Abstract
A 66-year-old man diagnosed with immunoglobulin G4-related sclerosing cholangitis (IgG4-SC) with diffuse intrahepatic bile duct stenosis and elevated serum IgG4 levels was referred for a further examination because of elevated serum carbohydrate antigen 19-9 levels despite treatment with corticosteroids. An umbilical nodule was found on a physical examination and a biopsy showed adenocarcinoma. Although several imaging studies revealed no changes from prior studies, bile cytology collected by endoscopic retrograde cholangiopancreatography showed adenocarcinoma. Consequently, the patient was diagnosed with cholangiocarcinoma resembling IgG4-SC after detecting an umbilical metastasis, also known as Sister Mary Joseph's nodule.
Keywords: immunoglobulin G4-related sclerosing cholangitis, cholangiocarcinoma, immunoglobulin G4-related disease, Sister Mary Joseph's nodule
Introduction
Immunoglobulin G4-related sclerosing cholangitis (IgG4-SC) is a sclerosing cholangitis of unknown etiology characterized by elevated serum IgG4 levels, local fibrosis, and marked infiltration of IgG4-positive plasma cells (1). IgG4-SC is one manifestation of IgG4-related disease (IgG4-RD) and is sometimes found concurrently with other IgG4-RDs, such as autoimmune pancreatitis (AIP), lacrimal or salivary gland inflammation, and retroperitoneal fibrosis (1). The frequency of IgG4-SC related to AIP, which is the most common extra-pancreatic lesion, is reported to be 48.6%, and the frequency of IgG4-SC limited to the intrahepatic bile ducts is approximately 40.9% (2). Steroids, such as prednisolone, are commonly used to treat IgG4-RD (1,2). It is important to differentiate IgG4-SC from bile duct stenosis caused by primary sclerosing cholangitis, cholangiocarcinoma, or pancreatic cancer (3,4).
Umbilical metastasis of a malignant tumor, known as Sister Mary Joseph's nodule, is a rare clinical finding associated with a poor prognosis for abdominopelvic malignancies and metastatic umbilical nodules (5,6). Although the proportions of primary sites differ between men and women, with gastrointestinal cancer being more common in men and ovarian cancer in women (7-9), umbilical metastases originating from cholangiocarcinoma are rare.
We herein report a patient with cholangiocarcinoma with a suspected IgG4-SC background in whom an umbilical metastasis was among the triggers to establishing the diagnosis of cholangiocarcinoma.
Case Report
A 66-year-old man was diagnosed with IgG4-related dacryoadenitis by a histological examination and improved spontaneously without steroid administration three years before presentation. He had been admitted for the evaluation of liver dysfunction two years previously. Being asymptomatic, he was followed without examinations or medication. One year previously, IgG4-SC had been suspected because of an elevated serum IgG4 level [942 mg/dL (<135 mg/dL)] and diffuse intrahepatic bile duct stenosis identified by magnetic resonance cholangiopancreatography (MRCP) (Fig. 1a). Previous magnetic resonance imaging had revealed diffuse bile duct stenosis without findings compatible with bile duct cancer, such as dilated intrahepatic bile ducts or suspicious malignant-appearing nodules. The patient was advised to undergo further tests to confirm the presence of IgG4-SC; however, he did not follow these recommendations. At that time, the serum carbohydrate antigen (CA) 19-9 level had been 59 U/mL (<37 U/mL).
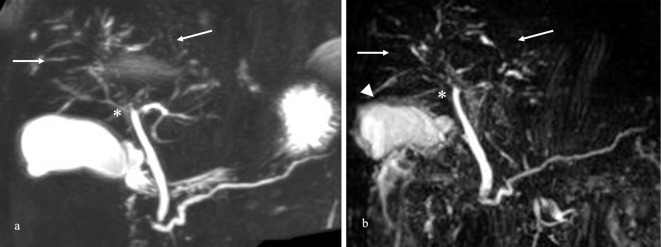
Figure 1.
