Abstract
Primary gastric rhabdomyosarcoma is extremely rare. An 87-year-old man visited our clinic with a chief complaint of abdominal pain. Computed tomography (CT) and 18F-fluorodeoxyglucose positron emission tomography-CT revealed a massive tumor originating from the muscularis propria of the stomach along with splenic vein tumor thrombosis. We diagnosed the patient with primary gastric rhabdomyosarcoma by an endoscopic ultrasound-guided fine-needle aspiration/biopsy.
Keywords: rhabdomyosarcoma, tumor thrombosis, endoscopic ultrasound-guided fine-needle aspiration/biopsy
Introduction
Rhabdomyosarcoma (RMS) is a soft-tissue sarcoma that can occur anywhere in the body, including in tissues devoid of skeletal muscles. RMS is common in children (1) but rare in adults, where soft-tissue sarcomas constitute less than 1% of all malignancies (2). RMS accounts for 3% of all soft-tissue sarcomas. RMS commonly occurs at the genitourinary (24%), parameningeal (16%), extremity (19%), orbit (9%), other head and neck (10%), and miscellaneous other sites (22%) (3). Most cases of gastrointestinal RMS are metastatic disease, so primary RMS is extremely rare. A few case reports of esophageal (4-7) and gastric RMS have been published (8,9).
We herein report an extremely rare case of primary gastric RMS with splenic vein tumor thrombosis and very rapid tumor growth despite radiotherapy in an adult. This case was also valuable for demonstrating the features of RMS on contrast-enhanced computed tomography (CT), 18F-fluorodeoxyglucose positron emission tomography-CT (FDG-PET/CT), and endoscopic ultrasound (EUS). We successfully diagnosed this case using an EUS-guided fine-needle aspiration/biopsy (EUS-FNA/B).
Case Report
An 87-year-old man presented to our clinic with a chief complaint of abdominal pain. He had a history of hypertension and valvular heart disease. He was admitted for a further examination of his abdominal pain.
On admission, his blood pressure was 127/58 mmHg, and his pulse was 68 beats/min. A large, hard mass was palpated from the epigastric lesion to the left side of the abdomen. The laboratory findings showed mild anemia, elevated fibrin/fibrinogen degradation products (FDP)-D-dimer (5.5 μg/mL), and elevated levels of the tumor marker α-fetoprotein (AFP; 24.9 ng/mL).
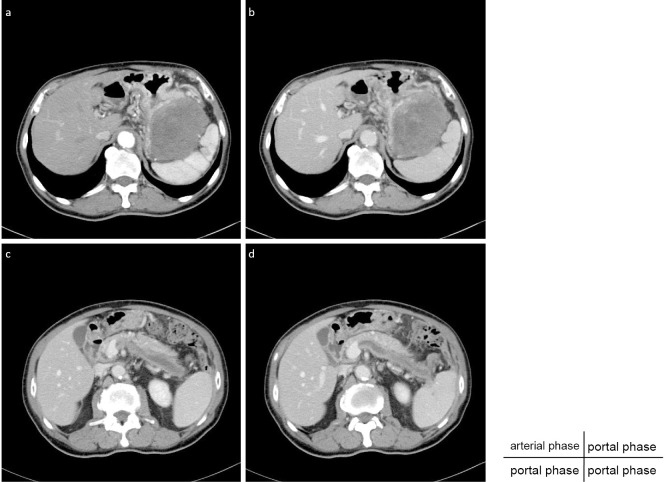
Contrast-enhanced CT revealed a 10.1×7.5-cm massive tumor in contact with the gastric wall (Fig. 1a, b). The tumor appeared to originate from the muscularis propria of the stomach. The inside of the tumor had a mosaic pattern, and hemorrhaging and tumor necrosis were suspected. Furthermore, CT revealed splenic vein thrombosis (Fig. 1c, d).
Figure 1.
The arterial phase (a) and portal phase (b-d) of dynamic CT show a massive tumor in contact with the gastric wall, which may originate from the muscularis propria of the stomach and splenic vein thrombosis.
