Abstract
Background MRI (magnetic resonance imaging) using low-magnet field strength has unique advantages for intraoperative use. We compared a novel, compact, portable MR imaging system to an established intraoperative 0.15 T system to assess potential utility in intracranial neurosurgery.
Methods Brain images were acquired with a 0.15 T intraoperative MRI (iMRI) system and a 0.064 T portable MR system. Five healthy volunteers were scanned. Individual sequences were rated on a 5-point (1 to 5) scale for six categories: contrast, resolution, coverage, noise, artifacts, and geometry.
Results Overall, the 0.064 T images (M = 3.4, SD = 0.1) had statistically higher ratings than the 0.15 T images (M = 2.4, SD = 0.2) ( p < 0.01). All comparable sequences (T1, T2, T2 FLAIR and SSFP) were rated significantly higher on the 0.064 T and were rated 1.2 points (SD = 0.3) higher than 0.15 T scanner, with the T2 fluid-attenuated inversion recovery (FLAIR) sequences showing the largest increment on the 0.064 T with an average rating difference of 1.5 points (SD = 0.2). Scanning time for the 0.064 T system obtained images more quickly and encompassed a larger field of view than the 0.15 T system.
Conclusions A novel, portable 0.064 T self-shielding MRI system under ideal conditions provided images of comparable quality or better and faster acquisition times than those provided by the already well-established 0.15 T iMR system. These results suggest that the 0.064 T MRI has the potential to be adapted for intraoperative use for intracranial neurosurgery.
Keywords: intraoperative MRI, portable MRI, hyperfine, low field, point of care imaging
Introduction
Since its introduction by Black et al in 1997, intraoperative magnetic resonance imaging (iMRI) has become a valuable tool in guiding neurosurgical procedures, particularly for brain tumor resection. 1 The iMRI can detect residual tumor that is not visible in the surgeon's field of view, thereby allowing for additional resection while also correcting for inevitable brain shift. 2 3 4 5 6 7 8 9 10 11 Because a positive correlation exists between the extent of tumor resection and overall survival, the use of iMRI has been shown to improve patient outcomes in a cost-effective manner. 11 12 This success has inspired use of iMRI beyond neurosurgical oncology, with utility demonstrated in functional and epilepsy neurosurgery. 13 14 15
Both high (1.5 or 3 T) and low-field (< 1.5 T) strength MR systems have exhibited surgical and clinical benefits. 4 6 7 9 10 12 16 However, differences exist in image quality and usability of these iMRI systems based upon their magnetic field strengths. For example, high-field strength systems produce higher quality images, but require at least a partial breakdown of the operative field, interrupting workflow and adding significant operating time. 17 Low-field strength systems, meanwhile, are less expensive and allow for better patient access and easier instrument use during surgery, though image quality is compromised. 4 Therefore, low-field devices have emerged with the goal of maximizing intraoperative usability. 4 18 The Polestar (Medtronic Surgical Technologies, Louisville, CO, USA) was one such low field (0.15T) system that was developed to attempt to overcome some of the difficulties of high field systems. Despite the obvious limitations in image quality (smaller field of view, lower signal to noise ratio (SNR) and smaller tissue contrast), these imaging systems have found some utility in improving functional outcomes in tumor surgery and this improved clinical benefit is found to be cost-effective. 19 20
Recently, a 0.064 T portable MR imaging system (Swoop, Hyperfine, Inc., Guilford, CT, USA) was approved for clinical use in the USA as a point of care device for head imaging. During the COVID-19 pandemic, the device was utilized in various ICU settings demonstrating its adaptability in different clinical environments. 21 This device allowed patients to remain in their rooms, attached to all necessary lines and mechanical ventilation, and still have brain imaging performed using various sequences (T1, T2, FLAIR, and DWI) that provide information not seen on portable CT scanners. 22 This 0.064 T system also has begun to see use in emergency department and outpatient settings but its use in the operating room has not yet been documented. We hypothesized that image quality at 0.064 T would be adequate for intraoperative decision making, and the aim of this study was to compare images acquired at 0.064 T and a validated 0.15 T iMRI.