Magnetic resonance cholangiopancreatography (MRCP) findings. (a) MRCP images from a year ago showing diffuse stenosis from the hilar to the intrahepatic bile duct (arrows) without diffuse narrowing of the main pancreatic duct. These findings revealed the stenosis of the bile duct in the perihilar portion (asterisk) without a dilated intrahepatic bile duct or cancerous nodules. (b) MRCP on admission revealed images similar to those of the intrahepatic bile duct a year ago and wall thickening of the gallbladder (arrowhead).
Three months previously, he had been treated with steroids as a diagnostic maneuver (prednisolone 30 mg) to diagnose IgG4-SC due to a lack of improvement in biliary stenosis detected using MRI. The patient's laboratory data before steroid administration were as follows: aspartate transaminase (AST): 23 U/L (13-30 U/L); alanine aminotransferase (ALT): 28 U/L (10-30 U/L); alkaline phosphatase (ALP): 316 U/L (106-322 U/L); γ-glutamyl transpeptidase (γ-GTP): 84 IU/dL (13-64 U/L); total bilirubin (T-bil): 0.65 mg/dL (0.40-1.50 mg/dL); direct bilirubin (D-bil): 0.10 mg/dL (0.00-0.20 mg/dL); carcinoembryonic antigen (CEA): 2.1 ng/dL (<5.0 ng/dL); CA19-9 491 U/mL (<37 U/mL); and serum IgG4: 791 mg/dL (11-121 mg/dL). Computed tomography (CT) three months after starting and gradually tapering prednisolone showed that the bile duct stenosis with wall thickening was unchanged without findings suggestive of malignancy, such as masses or enlarged lymph nodes. At that time, there were no umbilical lesions (Fig. 2b). However, the patient was referred to our institution due to an increased serum CA19-9 level of 4,994 U/mL (<37 U/mL).
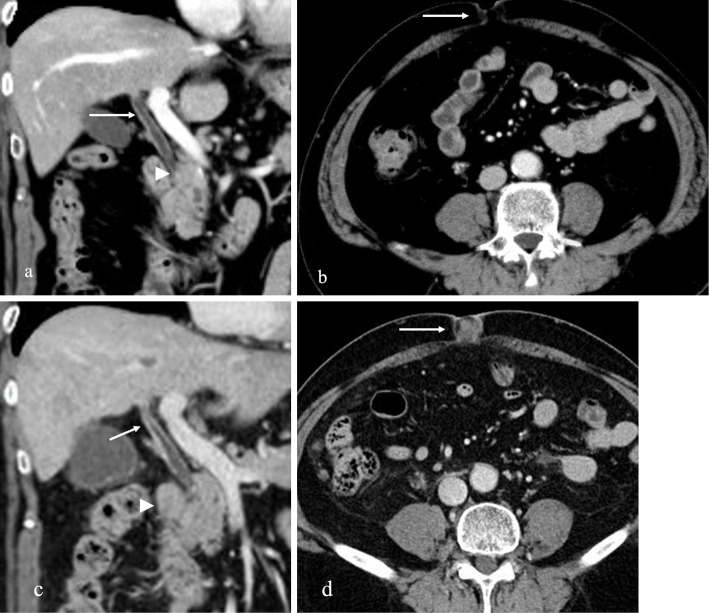
Figure 2.
Abdominal computed tomography (CT) findings. (a) CT revealed the absence of pancreatic enlargement suggestive of autoimmune pancreatitis or swelling of the lymph nodes (arrowhead). The findings revealed wall thickening without a localized solid mass in the perihilar bile ducts (arrow). (b) No solid mass was observed in the umbilical region (arrow). (c) CT revealed worsening wall thickening of the common bile duct with a contrast effect (arrow) and enlarged lymph nodes (arrowhead). (d) A solid mass appeared in the umbilical region (arrow).