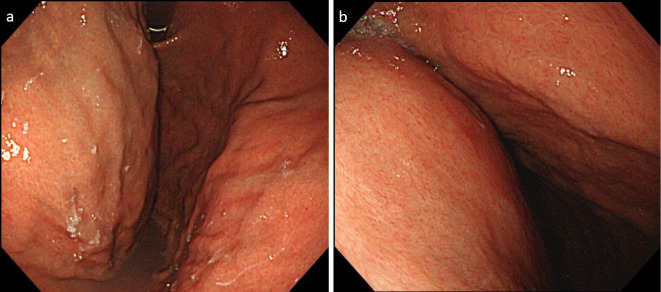
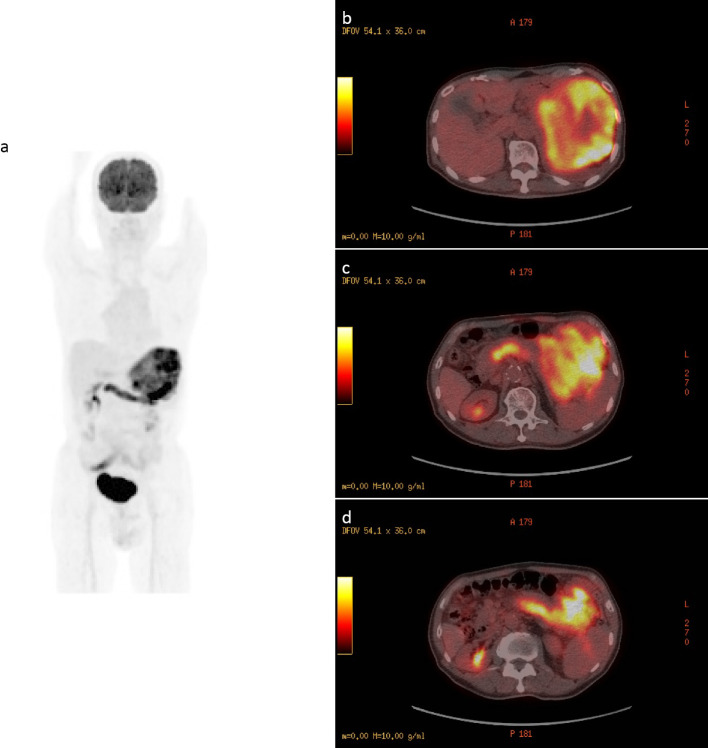
We therefore performed esophagogastroduodenoscopy (EGD), and a prominent submucosal bulge was observed at the posterior wall of the gastric body, but we were unable to make a diagnosis by a biopsy because no tumor was exposed (Fig. 2). We next performed FDG-PET/CT, which revealed that the tumor showed prominent FDG accumulation and a similar accumulation in the splenic vein (Fig. 3). CT and FDG-PET/CT showed no findings suggestive of a primary lesion other than in the stomach.
Figure 2.
Esophagogastroduodenoscopy images (a, b) show a prominent submucosal bulge at the posterior wall of the gastric body.
Figure 3.
FDG-PET/CT revealed that the tumor had prominent FDG accumulation (SUVmax 14.2) and a similar accumulation in the splenic vein (SUVmax 9.9). Maximum intensity projection (a) and fused images (b-d).
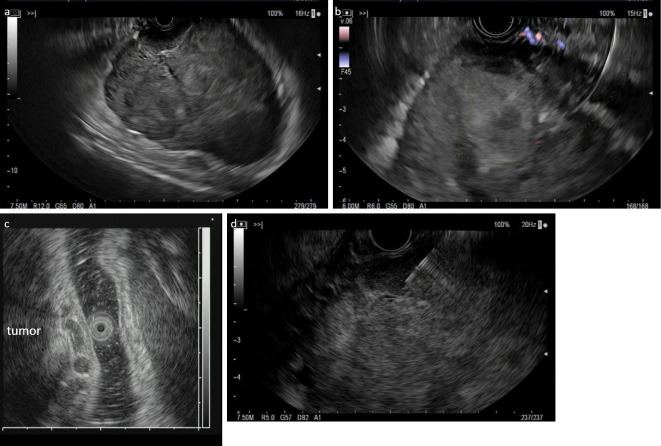
To make a definitive diagnosis of the tumor, EUS-FNA/B was performed (Fig. 4a-c). FNA/B was performed with a 22-gauge FNA needle (EZ Shot 3 Plus; Olympus, Tokyo, Japan) and the Aloka ProSound F75 color Doppler (Hitachi, Tokyo, Japan) (Fig. 4d). The punctures were performed three times with a rapid on-site cytological evaluation (ROSE).
