Key Features.
Project Viva, a pre-birth cohort of 2128 mother-child pairs, was initiated in 1999–2002 in Massachusetts to examine the extent to which factors and events during pregnancy and the perinatal period affect health outcomes over a lifetime among women and their offspring.
This updated cohort profile follows up women through mid-life. Two decades of continual follow-up enable us to evaluate women’s health across their reproductive years and health conditions that progress or emerge during mid-life.
New measures in women include assessments of lifetime fertility, biomarkers of cardiometabolic health and bone turnover, plasma concentrations of per- and polyfluoroalkyl substance (PFAS) chemicals, dual-energy X-ray absorptiometry (DXA) scans for body composition and bone density, and menopause-related symptoms. We also collected 24-h dietary recalls, analysed DNA, assessed impact of the COVID pandemic, and measured multiple environmental chemicals and contextual factors based on residential addresses.
A total of 843 women had any Mid-Life Visit data (2017–21; median age 51.1 years).
At the start of the ongoing Women’s Health Visit 1 in April 2022, 1356 women (median age 54.0 years) were eligible to participate.
Investigators interested in learning more about how to obtain Project Viva data can contact the Project Viva Principal Investigator Emily Oken at [project_viva@hphci.harvard.edu] or can get more information at [https://www.projectviva.org].
The original cohort
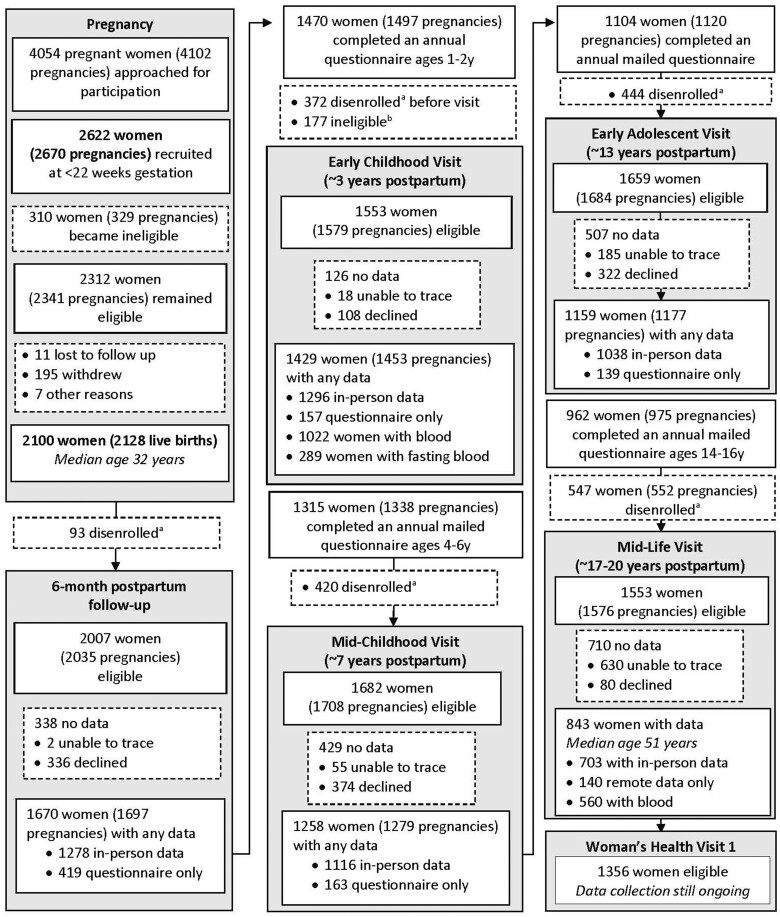
We established Project Viva to examine the extent to which events during the perinatal period may affect health outcomes over a lifetime. The cohort recruited pregnant women and follows women and their children over time. Research assistants recruited women at their initial prenatal visit (median 9.9 weeks’ gestation) April 1999–July 2002 from eight obstetric offices of Atrius Harvard Vanguard Medical Associates, a multispecialty group practice in suburban/urban Eastern Massachusetts.1 Women with a singleton pregnancy were eligible if they enrolled before 22 weeks’ gestation, planned to deliver at one of the two study hospitals and were able to answer questionnaires in English. We recruited 2622 women. After recruitment, we found 310 of them to be ineligible (moved out of study area, multiple gestation, miscarriage or stillbirth), leaving 2312 eligible women of whom 2100 were still enrolled at the time of delivery and had a live birth (Figure 1).
Figure 1.