On admission, a physical examination revealed a palpable, indurated umbilical area (Fig. 3a); however, there were no other abnormal findings, such as swelling of the salivary or lacrimal glands. The patient's laboratory data on admission showed: AST: 34 U/L; ALT: 52 U/L; ALP: 128 U/L; γ-GTP: 176 IU/dL; amylase: 68 U/L; lipase: 19 U/L; T-bil: 0.75 mg/dL; D-bil: 0.12 mg/dL; and serum IgG4: 384 mg/dL. After prednisolone administration, the serum IgG4 decreased, but was still higher than the reference values. Although the serum CEA level was normal [2.3 ng/dL (<5.0 ng/dL)], CA19-9 further increased (7,178 U/mL) compared to the previous evaluation.
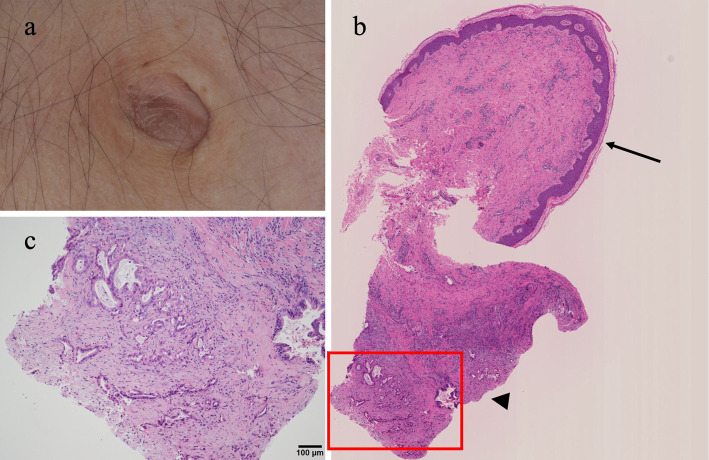
Figure 3.
Histological findings at the umbilicus. (a) The skin is normal, but a 1-cm mass is palpable. (b) An umbilical biopsy was performed from the epidermis to the dermis (macroscopic findings). The sites indicated by the arrow and the arrowhead are the skin-surrounded squamous cells and carcinoma in the subcutaneous tissue, respectively. (c) Histological findings show enlarged nuclei and atypical cells with obscured nucleoli proliferating in an irregular glandular duct structure on the deep side, with fibrosis and inflammatory cell infiltration in the surrounding area, consistent with adenocarcinoma (Hematoxylin and Eosin staining, 200×).
CT revealed increasing wall thickening of the common bile duct with a contrast effect, enlarged upper abdominal lymph nodes, an intra-abdominal soft tissue shadow suspected of potentially being peritoneal dissemination, and nodules at the umbilicus without intrahepatic bile duct dilation or pancreatic swelling (Fig. 2c, d). MRCP showed diffuse stenosis of the intrahepatic bile ducts, with no marked change from the previous study findings (Fig. 1b). MRCP revealed wall thickening of the gallbladder. The lesion of the gallbladder might have been due to IgG4-related gallbladder changes (Fig. 1b). Endoscopic ultrasound (EUS) revealed wall thickening of the extrahepatic bile duct (Fig. 4). EUS-fine needle aspiration was performed using a 22-gauge Franseen-type needle (Boston Scientific, Marlborough, USA) with two passages and no complications. Although sufficient tissue samples were acquired from lymph nodes, these samples included only benign lymphocytes without malignant cells. Endoscopic retrograde cholangiopancreatography (ERCP) detected diffuse stenosis of the intrahepatic bile ducts (Fig. 5). Based on the findings of biliary stenosis with Nakazawa classification type 2b (4), high serum IgG4 levels, and a history of IgG4-related sialadenitis, this constellation of findings was consistent with IgG4-SC according to the clinical diagnostic criteria of IgG4-SC (2).
Figure 4.