Figure 4.
EUS and color Doppler imaging of the tumor (a: 7.5 MHz, b: 6 MHz, c: 12 MHz) and an EUS-FNA/B was performed (d: 7.5 MHz). The tumor originated from the muscularis propria, and dilated vessels were present in the submucosa.
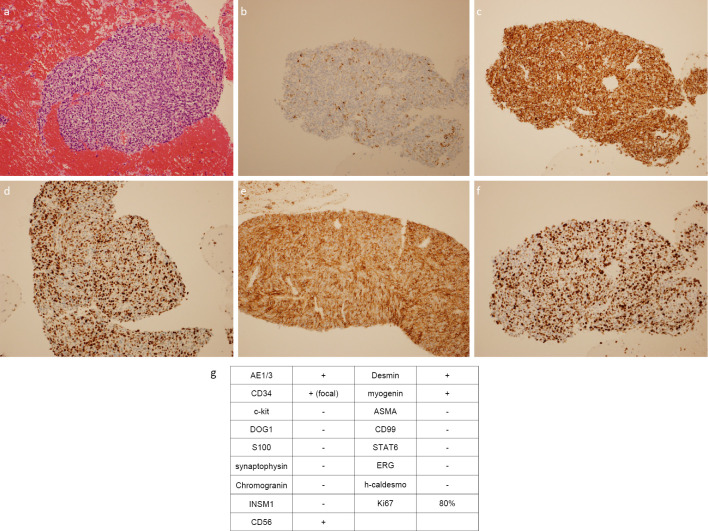
A histologic examination revealed tissue fragments composed of small round to spindle-shaped cells in the background of the blood clot. Tumor cells had atypical nuclei showing dense chromatin and pale to eosinophilic cytoplasm (Fig. 5a). Mitoses were found. Immunohistochemically, the tumor was focal positive for AE1/3, diffusely positive for desmin, myogenin, and CD56 (Fig. 5b-e). Ki-67 index was 80% (Fig. 5f). Detailed immunostaining results were tabulated (Fig. 5g). We diagnosed the patient with primary gastric RMS with splenic vein tumor thrombosis.
Figure 5.
A histologic examination revealed tissue fragments composed of small round to spindle-shaped cells in the background of a blood clot. Tumor cells had atypical nuclei showing dense chromatin and pale-to-eosinophilic cytoplasm (Hematoxylin and Eosin staining; a). Immunohistochemically, the tumor was focally positive for AE1/3 (b) and positive for desmin (c), myogenin (d), and CD56 (e). The Ki-67 index was 80% (f). Detailed immunostaining results are presented (g).
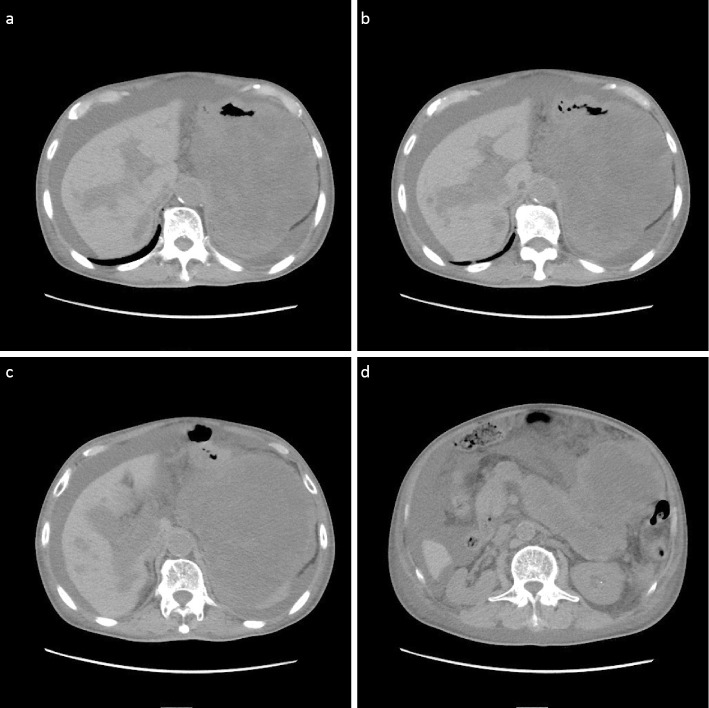
Because of his advanced age, surgery and chemotherapy were considered difficult, so he opted for palliative radiation therapy. Radiation therapy was conducted (30 Gy/10 fr). After radiation therapy, his symptoms temporarily improved, and he was discharged. However, two months after radiation therapy, the tumor grew rapidly. CT showed that the tumor had spread into the portal and intrahepatic portal veins, and ascites and pleural effusion appeared (Fig. 6). The patient ultimately died three months after his treatment.