Flow of Project Viva mothers’ involvement through the Mid-Life Visit. aCumulative number disenrolled from birth to visit. bIneligible for visit because no information on diet in pregnancy. We adapted Figure 1 from our original cohort profile1
Research assistants conducted mid-pregnancy in-person visits at the time of clinical glycaemic screening (median 27.9 weeks’ gestation) and delivery visits with mother and infant dyads in the hospital during the birth admission (median 39.7 weeks’ gestation). We obtained birth information on 2128 live-born singleton infants born to 2100 women (28 women enrolled with two separate pregnancies). We conducted additional in-person visits with mother and child dyads during infancy (median offspring age 6.2 months), early childhood (median 3.3 years), mid-childhood (median 7.7 years) and early adolescence (median 12.9 years), as described in our original cohort profile.1 Between visits, we administered annual mailed or online questionnaires.
Since our original cohort profile,1 we completed additional research visits with women (‘Mid-Life Visit’, median age 51.1 years) and adolescents (‘Mid-Adolescent Visit’, median 17.5 years) over July 2017–August 2021. By ‘mid-life’, we mean the middle years of adulthood, roughly from ages 35 to 65.2 We also administered four additional questionnaire-only visits: the Year 19 Questionnaire (December 2019 to June 2022), the 2020 Questionnaire (May to September 2020), the 2021 Questionnaire (February to August 2021) and the 2022 Questionnaire (January to December 2022). Project Viva is now in a new chapter where we are focusing on the women and young adult offspring as separate study participants instead of as pairs. As such, in April 2022, we launched the Women’s Health Visit (‘WHV1’) among 1356 eligible women (median age 54.0 years among those still enrolled). This updated cohort profile focuses on Project Viva women as they transition through mid-life. For simplicity we call the Viva mothers ‘women’ throughout, although we did not collect gender identity and some may have other identities.
Project Viva has shared data with numerous research consortia, including the Environmental influences on Child Health Outcomes (ECHO) programme sponsored by the US National Institutes of Health, which has provided a public access database [https://dash.nichd.nih.gov/].3 Other collaborations include the Maternal Obesity and Childhood Outcomes (MOCO) group,4 Consortium on Thyroid and Pregnancy (CTP),5 Early Growth and Genetics (EGG) consortium,6 Pregnancy And Childhood Epigenetics (PACE) consortium7 and Genetics of Diabetes In Pregnancy (GenDIP).
What is the reason for the new data collection?
We wanted to follow Project Viva women through key stages of the life course. Project Viva women are in mid-life, an important transition period. During the perimenopause, women experience marked physical, physiological and neuropsychiatric changes that extend beyond cessation of menses, including alterations that accelerate the aging process.8 Metabolic risk factors that may emerge or worsen include central adiposity, hypertension, insulin resistance, hyperglycaemia and hyperlipidaemia.9–13 Concomitantly, middle adulthood is a time of progressive loss of muscle and bone mass.14 Many perimenopausal women also experience vasomotor instability and neuropsychiatric changes, including symptoms of depression, anxiety, memory problems and sleep disturbance, which represent major detriments to quality of life and may be important sex-specific risk markers for cardiometabolic diseases and cognitive decline.15 Data collection on women from pregnancy through the menopausal transition allows us to examine how health in the peripartum period and beyond can influence health across adulthood.
What will be the new areas of research?
Current aims focus on testing our hypotheses that perimenopausal women with higher exposure to physiological, social and environmental stressors during important reproductive transitions—pregnancy, the peripartum period and the perimenopause—will have poorer health outcomes in mid-life, including greater cardiometabolic risk, unfavourable neuropsychiatric symptoms and poorer sleep duration and quality. We are evaluating pregnancy as a sensitive window and stress test for later-life cardiometabolic health.16,17 We are also examining whether evidence of impaired fertility over the reproductive years affects various aspects of health during mid-life, including higher adiposity, lower muscle mass and bone density and greater cardiometabolic and mental health risk. Additionally, we are studying the extent to which exposure to per- and polyfluoroalkyl substances (PFAS) alter the trajectory of cardiovascular disease risk in women across their reproductive lives and alter bone mineral density in mid-life. Using the extensive dietary data collected via repeated administration of an automated 24-h recall, we will examine how dietary intake and practices in mid-life impact or modify a range of health outcomes.
Who is in the cohort?
Since our original cohort profile,1 we have completed the Mid-Life Visit and four additional questionnaire-only visits. At the start of the Mid-Life Visit, 1553 women (median age 51.1 years) were enrolled and therefore eligible for the visit. Of 1553 women, 843 (54%) had any Mid-Life Visit data. At the questionnaire-only visits, 653 of 1208 (54%) had any Year 19 data, 537 of 1164 (46%) had any 2020 questionnaire data, 584 of 1187 (49%) had any 2021 questionnaire data and 481 of 1254 (38%) had any 2022 questionnaire data; 795 had data from any of the four questionnaires. We only sent these questionnaires electronically. If we did not have an e-mail on file, the participant did not receive a questionnaire. This was because we were fully remote during the COVID pandemic, and we could not go into the office to mail questionnaires. There were a few other exclusions at each time point due to specific requests (e.g. some participants reported that they temporarily did not want to participate, others had unique circumstances that did not allow them to complete questionnaires, such as military service/deployment or jail time).