Materials and Methods
Study Participants
Five healthy participants were included in the study and were at least 18 years old with no known medical conditions. The study was approved by the institutional review board. Written consent was obtained from all participants before enrollment.
Devices
Participants were scanned with three MRI systems: 1.5 T (GE Healthcare, Waukesha, WI, USA), 0.15 T Polestar iMRI (Medtronic Surgical Technologies, Louisville, CO, USA), and 0.064 T Swoop (Mk 1.2, Hyperfine, Inc., Guilford, CT, USA). The Swoop and Polestar scanners are shown in Fig. 1 . The 1.5 T scans were used as a screening image to assess for any possible pathological changes that may not be visible on lower field MRIs. Given the obvious superiority over the low-field imaging systems, 1.5 T images were not used in final analysis.
Fig. 1.

( A ) Medtronic (0.15 T) scanner. ( B ) Hyperfine (0.064 T) scanner. Pictured in the bottom left is the joystick that enables easy maneuverability.
The hyperfine system is a portable, 0.064 T MR imaging system ( Fig. 1B ). Its height is 140 cm, width is 86 cm, power is 15A/110V, and weight is 639 kg. 23 The scanner has two horizontally oriented magnets that form two poles. Each pole contains coils that transmit radiofrequency pulses, while the receive coil is on a platform inside the gantry. All related computer equipment is contained within the base of the MRI. Minimal training was needed to operate the device using a commercially available tablet device. It is mobile, self-shielding, and FCC Class A compliant, so other Class A compliant equipment outside the 5G boundary can be used while scanning.
Imaging
Various pre-configured clinical sequences were acquired, including T1, T2 FSE, T2 FLAIR, and SSFP. The 0.064 T system also acquired diffusion weighted images (DWI). Scan times for each study were recorded and compared.
Assessment
Four experienced observers (neurologists, radiologists, and neurosurgeons) rated each image series based on six categories: contrast, resolution, coverage, noise, artifacts, and geometry, with each category receiving its own rating. A 5-point (1 to 5) scale was used to rate each category, with 5–clinically acceptable with no limitations; 4–clinically acceptable with minor limitations; 3–clinically acceptable with moderate limitations; 2–clinically acceptable with major limitations; 1–clinically unacceptable scans. It was not possible to completely blind the raters due to differences in the imaging field of view acquired by the scanners (the Polestar has a limited field of view that does not capture the entire head).
Statistical Analysis
Univariate statistics were used to assess image quality scores, including mean and standard deviation. For each individual rater, scores from each subscale were collapsed into a mean score. These mean scores were then collapsed across raters. A paired t -test was then used to compare means between analogous 0.15 T and 0.064 T sequences. Additionally, means from each sequence were further collapsed into a 0.15 T overall mean score and a 0.064 T overall mean score. These overall mean scores were compared using the t -test. Findings with a p -value less than 0.01 were deemed statistically significant.
Results
The overall and individual sequence means (M) and standard deviations (SD) for image quality scores can be found in Table 1 . Overall, the 0.064 T images (M = 3.5, SD = 0.1) were given statistically higher ratings than the 0.15 T images (M = 2.4, SD = 0.2, p < 0.01) ( Fig. 2 ). The T2FSE-weighted images at 0.064 T (M = 3.5, SD = 0.2) were given statistically higher ratings than those at 0.15 T (M = 2.3, SD = 0.2, p < 0.01). All comparable sequences (T1, T2, T2 FLAIR, and SSFP) were rated significantly higher on the 0.064 T scanner compared to 0.15 T ( Table 1 ). On average, sequences on the 0.064 T scanner were rated 1.2 points (SD = 0.3) higher than 0.15 T scanner, with the T2 FLAIR sequences showing the largest increment on the 0.064T with an average rating difference of 1.5 points (SD = 0.2). The 0.15 T system was unable to acquire a DWI image and was overall rated the lowest amongst the 0.064 T sequences acquired. Regarding scanning time, the 0.064 T MRI acquired a complete set of images, including DWI, in 38 minutes and 1 second. The 0.15 T iMRI acquired its set of images, excluding DWI, in 42 minutes. Additionally, the 0.064 T system field of view encompassed the entire head, while only partial coverage was acquired by the 0.15 T iMRI. Fig. 2 shows T2W images from all 3 scanners, while Fig. 3 shows a variety of sequences acquired in two subjects using the 0.064 T and 0.15T MRIs.