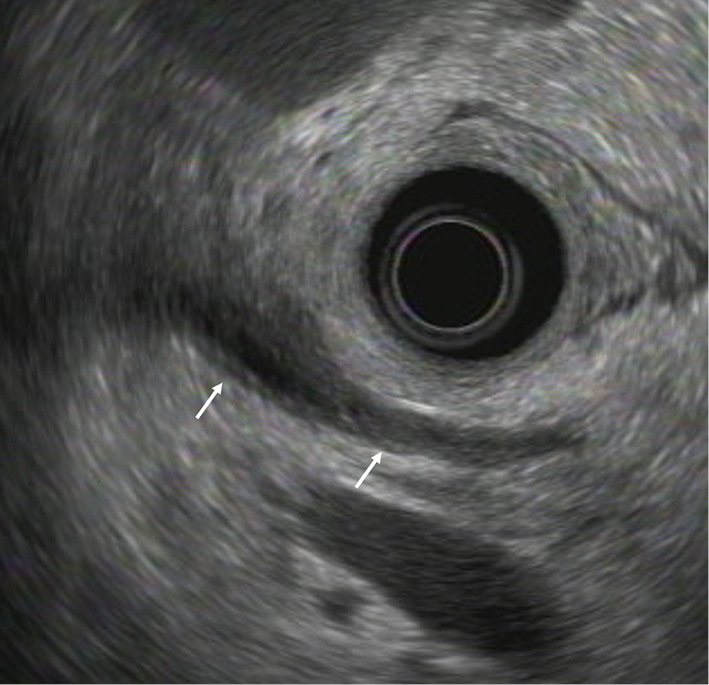
Endoscopic ultrasound findings show bile duct wall thickening, mainly at the confluence of the three ducts (arrows). There is no mass or localized stricture.
Figure 5.
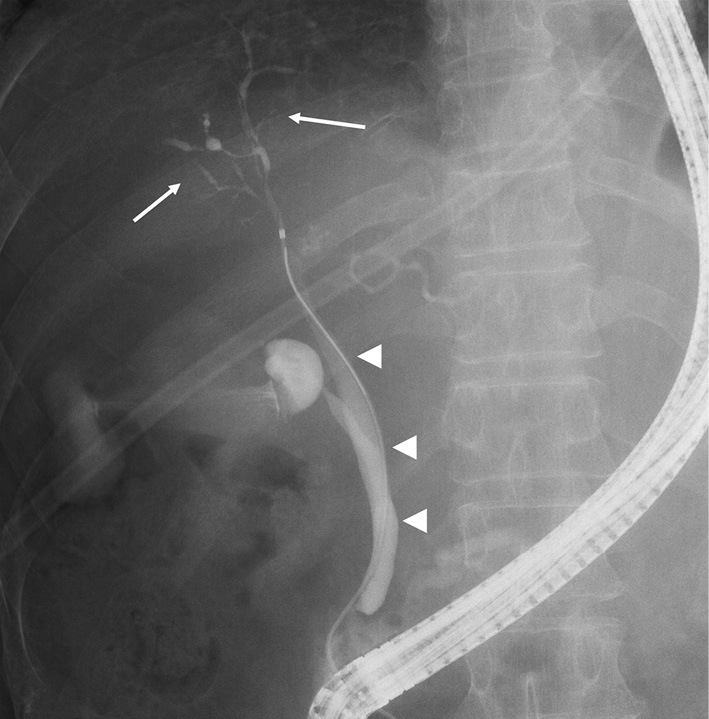
Endoscopic retrograde cholangiopancreatography findings show diffuse stenosis of the intrahepatic bile duct from the hilar bile duct (arrows), with no obvious mass noted in the bile duct (arrowheads). These findings indicate immunoglobulin G4-related sclerosing cholangitis, Nakazawa classification type 2b.
Although a histological examination should be performed to differentiate biliary cancer, a histological biopsy could not be performed because there was no obvious mass in the bile duct. Instead, an endoscopic naso-biliary drainage tube was placed in the common bile duct to obtain bile for a cytological examination. Cytologic findings from the bile revealed adenocarcinoma (Fig. 6). In addition, a histological examination of the indurated tissue in the umbilical region at the time of admission showed adenocarcinoma (Fig. 3b, c). Immunostaining results showed CK7 (+), CK20 (+: a few weak), CDX-2 (+: focal weak), PSA (-), and TTF1 (-) staining. These findings from the umbilical region revealed metastatic carcinoma consistent with an origin from an abdominal organ, such as the pancreas, stomach, or biliary tract. According to the cytological findings from bile, the umbilical metastasis, also known as Sister Mary Joseph's nodule, is derived from bile duct cancer.