We are currently conducting the WHV1 (2022–24). This visit is not complete and is in progress. At the start of the WHV1, 1356 women (median age 54.0 years) remained enrolled and therefore eligible for the visit. We summarize characteristics of Project Viva women enrolled at baseline and those who provided any data from the Mid-Life Visit in Table 1; most characteristics do not notably differ. However, women who completed a Mid-Life Visit tended to be older and more likely to be college graduates, non-smokers and have higher household income.
Table 1.
Selected baseline characteristics of Project Viva women at enrolment (1999–2002) and those who completed any component of the Mid-Life Visit (2017–21)
| Characteristic | Enrolled at index pregnancy (n=2100) | Any Mid-Life Visit |
|
|---|---|---|---|
| Yes (n=843) | No (n=1257) | ||
| At enrolment | |||
| Age, years, mean (SD) | 31.8 (5.2) | 32.5 (5.0) | 31.3 (5.3) |
| Pre-pregnancy BMI, kg/m2, mean (SD) | 24.9 (5.5) | 24.6 (5.2) | 25.1 (5.8) |
| Pre-pregnancy BMI categories, kg/m2 | |||
| <18.5 | 81 (4%) | 23 (3%) | 58 (5%) |
| 18.5–24.9 | 1222 (59%) | 522 (62%) | 700 (56%) |
| 25–29.9 | 453 (22%) | 184 (22%) | 269 (22%) |
| ≥30 | 330 (16%) | 113 (13%) | 217 (17%) |
| Race and ethnicity | |||
| Hispanic | 152 (7%) | 56 (7%) | 96 (8%) |
| White | 1379 (66%) | 576 (69%) | 803 (65%) |
| Black | 346 (17%) | 125 (15%) | 221 (18%) |
| Asian | 118 (6%) | 45 (5%) | 73 (6%) |
| Other | 81 (4%) | 38 (5%) | 43 (3%) |
| Nulliparous | 1017 (48%) | 411 (49%) | 606 (48%) |
| Education ≥college graduate | 1340 (65%) | 621 (74%) | 719 (58%) |
| Household income/year | |||
| <$40 000 | 290 (16%) | 112 (14%) | 178 (17%) |
| $40–$70 000 | 435 (24%) | 158 (20%) | 277 (26%) |
| > $70 000 | 1124 (61%) | 504 (65%) | 620 (58%) |
| Smoking | |||
| Never | 1425 (69%) | 601 (72%) | 824 (67%) |
| Before pregnancy | 389 (19%) | 161 (19%) | 228 (18%) |
| During pregnancy | 265 (13%) | 78 (9%) | 187 (15%) |
| Pregnancy characteristics | |||
| Pregnancy glucose status | |||
| Normal | 1687 (83%) | 697 (84%) | 990 (82%) |
| Isolated hyperglycaemia | 176 (9%) | 70 (8%) | 106 (9%) |
| Impaired glucose tolerance | 63 (3%) | 29 (3%) | 34 (3%) |
| Gestational diabetes mellitus | 113 (6%) | 35 (4%) | 78 (6%) |
| Pregnancy hypertension status | |||
| Normal | 1833 (89%) | 738 (89%) | 1095 (88%) |
| Gestational hypertension | 135 (7%) | 55 (7%) | 80 (6%) |
| Pre-eclampsia | 74 (4%) | 26 (3%) | 48 (4%) |
| Chronic hypertension | 27 (1%) | 12 (1%) | 15 (1%) |
| Gestational weight gaina | |||
| Inadequate | 267 (13%) | 115 (14%) | 152 (13%) |
| Adequate | 586 (29%) | 236 (28%) | 350 (29%) |
| Excessive | 1187 (58%) | 481 (58%) | 706 (58%) |
| Caesarean delivery | 496 (24%) | 189 (22%) | 307 (25%) |
| Preterm delivery <37 weeks | 152 (7%) | 56 (7%) | 96 (8%) |
Gestational weight gain per 2009 Institute of Medicine guidelines.
BMI, body mass index.
Challenges of long-term follow-up
Early in the cohort study, we found the postpartum transition to be a particularly challenging time for retention. More recent challenges were related to following up ‘linked’ mother-child dyads together, particulaly as the teens became more independent and decided for themselves whether and to what extent to continue their participation. From the postpartum period up until the Mid-Life/Mid-Adolescent visit, we primarily focused on child health outcomes and collected minimal measures about the women. With our renewed focus on mid-life maternal health, we refined our outreach to confirm that both women and offspring are equally valuable participants, and that we are happy to retain women even if their young adult child no longer wishes to participate.
What has been measured?