Table 1. Mean quality ratings of 0.064 T (Swoop) and 0.15 T (Polestar) image series.
| Swoop (0.064 T)* | Polestar (0.15 T)* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject | T1 # | T2 FSE $ | T2 FLAIR ^ | SSFP (PSIF) + | SSFP (bSSFP) | DWI | T1 # | T2 FSE $ | T2 FLAIR ^ | SSFP + |
| 1 | 3.79 | 3.83 | 3.83 | 3.42 | 3.46 | 2.75 | 2.5 | 2.25 | 2.46 | 2.54 |
| 2 | 3.67 | 3.42 | 3.83 | 3.33 | 3.39 | 2.5 | 3.11 | 2.75 | 2.06 | 2.92 |
| 3 | 3.38 | 3.54 | 3.5 | 3.67 | 3.17 | 2.58 | 2.08 | 1.96 | 2.29 | 2.21 |
| 4 | 3.5 | 3.42 | 3.54 | 3.38 | 3 | 2.96 | 2.58 | 2.46 | 2 | 2.46 |
| 5 | 3.29 | 3.46 | 3.54 | 3.38 | 3.04 | 2.83 | 2.33 | 2.25 | 1.96 | 2.38 |
| Mean (± SD) |
3.53 (0.21) |
3.53 (0.17) |
3.65 (0.17) |
3.44 (0.13) |
3.21 (0.21) |
2.72 (0.19) |
2.52 (0.38) |
2.33 (0.29) |
2.15 (0.21) |
2.5 (0.26) |
.
Abbreviations: bSSFP, balanced SSFP; DWI, diffusion-weighted imaging; FLAIR, fluid-attenuated inversion recovery; FSE, fast spine echo; PSIF, time-reversed fast imaging with steady state precession; SSFP, steady-state free processing.
Fig. 2.
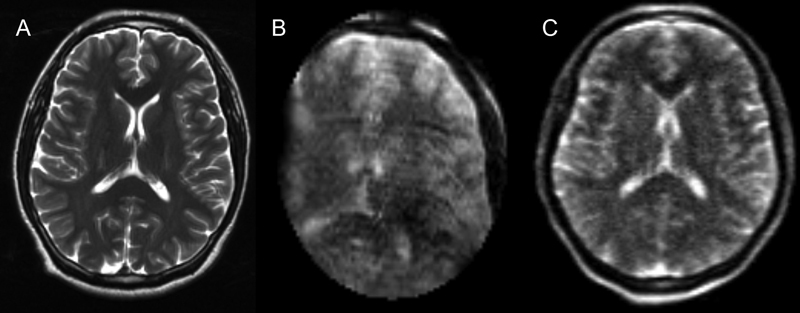
All the following T2W images were taken from the same subject. From left to right, these were acquired with the 1.5 T, 0.15 T iMRI, and 0.064 T MRI.
Fig. 3.
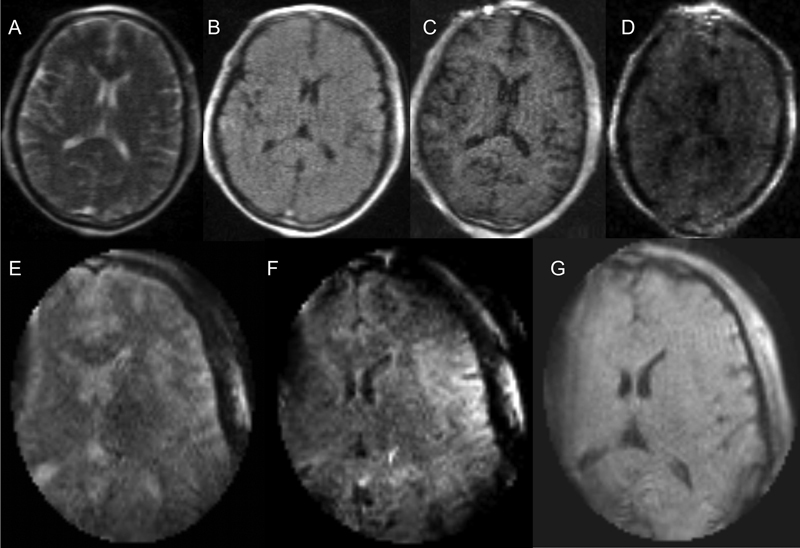
The following images were taken using the 0.064 T Hyperfine MRI and 0.15 T MRI from one healthy subject. ( A – D ) left to right shows T2, T2 FLAIR, T1 MPRAGE, DWI Trace images respectively from the 0.064 T. ( E – G ) from left to right shows T2, T2 FLAIR, and T1W MRI from 0.15 T.
