PubMed] [Google Scholar]
- 45. Manolopoulos KN, Karpe F, Frayn KN.. Marked resistance of femoral adipose tissue blood flow and lipolysis to adrenaline in vivo. Diabetologia 2012;55:3029–37. [DOI] [PubMed] [Google Scholar]
- 46. Pennells L, Kaptoge S, Wood A. et al. ; Emerging Risk Factors Collaboration. Equalization of four cardiovascular risk algorithms after systematic recalibration: individual-participant meta-analysis of 86 prospective studies. Eur Heart J 2019;40:621–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stanaway JD, Afshin A, Gakidou E. et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1923–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lyall DM, Celis-Morales C, Ward J. et al. Association of body mass index with cardiometabolic disease in the UK Biobank: a Mendelian randomization study. JAMA Cardiol 2017;2:882–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by UKB under application number 31461. Data can be accessed under the UKB data access process (https://www.ukbiobank.ac.uk/enable-your-research).