Abstract
Protruded signet-ring cell carcinoma (SRCC) is extremely rare. We herein report a rare case of flat elevated gastric SRCC in a patient without Helicobacter pylori infection. Esophagogastroduodenoscopy of a woman in her 50s revealed a flat, whitish lesion in the gastric body with elevation. Histological results of an endoscopically biopsied specimen led to a diagnosis of SRCC. Resection using endoscopic submucosal dissection was performed, and histology results revealed that the tumor was localized in the lamina propria. The size was 10×6 mm, and a protrusion had been formed by SRCC enlargement without destruction of the surface epithelium structure.
Keywords: signet-ring cell carcinoma, Helicobacter pylori, protruded type, early gastric cancer
Introduction
Signet-ring cell carcinoma (SRCC) is an undifferentiated type of cancer consisting of cells with ample cytoplasmic mucin that appear optically clear with Hematoxylin and Eosin staining and have an eccentrically placed nucleus (1). Early gastric SRCC tumors usually show a flat or depressed shape, and a protruded type is extremely rare (2-4). Gastric cancer is mainly present in patients with Helicobacter pylori infection or post-eradicated status, with few H. pylori-uninfected cases reported (5,6).
We herein report a rare case of elevated type of SRCC in the stomach of an H. pylori uninfected patient.
Case Report
A woman in her 50s visited a medical center for a health check-up. She did not have any gastrointestinal (GI) symptoms, and she underwent esophagogastroduodenoscopy (EGD) for upper GI screening. That was the first time she underwent EGD in our institute. She did not have a history of habitual smoking or drinking and had undergone surgery for a pituitary adenoma at 28 years old. The patient had also received medication for hypertension in the past, and her father had a history of bile duct cancer. There was no history of eradication therapy for H. pylori infection, and serum anti-H. pylori IgG antibody test results were negative. Serum pepsinogen I and II levels (57.1 and 9.7 ng/mL, respectively) and the I/II ratio (5.8) were normal, indicating no gastric mucosal atrophy, and her EGD findings also showed its absence. Thus, H. pylori-uninfected status was determined.
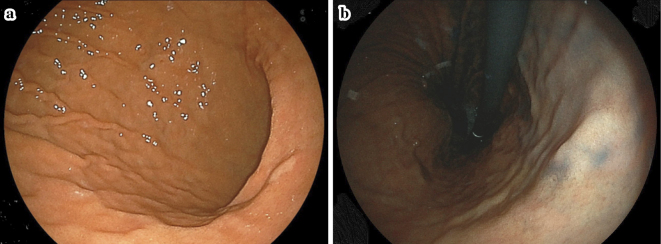
EGD showed a whitish, flat, elevated lesion in the lesser curvature of the gastric lower body (Fig. 1a, b). Although this protruded lesion was not endoscopically diagnosed as gastric cancer during EGD, an endoscopic biopsy was performed to determine the histological findings of this lesion. The biopsied sample obtained from the lesion showed SRCC in histology results (Fig. 2), and the patient was introduced to another hospital for detailed examinations. EGD at another hospital also showed a whitish, flat, elevated lesion in the lesser curvature of the gastric lower body (Fig. 3). Magnifying endoscopy with narrow-band imaging of the tumor did not show irregular microvascular and microsurface patterns, and the surface of tumor was considered to be covered by non-tumorous epithelium. Findings of submucosal invasion were not observed by endoscopic ultrasonography (Fig. 4), and there was no metastatic lesion on chest or abdominal computed tomography (CT). Thereafter, the lesion was removed via endoscopic submucosal dissection, as it was diagnosed as localized mucosal cancer.
Figure 1.
Representative endoscopic images of the lesser curvature of the gastric lower body showing a whitish, flat, elevated lesion. (a) White-light imaging. (b) Following indigo carmine spraying.
Figure 2.
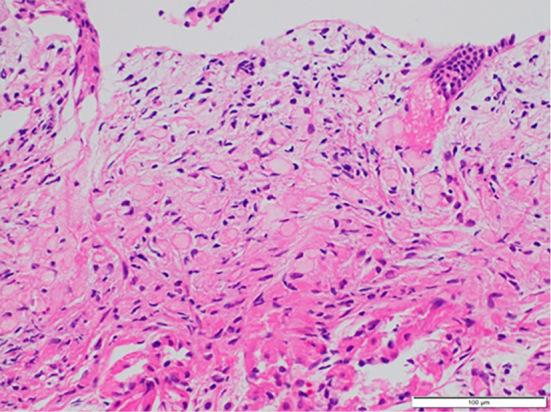
Representative histological image of biopsied specimen showing signet-ring cell carcinoma.