Discussion
Supplementary Video S1 Video demonstrating maneuverability and standard setup of the Hyperfine MRI on a regular OR bed.
Our results showed that the 0.064 T portable MR system provided images that were rated significantly higher quality than those of the established 0.15 T system. Using the pre-configured clinical sequences, the 0.064 T system was able to acquire a study (including the additional DWI) faster than the 0.15 T system and had a larger field of view.
Several generations of the 0.15 T systems have been used in the operating room and shown to be effective in increasing the extent of intra-cranial tumor resection. 4 However, this system had several limitations, necessitating the search for better intraoperative MR imaging system. For example, the 0.15 T system costs over $1 million to install, 24 while the 0.064 T device is available for a substantially lower price. 23 Another drawback of the 0.15 T iMRI was its need for external shielding to prevent interference from nearby electronic equipment. This also can interfere with image quality. 25 The 0.064 T MRI, in contrast, is self-shielding. Additionally, the 0.064 T scanner is mounted on a motorized cart that is controlled with a joystick, making maneuverability in and out of the surgical field within the operating room much easier than the 0.15 T ( Supplementary Video S1 ). This feature allows for the device to be shared between different operating room suites and could potentially be used as a portable MRI system even outside the OR, making the device significantly more cost-effective for purchase by healthcare facilities. The 0.15 T iMRI is no longer manufactured nor supported by Medtronic in any event.
On the other hand, while not tested in this cohort of healthy subjects, the 0.15 T scanner is able to acquire images enhanced with intravenous paramagnetic contrast agents, a method not currently available on the 0.064 T scanner. This would somewhat limits the utility in tumor surgery where, small amounts of residual enhancing tumor maybe harder to identify without contrast administration. Additionally, the 0.064 T scanner is not configured at present for truly intraoperative imaging, regarding such issues as patient position, head fixation, sterility, coil placement, among others. In its current state, to be imaged on the 0.064 T scanner, the sterile field would have to be broken down and the patient removed from any head fixation device, which can add significant time to the procedure if the surgical cavity needs to be re-explored after imaging. Another disadvantage of the 0.064 T system is that it is not currently feasible to acquire high resolution images (2 mm or less) that could be utilized for stereotaxy with surgical navigation systems. However, the recent work by Deoni et al on the 0.064 T scanner demonstrated the feasibility of using multiple low resolution anisotropic images acquired in orthogonal planes to reconstruct a high-resolution (1.5 × 1.5 × 1.5 mm 3 ) T2 sequence. 26 While this feature is not yet commercially available on the 0.064 T imaging system, this work demonstrates its feasibility for the future. The 0.15 T system, in contrast, comes with an integrated surgical navigation system that can acquire images at up to 2 mm slice thickness. Additionally, while the 0.064 T scanner was able to acquire DWI sequences (not available on the 0.15 T system), they were rated as having moderate limitations. How that would affect radiological interpretation and clinical utility remains to be seen on prospective patient focused studies.
Current versions of the 0.064 T system (not utilized in this study) are now implementing an FDA approved advanced image reconstruction strategy, utilizing deep learning—an enabling technology that uses artificial neural networks (ANNs). Using this technology, the 0.064 T software system software produces improved T1, T2, and FLAIR image quality by reducing image blurring and noise. The scanner now uses a unique advanced image reconstruction pipeline, introducing two steps to the linear image reconstruction process—advanced gridding and advanced denoising.