Figure 6.
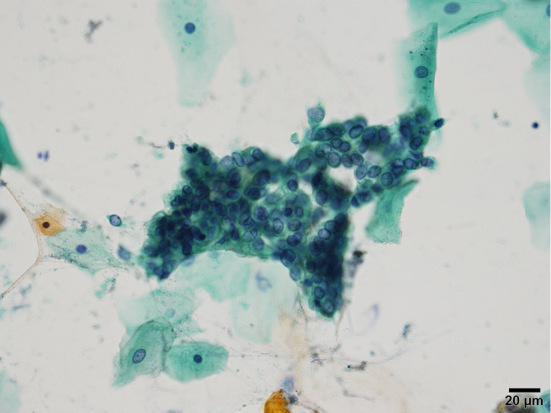
Cytological findings show adenocarcinoma (Papanicolaou staining, 200×).
The bile duct lesions were consistent with bile duct carcinoma resembling IgG4-SC. Since the primary cholangiocarcinoma lesion could not be identified because of the lack of a tumor or localized wall thickening, chemotherapy using gemcitabine and cisplatin was administered to treat cholangiocarcinoma with distant umbilical metastasis. Although the serum level of CA19-9 decreased to 458 U/mL after 5 months, cisplatin was discontinued because of a drug allergy. Four months later, lung metastases were found, and peritoneal dissemination was exacerbated. Administration of agent TS-1 was continued.
Discussion
In this patient, a Sister Mary Joseph's nodule and an elevated serum CA19-9 level supported the diagnosis of cholangiocarcinoma resembling IgG4-SC (3). The patient was assigned a likely diagnosis of IgG4-SC because of bile duct stenosis classified as Nakazawa classification type 2b, bile duct wall thickening, a high serum IgG4 level that decreased after treatment with steroids, and the existence of IgG4-related dacryoadenitis based on the diagnostic criteria for IgG4-SC (7). We diagnosed this patient with IgG4-SC based on these comprehensive findings. However, it is possible that the diagnosis was not IgG4-SC, as the bile duct stenosis did not improve after the administration of steroids, and the histological findings of IgG4-SC could not be confirmed despite several examinations.
Bile cytology revealed adenocarcinoma, leading to the diagnosis of cholangiocarcinoma. Although MRCP showed tight stenosis of the perihilar bile duct, the ERCP cholangiogram was atypical for cholangiocarcinoma due to the lack of an obvious localized tumor or dilation of the bile ducts. These findings might have been induced by stenosis due to IgG4-SC, which had already narrowed the intrahepatic bile ducts. A diffuse infiltrative type of cholangiocarcinoma, characterized by extensive irregular narrowing of the bile ducts and wall stiffening without masses or nodules (10), might have occurred in the liver two years previously. These findings might not be related to IgG4-SC, as patients with IgG4-SC tend to have elevated liver enzyme and bilirubin levels. However, why this patient did not have an abnormal liver function or significant symptoms is unclear. If this patient had undergone histological or cytological examinations by ERCP or a liver biopsy, cholangiocarcinoma might have been diagnosed at that time. However, this patient was deemed unresectable because of the unclear location of the cholangiocarcinoma.