Figure 3.
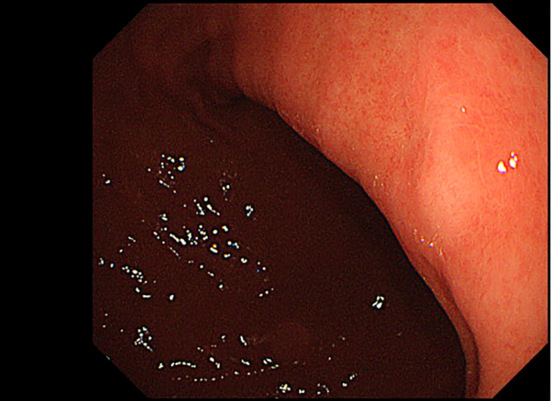
Representative endoscopic image at another hospital. A whitish, flat, elevated lesion was observed at the lesser curvature of the gastric lower body.
Figure 4.
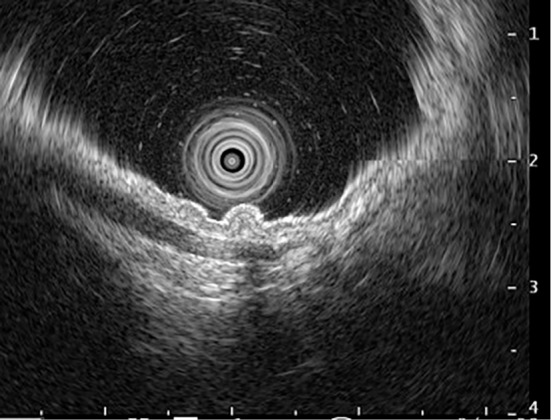
Representative image of endoscopic ultrasonography. Findings indicating submucosal invasion were not observed.
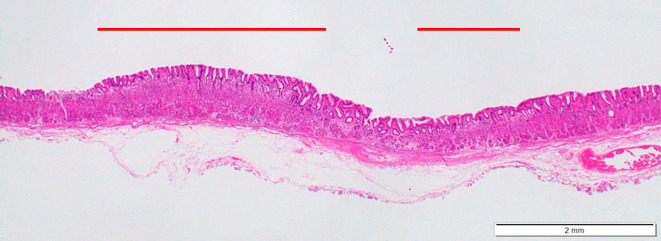
The tumor size was 10×6 mm, and histology results indicated SRCC localized in a superficial portion of the lamina propria. The surface of the lesion was covered by a normal epithelial structure, and the protrusion had been formed by enlargement of the SRCC (Fig. 5). No lymphovascular invasion was observed in histology results, and complete remission was considered to have been achieved by dissection. Histological results showed that the surface of normal epithelium covering the lesion was intact, with mild inflammatory cell infiltration of the lamina propria (Fig. 6). Local or metastatic recurrence was not observed by periodical EGD or CT during the two years after dissection.
Figure 5.
Representative histological image of an endoscopically resected specimen showing slight elevation of the lesion, while cancer invasion to the submucosal layer was not observed.
Figure 6.
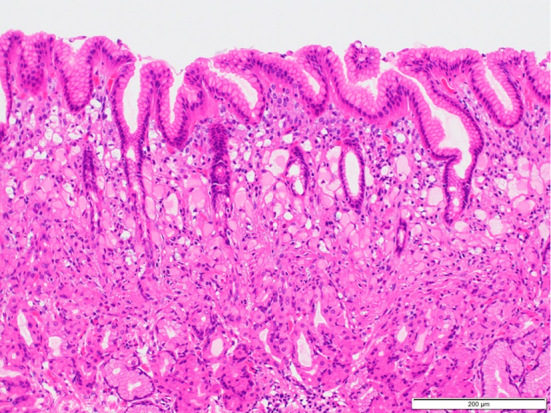
Representative histological image of an endoscopically resected specimen showing localization of signet-ring cell carcinoma in the superficial portion of the lamina propria. The surface of the normal epithelium was not destroyed by the lesion, and the degree of inflammatory cell infiltration was mild.
Discussion
We encountered a rare case of elevated-type SRCC in an H. pylori-uninfected patient. SRCC develops in the glandular neck zone of the lamina propria and then spreads horizontally (7). Following replacement of the normal gastric epithelial structure with cancer tissue, a depression is easily created along with tumor enlargement (2,8,9). Therefore, in its early stage, SRCC is usually flat or depressed, and elevation is rare. Indeed, Anzai et al. reported that the protruded type was found in only 5.6% of cases of undifferentiated gastric early cancer (2). In addition, an elevated undifferentiated gastric cancer tumor remaining in the mucosal layer has been reported to be extremely rare (2).