Figure 6.
(a-d) CT shows that the tumor had spread into the portal and intrahepatic portal veins, and ascites and pleural effusion appeared.
Discussion
Sarcomas account for 1-2% of gastrointestinal malignancies. The primary site was the stomach (50%), small bowel (30%), colorectum (15%), and esophagus (5%) (10). Leiomyosarcomas are the most common type; while other types of sarcomas, such as synovial and granulocytic sarcomas, have been reported in the gastrointestinal tract, they are extremely rare (11,12). As in this case, RMS in adults is rare, and primary RMS in the gastrointestinal tract is extremely rare. Only a few cases of primary gastric RMS have been reported (8,9), and to our knowledge, there have been no cases reported of primary gastric RMS with splenic vein tumor thrombosis. Tumor thrombosis is a rare but serious complication of a solid tumor. It is important to differentiate between tumor thrombosis and venous thromboembolism (VTE). FDG-PET/CT can help differentiate tumor thrombosis from VTE owing to its similar metabolic uptake to that of the tumor (13). Furthermore, the linear and focal FDG uptake patterns with a high maximum standardized uptake value (SUVmax) values in FDG-PET/CT may be useful (14). In the present case, a linear FDG uptake and high SUVmax (9.9) in the splenic vein were associated with tumor thrombosis.
The differential diagnosis of tumors originating from the muscularis propria of the stomach have been reported to include leiomyoma, gastrointestinal stromal tumor (GIST), schwannoma (15), and sarcoma, including leiomyosarcomas and RMS. The CT features of leiomyoma and schwannoma show homogeneity (16). As in this case, the differential diagnoses of heterogenous tumors are GIST and sarcoma, including leiomyosarcoma and RMS.
The US features of abdominal RMS reportedly include an inhomogeneous echo structure, and color Doppler flow imaging has shown rich blood flow signals (17). To our knowledge, there have been no cases reports describing the EUS features in RMS. The CT features of RMS reported that density was lower than muscle density, necrosis was present but no calcification or hemorrhage (18). Furthermore, the contrast-enhanced CT feature reported heterogenous enhancement, and the enhancement in the delayed scan was more obvious; the peripheral enhancement was more significant than the central enhancement (16), but the CT features of RMS were nonspecific (19). FDG-PET/CT is a valuable tool for initial staging and may be a predictor of the outcome (20), but GISTs have been reported to show FDG accumulation, schwannoma also have been reported to show FDG accumulation (21). Therefore, in the present case, US, CT, and FDG-PET/CT features were as discussed above, but it is difficult to diagnose RMS using US, CT, and FDG-PET/CT without a histologic examination.
Furthermore, there are no cases of gastrointestinal RMS diagnosed using an EUS-FNA/B. The standard for the diagnosis of gastrointestinal cancers is an endoscopic biopsy; the same has been reported for RMS (5,8,9). The gold standard for a final diagnosis for RMS is the immunohistochemical staining of the endoscopic biopsy sample (9), but others have reported that a biopsy alone may not be sufficient to make a diagnosis (8), with surgical resection required. In the present case, no tumor was exposed, so an EUS-FNA/B was performed. Furthermore, because surgery was not possible due to his advanced age, only an EUS-FNA/B specimen was used to make the diagnosis. This specimen was a small one, so the whole tumor was not assessed. We consider the lesion to be a possible RMS, as far as we could tell from this specimen. However, although extremely rare, the possibility that an RMS component of carcinosarcoma was collected cannot be ruled out.
