Abstract
Introduction:
The hepatitis C virus (HCV) epidemic remains a public health problem worldwide. A systematic review and meta-analysis were conducted to provide evidence of outcomes attained across the HCV care cascade in the era of direct-acting antivirals.
Methods:
Studies from North America, Europe, and Australia (January 2014 through March 2021) reporting on HCV care cascade outcomes (screening to cure) were included. When calculating the proportions of individuals completing each step, the numerator for Steps 1–8 was the number of individuals completing each step; the denominator was the number of individuals completing the previous step for Steps 1–3 and Step 3 for Steps 4–8. In 2022, random effects meta-analyses were conducted to estimate pooled proportions with 95% CIs.
Results:
Sixty-five studies comprising 7,402,185 individuals were identified. Among individuals with positive HCV ribonucleic acid test results, 62% (95% CI=55%, 70%) attended their first care appointment, 41% (95% CI=37%, 45%) initiated treatment, 38% (95% CI=29%, 48%) completed treatment, and 29% (95% CI=25%, 33%) achieved cure. HCV screening rates were 43% (95% CI=22%, 66%) in prisons or jails and 20% (95% CI=11%, 31%) in emergency departments. Linkage to care rates were 62% (95% CI=46%, 75%) for homeless individuals and 26% (95% CI=22%, 31%) for individuals diagnosed in emergency departments. Cure rates were 51% (95% CI=30%, 73%) in individuals with substance use disorder and 17% (95% CI=17%, 17%) in homeless individuals. Cure rates were lowest in the U.S.
Discussion:
Despite the availability of effective all-oral direct-acting antiviral therapies, persistent gaps remain across the HCV care cascade, especially among traditionally marginalized populations. Public health interventions targeting identified priority areas (e.g., emergency departments) may improve screening and healthcare retention of vulnerable populations with HCV infection (e.g., substance use disorder populations).
INTRODUCTION
The hepatitis C virus (HCV) epidemic has developed into a global public health problem.1,2 In the U.S. alone, between 2.4 and 3.9 million people are infected with HCV, with increasing incidence (about 40 per 100 person-years) among young people who inject drugs (PWIDs) and with approximately 50% of individuals unaware of their infection status.3,4 HCV incidence is also higher among PWIDs in other developed countries: in Australia, the incidence ranges from 7.6 to 12.8 per 100 person-years, whereas the incidence in England is 8.7 per 100 person-years.5,6 In 2013, the advent of oral direct-acting antiviral (DAA) agents revolutionized the treatment of HCV infection. These new agents target different structures involved in the HCV replication process (e.g., they inhibit units of the replicase complex or the ribonucleic acid (RNA) chain polymerase);7 they have 95% or higher therapeutic efficacy and limited adverse events, which has turned HCV infection into a curable disease.2,8 Thus, the WHO’s goal of decreasing HCV infection incidence by 90% by 20309 appears feasible. However, individuals with HCV face multilevel barriers (e.g., comorbidities, job insecurity, lack of insurance),3,4,10 and thus, the availability of an effective treatment alone is insufficient for reaching such an ambitious goal if affected populations lack proper access to care and support from healthcare systems.11 Despite multiple efforts worldwide to improve screening and linkage to care for at-risk populations, persistent gaps have been identified in the HCV care cascade—which typically includes HCV antibody screening, HCV RNA confirmation testing, treatment initiation and completion, and sustained virologic response (SVR). Barriers are more challenging to overcome in large populations that include traditionally marginalized groups, such as PWIDs and incarcerated or homeless individuals.12
Although evidence from different studies exists on the need to strengthen access to care for individuals with HCV infection, to the investigators’ knowledge, no systematic review has compared HCV care cascade outcomes for various strategies and different venues implemented around the world to address this need. Therefore, a systematic review and meta-analysis were conducted to synthesize and evaluate the reported proportions of outcomes attained at each step of the HCV care cascade after the availability of DAAs.
METHODS
The investigators searched MEDLINE, Embase, Cochrane Central Register of Controlled Trials, CINAHL, and PsycINFO from January 2014 through March 2021. The search strategy included terms for hepatitis C infection and each HCV care cascade step. The 8 steps of this study’s HCV care cascade included (1) HCV screening, (2) positive HCV antibody test results, (3) positive HCV RNA test results, (4) successful patient contact, (5) linkage to care at first appointment, (6) treatment initiation, (7) treatment completion, and (8) confirmed SVR. Appendix Table 1 (available online) provides the full search strategy using MeSH (Medical Subject Headings) terms and keywords. Observational studies, interventional studies, and clinical trials assessing outcomes at any point in the HCV care continuum, from screening to cure, were included. Additional manual searches were performed by reviewing the reference lists of the included studies. This systematic review was conducted according to PRISMA guidelines.13 The study’s protocol is registered in the International Prospective Register of Systematic Reviews (CRD42021243759).