Advanced gridding optimizes the imaging systems spatial frequency domain data (k-space data) before transforming the data into an image. This unique approach based on deep learning is superior to the traditional approach of using non-uniform fast Fourier transform (FFT-gridding) operations used by most conventional scanners. 27 Advanced denoising, the second application of deep learning that applies denoizing in small patches across the entire image. This process removes noise from the signal while preserving diagnostically critical information. The improvements in image quality using this reconstruction technique are shown in Fig. 4 .
Fig. 4.
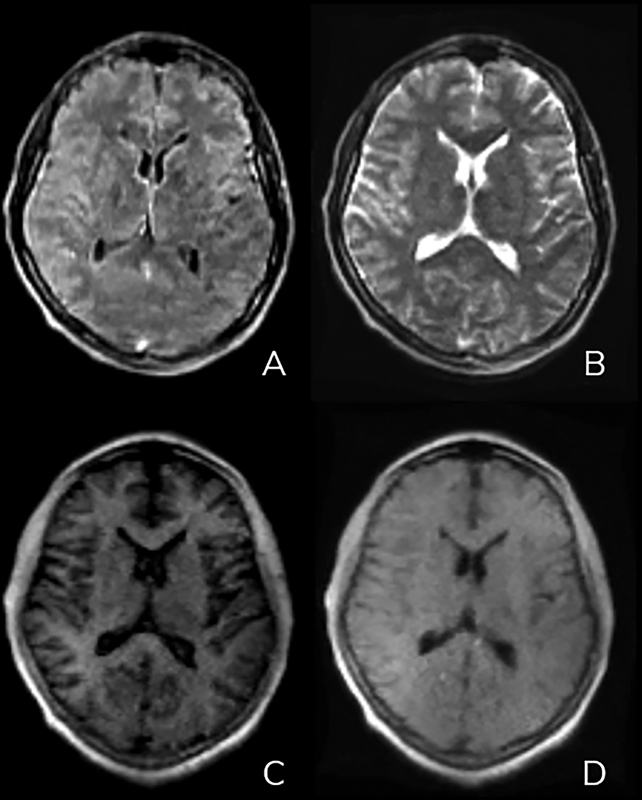
Images of a normal subject processed using Hyperfine's FDA approved deep learning algorithm. ( A ) Axial Flair, ( B ) Axial T2, ( C ) Axial T1 with gray-white image weighting, ( D ) Axial T1 with standard image weighting.
Findings from this study are in line with prior work demonstrating the benefit of this new portable 0.064 T imaging system, however these were performed in the ICU settings. 21 22 These other studies, however, lacked direct comparison of imaging with an already established MRI system. For example, while Sheth et al used conventional MRI images in their patients, images were taken at different time points of their ICU stays. 21 Because only patients with neurological injury were included, there was potential for improvement or worsening of their conditions over time, and so images taken from different time points may not be directly compared.
Our study, therefore, demonstrates the potential of this new technology with a direct comparison with an established low field MRI system. The main limitation of this study was the small sample size. Additionally, the raters could not be truly blinded to the MRI system used, creating the potential for some bias. The study also did not include images with intravenous (IV) contrast. The FDA has not yet approved the use of contrast at 0.064 T, a limitation regarding its immediate readiness for use during brain tumor resection, and we did not seek to use contrast in this group of healthy individuals. And, in this healthy cohort, our study could not directly compare the ability of the 0.064 T MRI to demonstrate abnormal pathological findings with the 0.15 T MRI. A larger study in various surgical patient populations is needed to truly understand it's utility in the operating suite. Lastly, although images were acquired in the operating room, this was without any surgery in progress and hence did not represent the actual surgical environment.
Conclusions
The results of our study demonstrate that a new, portable 0.064 T MRI system provides images of comparable quality or better, and at faster times than those provided by a well-established 0.15 T intraoperative MRI. Current advances in image reconstruction techniques with the 0.064 T MRI system have further improved the quality of the images acquired and broaden its clinical utility. This study suggests that with appropriate technical modifications a 0.064 T MRI system can be adapted as a potential next-generation intraoperative MRI.
Acknowledgments
The authors would like to acknowledge the contributions of Lori Arlinghaus PhD (Hyperfine, Inc.) for her contributions in data analysis and reviewing the manuscript. The study was approved by the Institutional Review Board, and written consent was obtained from all participants prior to enrollment.