Table 2 summarizes the data that we collected from women at the Mid-Life Visit (n = 843 with any data). We measured blood pressure and hand grip strength, collected anthropometric (weight, height, waist circumference) and dual X-ray absorptiometry (DXA) measures of body composition, and obtained fasting blood for bone turnover and cardiometabolic risk markers. We measured hip and spine bone mineral density using DXA. We collected data on self-reported clinical diagnosis of polycystic ovary syndrome, financial hardship, depressive and anxiety symptoms, diet and menopause symptoms, as well as lifetime fertility and pregnancy history. We will quantify the presence of 40 different PFAS in plasma from the Mid-Life Visit. Our earlier publication1 provides additional details about the measures collected at prior study visits.
Table 2.
Selected characteristics of Project Viva women at the Mid-Life Visit (2017–21)
| Characteristic | Completed any component of the Mid-Life Visit (n=843) a |
|---|---|
| Self-reported ever clinician diagnosis, % yes | |
| Depression (n=774) | 21% |
| Anxiety (n = 764) | 19% |
| Thyroid disease (n = 820) | 15% |
| Asthma (n = 820) | 17% |
| Hayfever, seasonal allergies or allergic rhinitis (n = 820) | 40% |
| Eczema (atopic dermatitis) (n = 819) | 16% |
| High blood pressure (hypertension) during a time when not pregnant (n = 819) | 15% |
| High blood pressure (hypertension) during a time when pregnant (n = 818) | 16% |
| Any cardiovascular disease (n = 820) | 4% |
| High cholesterol (n = 819) | 15% |
| Type 1 or 2 diabetes (n = 820) | 4% |
| Gestational diabetes (n = 819) | 7% |
| Autoimmune disease (n = 820) | 7% |
| Osteoporosis (n = 820) | 2% |
| Cancer (n = 819) | 10% |
| Polycystic ovary syndrome (n = 819) | 5% |
| Other self-reported dichotomous characteristics, % yes | |
| Any financial hardship in past 5 years (n = 748) | 12% |
| Life-time infertility (n = 751) | 35% |
| Postmenopause (n = 767) | 43% |
| Moderate to very severe hot flushes (n = 773) | 30% |
| Continuous characteristics, self-reported, mean (SD) | |
| Age at Mid-Life Visit, years (n = 843) | 50.9 (5.1) |
| Age at natural menopause, years (among those postmenopause) (n = 245) | 50.2 (3.9) |
| Menopause Rating Scale (0–30 possible range), points (n = 770) | 7.9 (5.9) |
| Sleep duration, h/day (n = 767) | 7.1 (1.0) |
| Light/moderate + vigorous physical activity, h/week (n = 773) | 5.6 (6.0) |
| Anxiety symptoms (GAD-7 score, 0–21 possible range), points (n = 741) | 2.0 (3.0) |
| Depression symptoms (PHQ-9 score, 0–27 possible range), points (n = 734) | 2.5 (3.4) |
| Healthy Eating Index 2015 total score (0–100 possible range), points (n = 748) | 60.1 (12.8) |
| Continuous characteristics, research measured, mean (SD) | |
| Hand grip strength dominant hand, kg (n = 674) | 27.9 (6.1) |
| Body mass index, kg/m2 (n = 692) | 28.2 (6.7) |
| Waist circumference, cm (n = 697) | 93.1 (15.8) |
| DXA total body fat, percent (n = 549) | 38.1 (7.0) |
| DXA total fat mass, kg (n = 549) | 30.2 (12.5) |
| DXA lean mass, kg (n = 549) | 46.4 (7.5) |
| DXA truncal fat mass, kg (n = 549) | 14.4 (7.3) |
| Total BMD L1–L4 spine, T-score (n = 542) | –0.15 (1.38) |
| Total BMD left hip, T-score (n = 531) | 0.00 (1.14) |
| Systolic blood pressure, mmHg (n = 695) | 117.1 (14.5) |
| Diastolic blood pressure, mmHg (n = 695) | 74.3 (11.0) |
| Total cholesterol, mg/dL (n = 558) | 208.5 (51.4) |
| Non-HDL cholesterol, mg/dL (n = 558) | 140.6 (43.7) |
| HbA1c, percent (n = 554) | 5.3 (0.6) |
| Fasting glucose, mg/dL (n = 526) | 91.9 (17.7) |
BMD, bone mineral density; DXA, dual X-ray absorptiometry; GAD-7, Generalized Anxiety Disorder-7; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; PHQ-9, Patient Health Questionnaire-9.
Numbers vary depending on questionnaire, interview, in-person measurements and blood tests.
We have linked geocoded residential address information to multiple measures of neighbourhood environment including social factors such as the Social Vulnerability Index,18 markers of air pollution including PM2.5 and black carbon daily from enrolment onwards,19 and measures of the built and natural environments, including Landsat Normalized Difference Vegetation Index (NDVI),20 a satellite-based objective indicator of the quantity of green vegetation on the ground, and Google Street View-based measures. We have also extracted DNA from blood samples to obtain genotypes using the Illumina Infinium Core Exome-24 genetic microarrays for 834 women who provided consent for genetic analysis of biospecimen samples. We assessed diet and analysed dietary intake data using the Automated Self-Administered 24-h (ASA24) Dietary Assessment Tool, versions 2016, 2018 and 2020, developed by the National Cancer Institute, Bethesda, MD.21 Annual questionnaires in 2020 and 2021 assessed behavioural, financial and psychosocial impacts of the COVID pandemic.