Studies were included if they (1) targeted adults with HCV screening or diagnosis; (2) reported at least 2 of the 8 steps in the HCV care cascade; (3) were conducted in the U.S., Europe, Australia, New Zealand, or Canada; (4) were published after January 2014 (all-oral DAA therapy era); (5) were conducted as clinical trials, interventional studies (i.e., studies where an intervention was implemented but which did not have a comparator group), and retrospective or prospective observational studies; and (6) were reported in English. Studies were excluded if they (1) focused on specific populations from other countries (e.g., immigrants); (2) focused on specific races or ethnicities (e.g., American Indian, Alaska Native); (3) included <100 individuals; (4) included interferon-based therapy; (5) did not report the study period; and (6) were systematic reviews, case studies, conference abstracts, or studies with surveys (e.g., convenience sample, probability sample). Two investigators (PHC, NO, MR, IU, HS, AJ, or XJ) independently screened titles, abstracts, and full-text articles and independently extracted data using a prespecified standardized form. Differences were reconciled by a third investigator. Information on authors, publication journal, publication year, study design, population, study setting, study location, sample size, intervention description (when applicable), results, and conclusions was extracted.
First, the number of individuals completing each step in the HCV care cascade step was obtained. Then, the investigators calculated the number of individuals screened among those eligible for HCV screening (Step 1), with positive antibody test results among those screened (Step 2), and with positive HCV RNA test results (HCV infection) among those with positive HCV antibody test results (Step 3). Next, among individuals with positive HCV RNA test results, the investigators obtained the number of individuals who were successfully contacted by healthcare providers (Step 4), attended their first clinic appointment (Step 5), initiated (Step 6) and completed (Step 7) DAA treatment, and achieved SVR at 12 weeks after therapy completion (Step 8). The proportion of individuals completing Steps 1–3 was calculated by dividing the number of individuals completing each step (numerator) by the number of individuals completing the previous step (denominator). Similarly, the proportion of HCV-infected individuals completing Steps 4–8 was calculated by dividing the number of individuals completing each step (numerator) by the number of individuals with a positive HCV RNA test result (denominator), defined as receiving an HCV diagnosis.
The metaprop command in Stata was used to calculate pooled prevalence estimates with exact binomial and score test–based 95% CIs for Steps 1–8 of the HCV care cascade using a random-effects model; this method appropriately combines rates close to margins using Freeman—Tukey Double Arcsine Transformation to stabilize variances.14 Then, heterogeneity across studies using Cochran’s Q test and I2 statistics was assessed.15
To account for potential sources of heterogeneity, the meta-analyses were stratified by (1) healthcare setting (i.e., emergency department [ED], ambulatory care, sexually transmitted disease [STD]/substance use disorder [SUD]/syringe exchange program, jail or prison), (2) population (i.e., individuals with HIV, with SUD, or experiencing homelessness or populations with ages ranging from 59 to 77 years as of 2023 [born in 1946 — 1964 or baby boomers]), and (3) country or territory (i.e., U.S., Europe, Australia, Canada). The meta-analyses were also stratified by country or territory owing to differences in access to care and access to DAA therapy among countries or territories included in this study (e.g., universal access to DAAs has been available since 2015 in Spain, 2016 in Australia, 2017 in France and Italy, and 2018 in Canada; access to DAAs has been scaling-up in the United Kingdom since 2017, and access remains restricted in the U.S., largely depending on the type of health insurance).5,16–21 Funnel plots were used to assess for publication bias. All meta-analyses were performed in 2022, using Stata 16 statistical software (StataCorp LP, College Station, TX).
RESULTS
After applying exclusion criteria, 65 full-text articles comprising 7,402,185 total participants were included in the analysis (Figure 1). A total of 49 studies (75%) were conducted in the U.S., 11 studies (17%) were conducted in Europe (3 studies in Italy and the United Kingdom each; 2 studies in Ireland; and 1 study in Finland, France, and Spain each), 3 studies (5%) were conducted in Australia, and 2 studies (3%) were conducted in Canada. In addition, 43 studies (66%) were interventional studies, 20 studies (31%) were retrospective or prospective observational studies, and 2 studies (3%) were clinical trials (Appendix Table 2, available online). Most studies assessed outcomes in ambulatory care settings (42%). Other settings included SUD, STD, and syringe exchange programs (12%); EDs (12%); prisons or jails (8%); and other settings (e.g., in-patient, healthcare systems [23%]). Two studies (3%) assessed outcomes in more than 1 setting.
Figure 1.