RMS has been classified by the World Health Organization into four histologic subtypes: embryonal, alveolar, pleomorphic, and spindle cell/sclerosing RMS (22). In adults, the histologic subtypes of RMS are the pleomorphic subtype (45%), alveolar (40%), and embryonal (15%) (23). In the present case, pathological examinations showed round to spindle-shaped tumor cells with concentrated chromatin, so the subtype was thought to be embryonal RMS.
The management of RMS in adults is unknown because its frequency is extremely low. The standard treatment for adult RMS follows that created for children, as proposed by the Intergroup Rhabdomyosarcoma Studies (IRS) (9). These treatments include multimodality treatment consisting of surgery, chemotherapy, and radiation. Surgery is the mainstay treatment for adult RMS. IRS protocols recommend radiotherapy to control the tumor (10), with reduced recurrence and mortality rates (24). Furthermore, IRS protocols recommend chemotherapy to improve the survival rate. The recommended regimen is combination chemotherapy of vincristine, actinomycin, etoposide or ifosfamide, and cyclophosphamide.
In the present patient, because of his advanced age, surgery and chemotherapy were not feasible, so he underwent palliative radiation therapy.
The prognosis of RMS in adults is worse than in children (25). Pleomorphic RMS has been reported to have the worst prognosis (26), while alveolar RMS had a better prognosis than other subtypes (23). Others have reported that independent prognostic factors for the overall survival were alveolar RMS, R0 resection, and adjuvant radiotherapy (27). However, the prognosis of gastric RMS is unclear, so the accumulation of more such cases is needed in the future.
In conclusion, primary gastric RWS with splenic vein tumor thrombosis diagnosed by an EUS-FNA/B is extremely rare. The accumulation of more cases is needed to clarify further clinical characteristics and establish a treatment strategy for RMS in adults.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Pastore G, Peris-Bonet R, Carli M, Martínez-García C, Sánchez de Toledo J, Steliarova-Foucher E. Childhood soft tissue sarcomas incidence and survival in European children (1978-1997): report from the Automated Childhood Cancer Information System project. Eur J Cancer 42: 2136-2149, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Weiss SW, Goldblum JR. Enzinger and Weiss's Soft Tissue Tumors. 4th ed. CV Mosby, St. Louis, 2001: 785-835. [Google Scholar]
- 3.Meyer WH, Spunt SL. Soft tissue sarcoma of childhood. Cancer Treat Rev 30: 269-280, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Wobbes T, Rinsma SG, Holla AT, Rietberg M, Leezenberg JA, Collenteur JC. Rhabdomyosarcoma of the esophagus. Arch Chir Neerl 27: 69-75, 1975. [PubMed] [Google Scholar]
- 5.Batoroev YK, Nguyen GK. Esophageal rhabdomyosarcoma: report of a case diagnosed by imprint cytology. Acta Cytol 50: 213-216, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Chetty R, Learmonth GM, Price SK, Taylor DA. Primary oesophageal rhabdomyosarcoma. Cytopathology 2: 103-108, 1991. [DOI] [PubMed] [Google Scholar]
- 7.Gandhi JS, Pasricha S, Gupta G, et al. Synchronous embryonal rhabdomyosarcoma (NOS) of the mid-oesophagus and stomach. J Gastrointest Cancer 43: S217-S220, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Fox KR, Moussa SM, Mitre RJ, Zidar BL, Raves JJ. Clinical and pathologic features of primary gastric rhabdomyosarcoma. Cancer 66: 772-778, 1990. [DOI] [PubMed] [Google Scholar]
- 9.Palermo M, Mastronardi LM, García RH, Solari I, Tarsitano FJ. Primary gastric rhabdomyosarcoma. Case report. Acta Gastroenterol Latinoam 42: 131-134, 2012. [PubMed] [Google Scholar]