The association between IgG4-SC and cholangiocarcinoma is unknown at present, as only a few patients have been reported. AIP, which is a manifestation of IgG4-RD, occurs concurrently with carcinoma in various organs (1). Shiokawa et al. determined the standardized incidence ratio of cancer to be 2.7 at the time of establishing the diagnosis of AIP, suggesting that IgG4-RD might be a paraneoplastic syndrome (11). Recently, pancreatic cancer has been the most common carcinoma reported to occur in patients with AIP (1). There may also be a mechanism, such as carcinogenesis, associated with chronic inflammation, as with pancreatic cancer in patients with chronic pancreatitis (12). To our knowledge, only five such patients, including the present patient, have been reported (10,13-15) (Table). The level of IgG4 and tumor markers, the Nakazawa classification of IgG4-SC, and the presence of AIP were different in each patient. Kurita et al. reported that the standardized incidence ratio of bile duct cancer in the patient with IgG4-SC was high (8.88) (16). Therefore, tumor markers such as CEA and CA19-9 should be evaluated during follow-up of patients with bile duct stenosis. When bile duct stricture occurs in a patient with IgG4-related disease, it is important to perform further studies, including ERCP with a biopsy and cytology from the bile duct, without assuming a diagnosis of IgG4-SC. The association between IgG4-SC and cholangiocarcinoma also requires further patient accumulation and investigations.
Table.
Previous Reports of Cholangiocarcinoma with IgG4-SC.
| Reference | Serum IgG4 (mg/dL) | CEA (ng/dL) | CA19-9 (U/mL) | Nakazawa classification of IgG4-SC | AIP present |
|---|---|---|---|---|---|
| (10) | 84 | 2.3 | 40.8 | Type 2a | - |
| (13) | 1,370 | 2.7 | 98 | Type 1 | + |
| (14) | 299 | N.A. | N.A. | Type 1 | + |
| (15) | 216 | 1.55 | 48.06 | Type 4 | - |
| Present patient | 942 | 2.7 | 7,178 | Type 2a | - |
AIP: autoimmune pancreatitis, CA19-9: carbohydrate antigen 19-9, CEA: carcinoembryonic antigen, IgG4-SC: immunoglobulin G4-related sclerosing cholangitis, N.A.: not acquired
Umbilical metastases of visceral malignancies are a rare clinical condition known as Sister Mary Joseph's nodule (3). Ishizawa et al. (17) reported that the rate of primary sites of Sister Mary Joseph's nodule was 40%, 19%, 13%, 11%, and 2.5% for the stomach, pancreas, ovary, colon, and bile duct, respectively. The mechanism of Sister Mary Joseph's nodule has not been clarified, although several hypotheses have been put forth, such as spreading in a hematogenous, lymphomatous, and disseminated manner (18). In this patient, an umbilical biopsy and a histological examination led to the diagnosis of adenocarcinoma detected by bile cytology only, although the location of the primary lesion was unknown. The umbilical nodule was a possibility of IgG4-related pseudotumor because this patient was complicated with IgG4-RD (19). Although Sister Mary Joseph's nodule is a rare clinical finding, when suspected, it is important to perform an aggressive biopsy to diagnose and differentiate umbilical metastases from malignancy.
Elevated serum CA19-9 levels and the presence of umbilical metastasis strongly suggested visceral malignancy, although the primary tumor could not be identified, and neither MRCP nor ERCP showed a tumor or localized stricture. The elevation of serum CA19-9 levels was affected by cholangiocarcinoma, as the level decreased after treatment with chemotherapy but was not improved by steroid treatment.
Several limitations associated with the present study warrant mention. Histological evidence of IgG4-SC could not be obtained. A histological examination can diagnose the patient with IgG4-SC, but it may be difficult to diagnose IgG4-SC using liver histology because the administration of prednisolone has already begun. The existence and location of cholangiocarcinoma could not be confirmed by several diagnostic modalities. However, the occurrence of lung metastases is corroborative evidence of malignancy during the clinical course.
In conclusion, we reported a patient with cholangiocarcinoma who may have had IgG4-SC by identifying Sister Mary Joseph's nodule and an elevated serum CA19-9 level. Imaging findings showed diffuse bile duct stenosis. IgG4-RD carcinogenesis and the mechanism causing Sister Mary Joseph's nodule should be further studied. We thought it is better when dealing with such patients to perform close monitoring and regular follow-up with biliary imaging and tumor markers for the early detection of any malignant changes and early management. Further prospective multicenter studies on the association between IgG4-SC, cholangiocarcinoma, and umbilical metastases are encouraged to be conducted.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This work was supported by MHLW Research Program on Rare and Intractable Diseases (grant number JPMH20FC1040).