A literature search of gastric elevated mucosal SRCC was performed by Japan Medical Abstracts Society (JAMAS) from 1984 to 2022, since this type of tumor was previously reported from Japan (2-4,10-17). The characteristics of the reported cases with gastric elevated mucosal SRCC, including three case reports from the English literature and our own case, are shown in Table. Elevated mucosal SRCC was considered to be mainly located in the antrum, although the lesion in our case was observed in the lower body. Various sizes of lesion were reported, and the shape of the elevated mucosal SRCC was macroscopically divided into flat-elevated, submucosal tumor-like, and polypoid.
Table.
Case Reports of Gastric Elevated Undifferentiated Signet-ring Cell Mucosal Cancer in Japanese.
| Reference | Reported year | Age | Sex | Location | Macroscopic type | Size (mm) | Estimated cause of elevation |
|---|---|---|---|---|---|---|---|
| (2) | 1988 | 64 | Female | Antrum | Flat elevated | 65 | Tumor growth |
| (2) | 1988 | 76 | Male | Antrum | Flat elevated | 18 | Tumor growth |
| (10) | 1991 | 70 | Female | Antrum AW | Flat elevated | 80*65 | Tumor growth |
| (11) | 1995 | 62 | Male | Antrum LC | Submucosal tumor-like | 6*4 | Tumor growth |
| (12) | 2005 | 61 | Male | Not mentioned | Polypoid | 10 | Tumor in hyperplastic polyp |
| (13) | 2008 | 77 | Female | Low body GC | Polypoid | 20*17*12 | Tumor growth |
| (14) | 2012 | 30s | Female | Middle body PW | Polypoid | 16*14 | Tumor in hyperplastic polyp |
| (15) | 2016 | 67 | Male | Antrum AW | Polypoid | 21*12 | Tumor growth |
| (16) | 2016 | 60s | Male | Upper body PW | Polypoid | 10*8 | Tumor in hyperplastic polyp |
| (3) | 2020 | 45 | Female | Body LC | Submucosal tumor-like | Not mentioned | Tumor growth |
| (4) | 2021 | 63 | Female | Antrum AW | Submucosal tumor-like | 8*5 | Fibromuscular obliteration |
| (17) | 2023 | 40s | Female | Antrum PW | Submucosal tumor-like | 4*3 | Tumor growth |
| Our case | 50s | Female | Low body LC | Flat elevated | 10*6 | Tumor growth |
AW: anterior wall, LC: lesser curvature, GC: greater curvature, PW: posterior wall. Submucosal tumor-like: submucosal tumor-like appearance with the depression on the top. Tumor growth: Expansive growth of signet-ring cell carcinoma in lamina propria
While the mechanism underlying the protrusion of undifferentiated gastric cancer has not been determined, four possibilities have been proposed: formation of fibrous tissue or mucinous nodules due to cancer invasion into a deep layer of the stomach, dense growth of cancer cells, generation of undifferentiated cancer in a gastric hyperplastic polyp, and fibromuscular obliteration of muscularis propria (Table) (2-4,10-17). In the present case, the formation of fibrous tissue and mucinous nodules in the submucosal layer under the cancer tissue was not noted on histological finings. In addition, hyperplastic polyp components and fibromuscular obliteration of muscularis propria were not observed. The protrusion in our case was histologically formed by the growth of the SRCC localized in the lamina propria, and the surface of the SRCC was covered by normal epithelium. SRCC is generated in the lamina propria, and the depression of the SRCC is caused by the destruction of the surface epithelial structure on the tumor (2-4). The surface of the epithelium on SRCC is considered to be easily damaged in the presence of gastric mucosal inflammation, which is mainly caused by H. pylori infection. Therefore, the H. pylori-uninfected status of our case might have correlated with the morphology of the lesion. Further studies are recommended to clarify the role of the H. pylori infection status in the morphology of SRCC, since SRCC is known to occasionally develop in H. pylori-uninfected cases (18,19).