At the start of the ongoing ‘Women’s Health Visit (WHV) 1’ in April 2022, 1356 women (median age 54 years) were eligible for the visit (Table 3). We invite participants to attend in-person visits at our research clinic in Boston, but also offer home or remote visits if travel to Boston is prohibitive or the participant is not comfortable with an in-person study visit. Participants have the option to complete the consent and questionnaire electronically. The WHV1 components include: (i) a questionnaire about health, behaviour, diet and environment; (ii) anthropometry measurements, including height, weight, bioimpedance, waist and hip circumferences and middle-upper arm circumference; (iii) blood pressure measurements using an automated cuff (Omron HEM-907XL); (iv) cognitive tests including the Montreal Cognitive Assessment (MoCA) and Cambridge Neuropsychological Test Automated Battery (CANTAB)22–25; (v) a short physical performance assessment26; (vi) actigraphy (GENEActiv® Original actigraph from Activinsights Corporation)27; and (vii) sleep diaries. Participants complete the actigraphy and sleep diary visit components at their home for 7 days after the visit date. In addition, for those who did not participate in these components during the Mid-Life Visit (2017–21), we also perform DXA whole-body, lumbar spine, and hip scans (Hologic model Discovery A, Bedford, MA) and collect blood and urine samples. As a thank you for participating, we give each participant a gift card and reimbursement for parking or transportation.
Table 3.
New data collection from women in Project Viva, a longitudinal pre-birth cohort started in 1999
| Mid-Life Visit | Questionnaire-only visits | Women’s Health Visit 1 | |
|---|---|---|---|
| July 2017—August 2021 (843 women with any data) | Year 19, 2020, 2021, 2022 | April 2022–24 (ongoing) (1356 eligible at start of visit) | |
| Median age | 51 years | 54 years | |
| Questionnaire | X | X | X |
| Home and neighbourhood environment | X | X | X |
| General health | X | X | X |
| Lifetime fertility and pregnancy history | X | ||
| Age at menopause and menopause-related symptoms | X | X | X |
| Depression and anxiety symptoms | X | X | X |
| Mood | X | X | X |
| Behaviours | X | X | X |
| Sleep | X | X | X |
| History of adverse childhood experiences | X | ||
| COVID-19 experiences | X | ||
| Medical history interview | X | ||
| Research measures | |||
| Anthropometry | X | X | |
| Blood pressure | X | X | |
| Fasting blood and urine collection | X | Xa | |
| Biomarkers of cardiometabolic health and bone turnover | X | Xa | |
| 2-h oral glucose tolerance test | X | Xa | |
| DXA whole-body, spine, hip scans | X | Xa | |
| Hand grip strength | X | ||
| Diet (ASA24 Dietary Assessment Tool) | X | ||
| Extracted DNA from blood samples to obtain genotypes | X | ||
| Cognition (MoCA and CANTAB) | X | ||
| Short physical performance battery | X | ||
| 7-day actigraphy and sleep diaries | X |
ASA24, Automated Self-Administered 24-h; CANTAB, Cambridge Neuropsychological Test Automated Battery; DXA, dual X-ray absorptiometry; MoCA, Montreal Cognitive A.
We performed DXA and collected blood and urine samples at Women’s Health Visit 1 only for women who did not participate in these components during the Mid-Life Visit.
What has it found?
The Project Viva team and collaborators have continued to be highly productive since the publication of the original cohort profile in 2015.1 From January 2016 through January 2023, there were about 200 Project Viva publications [https://www.projectviva.org/publications]. We have started to analyse and publish findings from the Mid-Life Visit, Year 19 questionnaire and annual survey data. In this section we summarize some of our notable findings among Project Viva women, published after our original cohort profile related to our new research focus.
Using new outcome data measured during the Mid-Life Visit, we have examined life course predictors of the timing and symptoms of menopause. We found that compared with women with normal menstrual cycle length of 26 to 34 days during their reproductive years, those with shorter menstrual cycle length had a higher frequency of menopausal symptoms in mid-life and reached menopause earlier.28 We also found that a history of psychosocial stressors in childhood, adolescence and early adulthood was associated with worse menopausal symptoms and poorer wellbeing two decades after initial report.29 We did not find consistent associations of a history of hypertensive disorders of pregnancy or gestational diabetes mellitus with menopausal symptoms in mid-life or the age at the onset of natural menopause.30
We also examined predictors of weight, body composition and cardiovascular risk into mid-life. We found that women who had their first birth at a younger (<23 years) or older age (≥40 years) and those who had four or more lifetime births had a higher trajectory of weight, waist circumference and body fat up to 20 years after their final birth.31 We also found that low anti-Müllerian hormone (AMH) at about 3 years postpartum was associated with greater adiposity concurrently and across approximately 9 years of follow-up into mid-life.32 Women with versus without infertility had higher average weight, waist circumference and body fat.33 Among younger (18–29 years), but not older (≥30 years) women, infertility was associated with higher systolic and diastolic blood pressure.33 Additionally, we found a small beneficial association between total lifetime breastfeeding duration and mid-life handgrip strength, a measure of muscle strength.34
What are the main strengths and weaknesses?