References
- 1.Masamune A, Kikuta K, Hamada S, et al. Nationwide epidemiological survey of autoimmune pancreatitis in Japan in 2016. J Gastroenterol 55: 462-470, 2020. [DOI] [PubMed] [Google Scholar]
- 2.Kamisawa T, Nakazawa T, Tazuma S, et al. Clinical practice guidelines for IgG4-related sclerosing cholangitis. J Hepatobiliary Pancreat Sci 26: 9-42, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakazawa T, Kamisawa T, Okazaki K, et al. Clinical diagnostic criteria for IgG4-related sclerosing cholangitis 2020: (Revision of the clinical diagnostic criteria for IgG4-related sclerosing cholangitis 2012). J Hepatobiliary Pancreat Sci 28: 235-242, 2021. [DOI] [PubMed] [Google Scholar]
- 4.Nakazawa T, Naitoh I, Hayashi K, et al. Diagnostic criteria for IgG4-related sclerosing cholangitis based on cholangiographic classification. J Gastroenterol 47: 79-87, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Mayo WJ. Metastasis in cancer. Proc Staff Meet Mayo Clin 3: 327, 1928. [Google Scholar]
- 6.Albano EA, Kanter J. Images in clinical medicine. Sister Mary Joseph's nodule. N Engl J Med 352: 1913, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Gabriele R, Conte M, Egidi F, Borghese M. Umbilical metastases: current viewpoint. World J Surg Oncol 3: 13, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubreuil A, Dompmartin A, Barjot P, Louvet S, Leroy D. Umbilical metastasis or Sister Mary Joseph's nodule. Int J Dermatol 37: 7-13, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Hugen N, Kanne H, Simmer F, et al. Umbilical metastases: real-world data shows abysmal outcome. Int J Cancer 149: 1266-1273, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Straub BK, Esposito I, Gotthardt D, et al. IgG4-associated cholangitis with cholangiocarcinoma. Virchows Arch 458: 761-765, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Shiokawa M, Kodama Y, Yoshimura K, et al. Risk of cancer in patients with autoimmune pancreatitis. Am J Gastroenterol 108: 610-617, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Lowenfels AB, Maisonneuve P, Cavallini G, et al.; International Pancreatitis Study Group. Pancreatitis and the risk of pancreatic cancer. N Engl J Med 328: 1433-1437, 1993. [DOI] [PubMed] [Google Scholar]
- 13.Toyohara T, Nakazawa T, Zakharia K, et al. IgG4-related sclerosing cholangitis complicated with cholangiocarcinoma and detected by forkhead box P3 immunohistochemical staining. Intern Med 60: 859-866, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douhara A, Mitoro A, Otani E, et al. Cholangiocarcinoma developed in a patient with IgG4-related disease. World J Gastrointest Oncol 5: 181-185, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang YA, Shen XZ, Zhu JM, et al. Extensive metastatic cholangiocarcinoma associated with IgG4-related sclerosing cholangitis misdiagnosed as isolated IgG4-related sclerosing cholangitis: a case report and literature review. Medicine 94: e2052, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurita Y, Fujita Y, Sekino Y, et al. IgG4-related sclerosing cholangitis may be a risk factor for cancer. J Hepatobiliary Pancreat Sci 28: 524-532, 2021. [DOI] [PubMed] [Google Scholar]
- 17.Ishizawa T, Mitsuhashi Y, Kondo S, Watabe S. Sister Joseph's nodule: a case report and review of the Japanese literature. J Dermatol 24: 662-665, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Salemis NS. Umbilical metastasis or Sister Mary Joseph's nodule as a very early sign of an occult cecal adenocarcinoma. J Gastrointest Cancer 38: 131-134, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Katerji R, Smoller BR. Immunoglobulin-G4-related skin disease. Clin Dermatol 39: 283-290, 2021. [DOI] [PubMed] [Google Scholar]