We encountered a rare case of an H. pylori-uninfected patient with a gastric flat mucosal SRCC that showed elevation. Endoscopists should recognize that a protruded SRCC can occur in the stomach. A future large-scale investigation is needed to more clearly elucidate the progression and prognosis of such lesions.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Lauwers GY, Carneiro F, Graham DY, et al. Gastric carcinoma. In: WHO Classification of Tumours of the Digestive System. 4th ed. Bosman FT, Carneiro F, Hruban RH, Theise ND, Eds. IARC Press, Lyon, 2010: 48-58. [Google Scholar]
- 2.Anzai H, Takagi K, Ohta H, Ohhashi E, Takahashi T, Nakajima T. Clinicopathological studies of elevated early gastric cancer showing histologically undifferentiated adenocarcinoma. Jpn J Gastroenterol Surg 21: 2094-2098, 1988(in Japanese). [Google Scholar]
- 3.Shiratori Y, Ikeya T, Suzuki K, Nakamura K, Fukuda K. The rare case of elevated signet ring cell gastric carcinoma with Helicobacter pylori-naïve mucosa. Clin J Gastroenterol 13: 736-739, 2020. [DOI] [PubMed] [Google Scholar]
- 4.Misumi Y, Ichihara S, Nonaka K, Onizuka H, Nagashima Y. Gastric signet-ring cell carcinoma that presented as an elevated lesion due to fibromuscular obliteration in the lamina propria. Case Rep Gastrointest Med 2021: 2887256, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum 61: 1-241, 1994. [PMC free article] [PubMed] [Google Scholar]
- 6.de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 13: 607-615, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Kumarasinghe MP, Lim TK, Ooi CJ, Luman W, Tan SY, Koh M. Tubule neck dysplasia: precursor lesion of signet ring cell carcinoma and the immunohistochemical profile. Pathology 38: 468-471, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Baba Y, Takagi K, Nakamura K, Kumakura K. A comparative study between histopathological and radiological findings od depressed early carcinoma of the stomach. Stomach Intest 10: 37-49, 1975(in Japanese with English abstract). [Google Scholar]
- 9.Shinohara N, Nakamura K, Kikuchi M, Makino T, Nishizawa M. Growing mode of the micro-carcinoma in incipient phase of cancer development. Stomach Intest 20: 431-439, 1985(in Japanese with English abstract). [Google Scholar]
- 10.Maguchi H, Orii Y, Saitoh Y, et al. IIa-aggregated polypoid type of poorly differentiated adenocarcinoma in gastric mucosa, report of a case. Stomach Intest 26: 209-215, 1991(in Japanese with English abstract). [Google Scholar]
- 11.Mochizuki T, Narisawa R, Honma T, et al. Signet-ring cell carcinoma of the stomach with submucosal tumor-like appearance, report of a case. Stomach Intest 30: 807-813, 1995(in Japanese with English abstract). [Google Scholar]
- 12.Miyazaki I, Ichimura S, Kuboki T, et al. A case of elevated signet ring cell carcinoma in hyperplastic polyp and inverted hyperplastic polyp. J Saitama Med Soc 40: 48-52, 2005(in Japanese). [Google Scholar]
- 13.Oku T, Maeda M, Ono K, et al. Pedunculated gastric signet ring cell carcinoma. Dig Endosc 20: 48-50, 2008. [Google Scholar]
- 14.Suganuma T, Hirasawa T, Shimizu T, et al. A case of the undifferentiated intramucosal carcinoma that it presented a elevated type, and was performed ESD. Prog Dig Endosc 81: 98-99, 2012(in Japanese with English abstract). [Google Scholar]
- 15.Oura R, Katayama Y, Gyotoku Y, et al. A case of undifferentiated intramucosal gastric cancer that exhibited elevated type. Dokkyo J Med Sci 43: 125-129, 2016. [Google Scholar]
- 16.Kakutani A, Shimodate Y, Mori H, et al. A type 0-I 0 low-grade differentiated-type adenocarcinoma with several signet ring cell components, report of a case. Stomach Intest 51: 1218-1229, 2016(in Japanese with English abstract). [Google Scholar]
- 17.Kishi K, Adachi K, Sakamoto U, et al. Gastric protruded type undifferentiated mucosal cancer in case with post-eradication for Helicobacter pylori. Gastroenterol Endosc. Forthcoming. (in Japanese with English abstract). [Google Scholar]
- 18.Yamamoto Y, Fujisaki J, Omae M, Hirasawa T, Igarashi M. Helicobacter pylori-negative gastric cancer: characteristics and endoscopic findings. Dig Endosc 27: 551-561, 2015. [DOI] [PubMed] [Google Scholar]
- 19.Yamada A, Kaise M, Inoshita N, et al. Characterization of Helicobacter pylori-naïve early gastric cancers. Digestion 98: 127-134, 2018. [DOI] [PubMed] [Google Scholar]