Funding Statement
Funding IRB costs and the Hyperfine 0.064 T System were supported by Hyperfine, Inc.
Conflict of Interest The corresponding author is a member of the Medical Advisory Board of Hyperfine.
These authors contributed equally to the manuscript and retain the first authorship.
References
- 1.Black P M, Moriarty T, Alexander E, IIIet al. Development and implementation of intraoperative magnetic resonance imaging and its neurosurgical applications Neurosurgery 19974104831–842., discussion 842–845 [DOI] [PubMed] [Google Scholar]
- 2.Martin C, Alexander E, III, Wong T, Schwartz R, Jolesz F, Black P M. Surgical treatment of low-grade gliomas in the intraoperative magnetic resonance imager. Neurosurg Focus. 1998;4(04):e8. doi: 10.3171/foc.1998.4.4.11. [DOI] [PubMed] [Google Scholar]
- 3.Hirschl R A, Wilson J, Miller B, Bergese S, Chiocca E. The predictive value of low-field strength magnetic resonance imaging for intraoperative residual tumor detection. Clinical article. J Neurosurg. 2009;111(02):252–257. doi: 10.3171/2008.9.JNS08729. [DOI] [PubMed] [Google Scholar]
- 4.Livne O, Harel R, Hadani M, Spiegelmann R, Feldman Z, Cohen Z R. Intraoperative magnetic resonance imaging for resection of intra-axial brain lesions: a decade of experience using low-field magnetic resonance imaging, Polestar N-10, 20, 30 systems. World Neurosurg. 2014;82(05):770–776. doi: 10.1016/j.wneu.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Olubiyi O I, Ozdemir A, Incekara F et al. Intraoperative magnetic resonance imaging in intracranial glioma resection: a single-center, retrospective blinded volumetric study. World Neurosurg. 2015;84(02):528–536. doi: 10.1016/j.wneu.2015.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatiboglu M A, Weinberg J S, Suki Det al. Impact of intraoperative high-field magnetic resonance imaging guidance on glioma surgery: a prospective volumetric analysis Neurosurgery 200964061073–1081., discussion 1081 [DOI] [PubMed] [Google Scholar]
- 7.Leuthardt E C, Lim C C, Shah M Net al. Use of movable high-field-strength intraoperative magnetic resonance imaging with awake craniotomies for resection of gliomas: preliminary experience Neurosurgery 20116901194–205., discussion 205–206 [DOI] [PubMed] [Google Scholar]
- 8.Bohinski R J, Kokkino A K, Warnick R Eet al. Glioma resection in a shared-resource magnetic resonance operating room after optimal image-guided frameless stereotactic resection Neurosurgery 20014804731–742., discussion 742–744 [DOI] [PubMed] [Google Scholar]
- 9.Senft C, Seifert V, Hermann E, Franz K, Gasser T.Usefulness of intraoperative ultra low-field magnetic resonance imaging in glioma surgery Neurosurgery 200863(4, Suppl 2)257–266., discussion 266–267 [DOI] [PubMed] [Google Scholar]
- 10.Leroy H A, Delmaire C, Le Rhun E, Drumez E, Lejeune J P, Reyns N. High-field intraoperative MRI and glioma surgery: results after the first 100 consecutive patients. Acta Neurochir (Wien) 2019;161(07):1467–1474. doi: 10.1007/s00701-019-03920-6. [DOI] [PubMed] [Google Scholar]
- 11.Salas S, Brimacombe M, Schulder M.Stereotactic accuracy of a compact intraoperative MRI system Stereotact Funct Neurosurg 200785(2–3):69–74. [DOI] [PubMed] [Google Scholar]
- 12.Abraham P, Sarkar R, Brandel M G et al. Cost-effectiveness of intraoperative MRI for treatment of high-grade gliomas. Radiology. 2019;291(03):689–697. doi: 10.1148/radiol.2019182095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui Z, Pan L, Song H et al. Intraoperative MRI for optimizing electrode placement for deep brain stimulation of the subthalamic nucleus in Parkinson disease. J Neurosurg. 2016;124(01):62–69. doi: 10.3171/2015.1.JNS141534. [DOI] [PubMed] [Google Scholar]
