Abstract
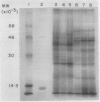
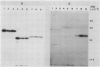
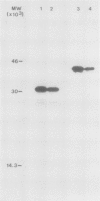
Polyclonal antibodies raised against barley (1→3,1→4)-β-d-glucanase, α-amylase and carboxypeptidase were used to detect precursor polypeptides of these hydrolytic enzymes among the in vitro translation products of mRNA isolated from the scutellum and aleurone of germinating barley. In the scutellum, mRNA encoding carboxypeptidase appeared to be relatively more abundant than that encoding α-amylase or (1→3,1→4)-β-d-glucanase, while in the aleurone α-amylase and (1→3,1→4)-β-d-glucanase mRNAs predominated. The apparent molecular weights of the precursors for (1→3,1→4)-β-d-glucanase, α-amylase, and carboxypeptidase were 33,000, 44,000, and 35,000, respectively. In each case these are slightly higher (1,500-5,000) than molecular weights of the mature enzymes. Molecular weights of precursors immunoprecipitated from aleurone and scutellum mRNA translation products were identical for each enzyme.
Full text
PDF
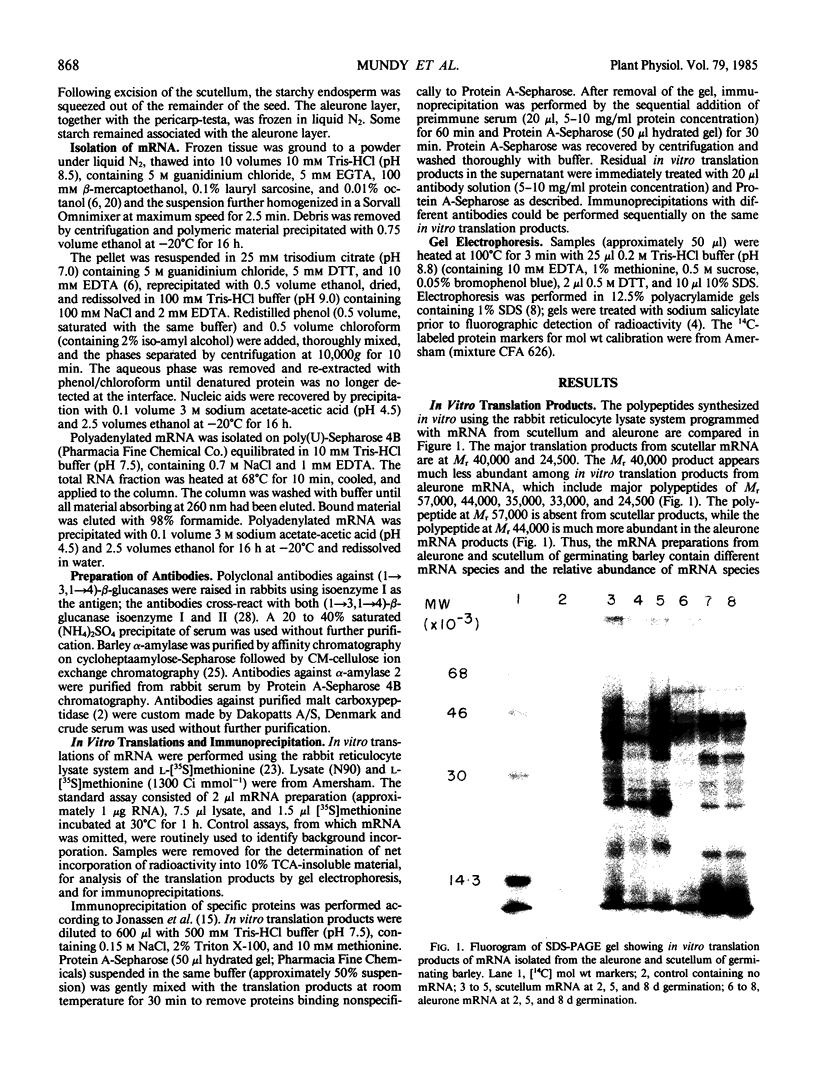



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chrispeels M. J., Varner J. E. Gibberellic Acid-enhanced synthesis and release of alpha-amylase and ribonuclease by isolated barley and aleurone layers. Plant Physiol. 1967 Mar;42(3):398–406. doi: 10.1104/pp.42.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Bennoun P. Thylakoid membrane polypeptides of Chlamydomonas reinhardtii: wild-type and mutant strains deficient in photosystem II reaction center. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2175–2179. doi: 10.1073/pnas.72.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J. V., Higgins T. J. Characterization of the alpha-Amylases Synthesized by Aleurone Layers of Himalaya Barley in Response to Gibberellic Acid. Plant Physiol. 1982 Dec;70(6):1647–1653. doi: 10.1104/pp.70.6.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macgregor A. W., Macdougall F. H., Mayer C., Daussant J. Changes in Levels of alpha-Amylase Components in Barley Tissues during Germination and Early Seedling Growth. Plant Physiol. 1984 May;75(1):203–206. doi: 10.1104/pp.75.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozer T. J. Partial purification and characterization of the mRNA for alpha-amylase from barley aleurone layers. Plant Physiol. 1980 May;65(5):834–837. doi: 10.1104/pp.65.5.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita T. W., Greene F. C. Wheat Storage Proteins : ISOLATION AND CHARACTERIZATION OF THE GLIADIN MESSENGER RNAs. Plant Physiol. 1982 Apr;69(4):834–839. doi: 10.1104/pp.69.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paleg L. G. Physiological Effects of Gibberellic Acid. II. On Starch Hydrolyzing Enzymes of Barley Endosperm. Plant Physiol. 1960 Nov;35(6):902–906. doi: 10.1104/pp.35.6.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ranki H., Sopanen T. Secretion of alpha-amylase by the aleurone layer and the scutellum of germinating barley grain. Plant Physiol. 1984 Jul;75(3):710–715. doi: 10.1104/pp.75.3.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanovich M. P., Hill R. D. Affinity chromatography of cereal alpha-amylase. Anal Biochem. 1976 Jun;73(2):430–433. doi: 10.1016/0003-2697(76)90191-3. [DOI] [PubMed] [Google Scholar]
- Winspear M. J., Preston K. R., Rastogi V., Oaks A. Comparisons of Peptide hydrolase activities in cereals. Plant Physiol. 1984 Jun;75(2):480–482. doi: 10.1104/pp.75.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward J. R., Fincher G. B. Purification and chemical properties of two 1,3;1,4-beta-glucan endohydrolases from germinating barley. Eur J Biochem. 1982 Jan;121(3):663–669. doi: 10.1111/j.1432-1033.1982.tb05837.x. [DOI] [PubMed] [Google Scholar]