As described previously,1 the primary strength of Project Viva is the in-person collection of exposure and outcome measures using research standard procedures conducted by trained and expert staff, which reduces likelihood of measurement bias, such as the anthropometric measurements which we have validated against gold-standard measures such as DXA.35,36 Another strength is the wealth of covariate information we have collected, which may be used in analytical models to minimize confounding and account for baseline and time-varying confounders.37 Two decades of continual follow-up enables us to evaluate women’s health across their reproductive years and conditions that emerge during mid-life, thereby enabling assessment/identification of sensitive exposure windows in relation to long-term health outcomes and identifying reproductive risk factors or risk markers for health status in mid-life.
Weaknesses include loss to follow-up, which can result in reduced sample size and thus lower statistical power for rare exposures or outcomes, as well as the possibility of selection bias. We have applied modern causal inference methods and analytical techniques to minimize these biases in several analyses.38–40
Other limitations include the extent to which findings are generalizable, since all participants resided in the greater Boston area and had health insurance at the time of enrolment, and many were college educated. A majority of our participants identified as being non-Hispanic White (Table 1), although the proportions of racial and ethnic minorities originally enrolled in Project Viva were higher than in Massachusetts as a whole, according to the 2000 US census.41
Can I get hold of the data? Where can I find out more?
We recognize that Project Viva provides a unique data resource, and we have a long track record of sharing data with investigators throughout the world. Please see [https://www.projectviva.org] for information and resources related to data collection.
We have a formal protocol that details procedures for individual investigators seeking to use Project Viva data, available at [https://www.projectviva.org]. For more information, investigators can also contact the Project Viva Principal Investigator, Emily Oken, at [project_viva@hphci.harvard.edu]. We are currently developing a new research portal through which investigators can access information and documentation related to our data.
Ethics approval
At each visit, we obtained written informed consent from the women. The Harvard Pilgrim Health Care Institutional Review Board approved all study protocols in line with ethical standards established by the Declaration of Helsinki.
Acknowledgements
We appreciate the Project Viva mothers, children and families for their ongoing participation and are grateful to the dozens of Project Viva staff, past and present, who collected such high-quality data. We are indebted to the vision of Matthew W Gillman, the founding PI of Project Viva.
Contributor Information
Sheryl L Rifas-Shiman, Division of Chronic Disease Research Across the Lifecourse, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, MA, USA.
Izzuddin M Aris, Division of Chronic Disease Research Across the Lifecourse, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, MA, USA.
Karen M Switkowski, Division of Chronic Disease Research Across the Lifecourse, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, MA, USA.
Jessica Young, Division of Chronic Disease Research Across the Lifecourse, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, MA, USA.
Abby F Fleisch, Pediatric Endocrinology and Diabetes, Maine Medical Center, Portland, ME, USA; Center for Interdisciplinary Population and Health Research, MaineHealth Institute for Research, Portland, ME, USA.
Tamarra James-Todd, Department of Environmental Health, Harvard T H Chan School of Public Health, Boston, MA, USA; Department of Epidemiology, Harvard T H Chan School of Public Health, Boston, MA, USA.
Ami R Zota, Department of Environmental Health Sciences, Columbia University Mailman School of Public Health, New York, NY, USA.
Wei Perng, Department of Epidemiology and the Lifecourse Epidemiology of Adiposity and Diabetes (LEAD) Center, Colorado School of Public Health, University of Colorado, Anschutz Medical Campus, Aurora, CO, USA.
Marie-France Hivert, Division of Chronic Disease Research Across the Lifecourse, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, MA, USA; Diabetes Unit, Massachusetts General Hospital, Boston, MA, USA.
Janet W Rich-Edwards, Department of Epidemiology, Harvard T H Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA; Connors Center for Women’s Health and Gender Biology, Brigham and Women’s Hospital, Boston, MA, USA.
Melissa Perez Capotosto, William F. Connell School of Nursing, Boston College, Boston, MA, USA.
Jorge E Chavarro, Department of Epidemiology, Harvard T H Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA; Department of Nutrition, Harvard T H Chan School of Public Health, Boston, MA, USA.
Emily Oken, Division of Chronic Disease Research Across the Lifecourse, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, MA, USA; Department of Nutrition, Harvard T H Chan School of Public Health, Boston, MA, USA.