- 14.Kurwale N S, Chandra S P, Chouksey P et al. Impact of intraoperative MRI on outcomes in epilepsy surgery: preliminary experience of two years. Br J Neurosurg. 2015;29(03):380–385. doi: 10.3109/02688697.2014.1003034. [DOI] [PubMed] [Google Scholar]
- 15.Tatsui C E, Nascimento C NG, Suki D et al. Image guidance based on MRI for spinal interstitial laser thermotherapy: technical aspects and accuracy. J Neurosurg Spine. 2017;26(05):605–612. doi: 10.3171/2016.9.SPINE16475. [DOI] [PubMed] [Google Scholar]
- 16.White T, Zavarella S, Jarchin L, Nardi D, Schaffer S, Schulder M. Combined brain mapping and compact intraoperative MRI for brain tumor resection. Stereotact Funct Neurosurg. 2018;96(03):172–181. doi: 10.1159/000488991. [DOI] [PubMed] [Google Scholar]
- 17.Hadani M, Spiegelman R, Feldman Z, Berkenstadt H, Ram Z.Novel, compact, intraoperative magnetic resonance imaging-guided system for conventional neurosurgical operating rooms Neurosurgery 20014804799–807., discussion 807–809 [DOI] [PubMed] [Google Scholar]
- 18.Gerlach R, du Mesnil de Rochemont R, Gasser Tet al. Feasibility of Polestar N20, an ultra-low-field intraoperative magnetic resonance imaging system in resection control of pituitary macroadenomas: lessons learned from the first 40 cases Neurosurgery 20086302272–284., discussion 284–285 [DOI] [PubMed] [Google Scholar]
- 19.Makary M, Chiocca E A, Erminy N et al. Clinical and economic outcomes of low-field intraoperative MRI-guided tumor resection neurosurgery. J Magn Reson Imaging. 2011;34(05):1022–1030. doi: 10.1002/jmri.22739. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Garcia S, García-Lorenzo B, Ramos P R et al. Cost-effectiveness of low-field intraoperative magnetic resonance in glioma surgery. Front Oncol. 2020;10:586679. doi: 10.3389/fonc.2020.586679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheth K N, Mazurek M H, Yuen M M, Cahn B A, Shah J T, Ward A, Kim J A, Gilmore E J, Falcone G J, Petersen N, Gobeske K T. Assessment of brain injury using portable, low-field magnetic resonance imaging at the bedside of critically ill patients. JAMA neurology. 2021;78(01):41–47. doi: 10.1001/jamaneurol.2020.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turpin J, Unadkat P, Thomas J et al. Portable magnetic resonance imaging for ICU patients. Crit Care Explor. 2020;2(12):e0306. doi: 10.1097/CCE.0000000000000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyperfine; 2021. Accessed November 16, 2022, at:https://hyperfine.io/product/#subscriptionservice
- 24.Schulder M. Intracranial surgery with a compact, low-field-strength magnetic resonance imager. Top Magn Reson Imaging. 2009;19(04):179–189. doi: 10.1097/RMR.0b013e31819637cc. [DOI] [PubMed] [Google Scholar]
- 25.Ntoukas V, Krishnan R, Seifert V.The new generation polestar n20 for conventional neurosurgical operating rooms: a preliminary report Neurosurgery 200862(3, Suppl 1)82–89., discussion 89–90 [DOI] [PubMed] [Google Scholar]
- 26.Deoni S CL, O'Muircheartaigh J, Ljungberg E, Huentelman M, Williams S CR. Simultaneous high-resolution T 2 -weighted imaging and quantitative T 2 mapping at low magnetic field strengths using a multiple TE and multi-orientation acquisition approach . Magn Reson Med. 2022;88(03):1273–1281. doi: 10.1002/mrm.29273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zwart N R, Johnson K O, Pipe J G. Efficient sample density estimation by combining gridding and an optimized kernel. Magn Reson Med. 2012;67(03):701–710. doi: 10.1002/mrm.23041. [DOI] [PubMed] [Google Scholar]