- 10.McGrath PC, Neifeld JP, Lawrence W Jr, Kay S, Horsley JS 3rd, Parker GA. Gastrointestinal sarcomas. Analysis of prognostic factors. Ann Surg 206: 706-710, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Billings SD, Meisner LF, Cummings OW, Tejada E. Synovial sarcoma of the upper digestive tract: a report of two cases with demonstration of the X;18 translocation by fluorescence in situ hybridization. Mod Pathol 13: 68-76, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Sekaran A, Darisetty S, Lakhtakia S, Ramchandani M, Reddy DN. Granulocytic sarcoma of the stomach presenting as dysphagia during pregnancy. Case Rep Gastrointest Med 2011: 627549, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad W, Asghar N, Zafar T, Bashir H. Complete splenic vein tumour thrombus in primary gastric malignancy on F18-fluorodeoxyglucose positron emission tomography-computed tomography. J Pak Med Assoc 71: 175-176, 2021. [PubMed] [Google Scholar]
- 14.Kara PO, Koc ZP, Sezer EY, Yilmaz EB, Citak EC. Role of FDG PET-CT in distinction of benign thrombus and tumor thrombus in oncological patients. Biomed J Sci Tech Res 2: 2758-2762, 2018. [Google Scholar]
- 15.Akahoshi K, Oya M, Koga T, Shiratsuchi Y. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol 24: 2806-2817, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy AD, Quiles AM, Miettinen M, Sobin LH. Gastrointestinal schwannomas: CT features with clinicopathologic correlation. AJR Am J Roentgenol 184: 797-802, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Shi J, Du J, Wu W, Wang Q. Clinical and imaging features of abdominal rhabdomyosarcoma of non-organ origin in children. Zhonghua Zhong Liu Za Zhi 38: 845-851, 2016. [DOI] [PubMed] [Google Scholar]
- 18.Tian L, Cai Y, Li X, Cai J. Computed tomography (CT) features of pelvic rhabdomyosarcoma (RMS) in children. Curr Med Imaging 18: 299-304, 2022. [DOI] [PubMed] [Google Scholar]
- 19.Saboo SS, Krajewski KM, Zukotynski K, et al. Imaging features of primary and secondary adult rhabdomyosarcoma. AJR Am J Roentgenol 199: W694-W703, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Baum SH, Frühwald M, Rahbar K, Wessling J, Schober O, Weckesser M. Contribution of PET/CT to prediction of outcome in children and young adults with rhabdomyosarcoma. J Nucl Med 52: 1535-1540, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Hong IK, Kim DY. F-18 FDG PET/CT of a gastric schwannoma. Nucl Med Mol Imaging 45: 238-240, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, Eds. IARC Press, Lyon, 2013. [Google Scholar]
- 23.Liu YT, Wang CW, Hong RL, Kuo SH. Prognostic factors and treatment outcomes of adult patients with rhabdomyosarcoma after multimodality treatment. Anticancer Res 39: 1355-1364, 2019. [DOI] [PubMed] [Google Scholar]
- 24.Zhao R, Yu X, Feng Y, et al. The survival benefit of radiotherapy in localized primary adult rhabdomyosarcoma. Asia Pac J Clin Oncol 16: 266-272, 2020. [DOI] [PubMed] [Google Scholar]
- 25.Khosla D, Sapkota S, Kapoor R, Kumar R, Sharma SC. Adult rhabdomyosarcoma: clinical presentation, treatment, and outcome. J Cancer Res Ther 11: 830-834, 2015. [DOI] [PubMed] [Google Scholar]
- 26.Furlong MA, Mentzel T, Fanburg-Smith JC. Pleomorphic rhabdomyosarcoma in adults: a clinicopathologic study of 38 cases with emphasis on morphologic variants and recent skeletal muscle-specific markers. Mod Pathol 14: 595-603, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Bompas E, Campion L, Italiano A, et al. Outcome of 449 adult patients with rhabdomyosarcoma: an observational ambispective nationwide study. Cancer Med 7: 4023-4035, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]