Data availability
See ‘Can I get hold of the data?’ above. The data underlying this article are available on reasonable request to the corresponding author and appropriate approval from the Project Viva team and Institutional Review Board.
Author contributions
S.R.S. wrote the article and led the analysis. E.O. is the principal investigator of Project Viva, designed the study and drafted sections of the text. I.A., K.S., J.Y., A.F., T.J.T., A.Z., W.P., M.F.H., J.R.E., M.P.C. and J.C. are co-investigators of Project Viva and have led grants and conducted research related to Project Viva women. All authors have contributed to and reviewed the article for submission. S.R.S. and E.O. had primary responsibility for final content.
Funding
Project Viva and its team of co-investigators have been funded by grants from the National Institutes of Health (grant numbers R01 HD034568, UH3 OD023286, R01 ES031065, U54 AG062322, R24 ES030894, R01 HD096032, 3R01HD096032-04S1, R01 ES024765).
Conflict of interest
None declared.
References
- 1. Oken E, Baccarelli AA, Gold DR. et al. Cohort profile: project viva. Int J Epidemiol 2015;44:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Psychological Association. APA Dictionary of Psychology. https://dictionary.apa.org/mid-life-crisis (19 May 2023, date last accessed).
- 3. Tylavsky FA, Ferrara A, Catellier DJ. et al. Understanding childhood obesity in the US: the NIH environmental influences on child health outcomes (ECHO) program. Int J Obes (Lond) 2020;44:617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patro Golab B, Santos S, Voerman E. et al. ; MOCO Study Group Authors. Influence of maternal obesity on the association between common pregnancy complications and risk of childhood obesity: an individual participant data meta-analysis. Lancet Child Adolesc Health 2018;2:812–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Korevaar TIM, Derakhshan A, Taylor PN. et al. ; Consortium on Thyroid and Pregnancy—Study Group on Preterm Birth. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA 2019;322:632–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taal HR, Pourcain BS, Thiering E. et al. ; Early Growth Genetics (EGG) Consortium. Common variants at 12q15 and 12q24 are associated with infant head circumference. Nat Genet 2012;44:532–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Felix JF, Joubert BR, Baccarelli AA. et al. Cohort Profile: Pregnancy and Childhood Epigenetics (PACE) consortium. Int J Epidemiol 2018;47:22–23u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harlow SD, Gass M, Hall JE. et al. ; STRAW + 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop +10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab 2012;97:1159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zheng Y, Manson JE, Yuan C. et al. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA 2017;318:255–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Writing Group M, Mozaffarian D, Benjamin EJ. et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 2016;133:e38–360. [DOI] [PubMed] [Google Scholar]
- 11. Huang WY, Chang CC, Chen DR, Kor CT, Chen TY, Wu HM.. Circulating leptin and adiponectin are associated with insulin resistance in healthy postmenopausal women with hot flashes. PLoS One 2017;12:e0176430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Woodard GA, Brooks MM, Barinas-Mitchell E, Mackey RH, Matthews KA, Sutton-Tyrrell K.. Lipids, menopause, and early atherosclerosis in Study of Women's Health Across the Nation Heart women. Menopause 2011;18:376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thurston RC, El Khoudary SR, Sutton-Tyrrell K. et al. Vasomotor symptoms and lipid profiles in women transitioning through menopause. Obstet Gynecol 2012;119:753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Doherty TJ. The influence of aging and sex on skeletal muscle mass and strength. Curr Opin Clin Nutr Metab Care 2001;4:503–508. [DOI] [PubMed] [Google Scholar]
- 15. Woods NF, Mitchell ES.. Symptoms during the perimenopause: prevalence, severity, trajectory, and significance in women's lives. Am J Med 2005;118(Suppl 12B):14–24. [DOI] [PubMed] [Google Scholar]
- 16. Rich-Edwards JW, McElrath TF, Karumanchi SA, Seely EW.. Breathing life into the lifecourse approach: pregnancy history and cardiovascular disease in women. Hypertension 2010;56:331–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sattar N, Greer IA.. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ 2002;325:157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention, Agency for Toxic Substances and Disease Registry, Geospatial Research Analysis and Services Program. CDC Social Vulnerability Index Database. https://www.atsdr.cdc.gov/placeandhealth/svi/data_documentation_download.html (18 November 2020, date last accessed).
- 19. Fleisch AF, Aris IM, Rifas-Shiman SL. et al. Prenatal exposure to traffic pollution and childhood body mass index trajectory. Front Endocrinol (Lausanne) 2018;9:771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jimenez MP, Oken E, Gold DR. et al. Early life exposure to green space and insulin resistance: An assessment from infancy to early adolescence. Environ Int 2020;142:105849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ASA24® Dietary Assessment Tool | EGRP/DCCPS/NCI/NIH. https://epi.grants.cancer.gov/asa24// (17 May 2021, date last accessed).
- 22. O'Driscoll C, Shaikh M.. Cross-cultural applicability of the Montreal Cognitive Assessment (MoCA): a systematic review. J Alzheimers Dis 2017;58:789–801. [DOI] [PubMed] [Google Scholar]
- 23. Nasreddine ZS, Phillips NA, Bedirian V. et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–99. [DOI] [PubMed] [Google Scholar]
- 24. Sprowles JLN, Monaikul S, Aguiar A. et al. Associations of concurrent PCB and PBDE serum concentrations with executive functioning in adolescents. Neurotoxicol Teratol 2022;92:107092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lenehan ME, Summers MJ, Saunders NL, Summers JJ, Vickers JC.. Does the Cambridge Automated Neuropsychological Test Battery (CANTAB) distinguish between cognitive domains in healthy older adults? Assessment 2016;23:163–72. [DOI] [PubMed] [Google Scholar]
- 26. Freiberger E, de Vreede P, Schoene D. et al. Performance-based physical function in older community-dwelling persons: a systematic review of instruments. Age Ageing 2012;41:712–21. [DOI] [PubMed] [Google Scholar]
- 27. Jenkins CA, Tiley LCF, Lay I, Hartmann JA, Chan JKM, Nicholas CL.. Comparing GENEActiv against actiwatch-2 over seven nights using a common sleep scoring algorithm and device-specific wake thresholds. Behav Sleep Med 2022;20:369–79. [DOI] [PubMed] [Google Scholar]
- 28. Mínguez-Alarcón L, Rifas-Shiman SL, Soria-Contreras DC. et al. Self-reported menstrual cycle length during reproductive years in relation to menopausal symptoms at mid-life in Project Viva. Menopause 2022;29:1130–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Faleschini S, Tiemeier H, Rifas-Shiman SL. et al. Longitudinal associations of psychosocial stressors with menopausal symptoms and well-being among women in mid-life. Menopause 2022;29:1247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Soria-Contreras DC, Perng W, Rifas-Shiman SL. et al. Associations of hypertensive disorders of pregnancy and gestational diabetes mellitus with menopausal symptoms at mid-life in Project Viva. Menopause 2022;29:1021–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soria-Contreras DC, Aris IM, Rifas-Shiman SL. et al. Associations of age at first birth and lifetime parity with weight and adiposity across mid-life in women from Project Viva. Obesity (Silver Spring) 2023;31:2407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Francis EC, Oken E, Hivert MF, Rifas-Shiman SL, Chavarro JE, Perng W.. Antimüllerian hormone and adiposity across mid-life among women in Project Viva. Menopause 2023;30:247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Soria-Contreras DC, Oken E, Tellez-Rojo MM, Rifas-Shiman SL, Perng W, Chavarro JE.. History of infertility and long-term weight, body composition, and blood pressure among women in Project Viva. Ann Epidemiol 2022;74:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paster IC, Lin PD, Rifas-Shiman SL, Perng W, Chavarro JE, Oken E.. Association of total lifetime breastfeeding duration with mid-life handgrip strength: findings from Project Viva. BMC Womens Health 2022;22:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Louer AL, Simon DN, Switkowski KM, Rifas-Shiman SL, Gillman MW, Oken E.. Assessment of child anthropometry in a large epidemiologic study. J Vis Exp 2017:54895. doi: 10.3791/54895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boeke CE, Oken E, Kleinman KP, Rifas-Shiman SL, Taveras EM, Gillman MW.. Correlations among adiposity measures in school-aged children. BMC Pediatr 2013;13:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chiu Y-H, Rifas-Shiman SL, Kleinman K, Oken E, Young JG.. Effects of intergenerational exposure interventions on adolescent outcomes: an application of inverse probability weighting to longitudinal pre-birth cohort data. Paediatr Perinat Epidemiol 2020;34:366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aris IM, Sordillo JE, Rifas-Shiman SL. et al. Childhood patterns of overweight and wheeze and subsequent risk of current asthma and obesity in adolescence. Paediatr Perinat Epidemiol 2021;35:569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oken E, Aris IM, Young JG.. Pre-pregnancy weight and preterm birth: a causal relation? Lancet Diabetes Endocrinol 2019;7:663–65. [DOI] [PubMed] [Google Scholar]
- 40. Aris IM, Sarvet AL, Stensrud MJ. et al. Separating algorithms from questions and causal inference with unmeasured exposures: an application to birth cohort studies of early BMI rebound. Am J Epidemiol 2021;190:1414–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.U.S. Department of Commerce Economics and Statistics Administration. U.S. Census Bureau. Massachusetts: Census 2000 Profile. https://usa.ipums.org/usa/resources/voliii/pubdocs/2000/c2kprof00-ma.pdf (27 September 2023, date last accessed).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
See ‘Can I get hold of the data?’ above. The data underlying this article are available on reasonable request to the corresponding author and appropriate approval from the Project Viva team and Institutional Review Board.