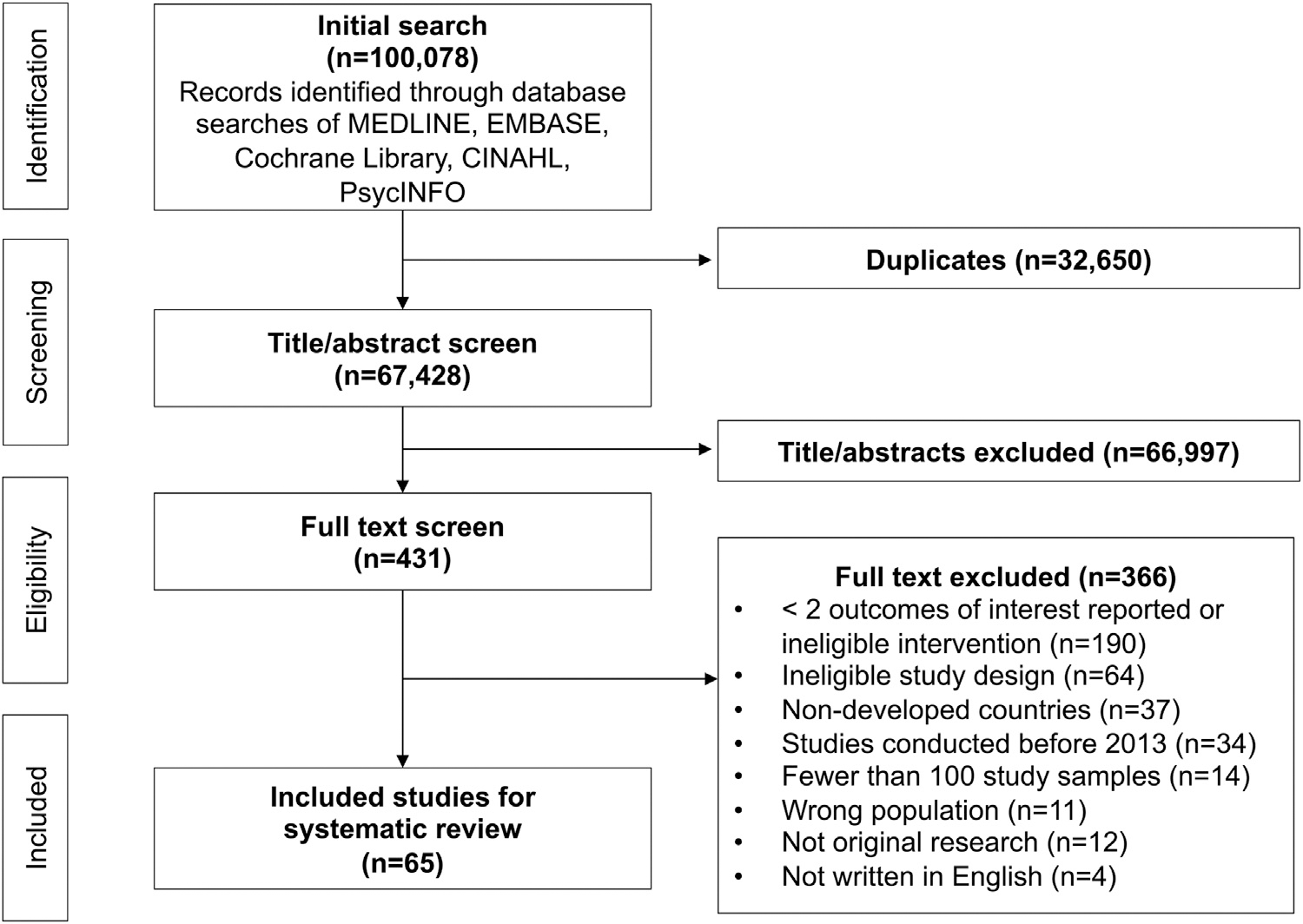
Flow diagram of study selection.
Table 1 summarizes the pooled proportion of individuals completing each step of the HCV care cascade. Overall, 49% (95% CI=37%, 61%, I2=100%, p<0.01) of individuals were screened for HCV (Step 1), and 15% (95% CI=12%, 18%, I2=100%, p<0.01) of individuals had positive HCV antibody tests (Step 2); of those, 53% (95% CI=48%, 59%, I2=99%, p<0.01) had positive HCV RNA test results (Step 3). Among individuals with positive RNA test results (HCV diagnosis), 82% (95% CI=76%, 88%, I2=97%, p<0.01) were successfully contacted (Step 4), 62% (95% CI=55%, 70%, I2=99%, p<0.01) attended their first appointment (Step 5), 41% (95% CI=37%, 45%, I2=99%, p<0.01) initiated HCV treatment (Step 6), 38% (95% CI=29%, 48%, I2=99%, p<0.01) completed treatment (Step 7), and 29% (95% CI=25%, 33%, I2=99%, p<0.01) achieved SVR (Step 8).
Table 1.
Pooled Proportions of Individuals Completing Each of the 8 Steps in the HCV Care Cascade
| Step in the HCV care cascade | Number of studies (N=65) | Number of individuals | Random effects, estimate (95% CI) | I 2 |
|---|---|---|---|---|
|
| ||||
| Step 1: screening rate (among persons who were eligible for hepatitis C screening) | 32 | 6,396,187 | 0.49(0.37,0.61) | 100%a |
| Step 2: prevalence of positive antibody test (among persons who were screened for HCV) | 48 | 508,412 | 0.15 (0.12, 0.18) | 100%a |
| Step 3: prevalence of positive HCV RNA test (among persons with positive HCV antibody test) | 47 | 42,163 | 0.53 (0.48, 0.59) | 99%a |
| Step 4: successful contact rate (among persons with positive HCV RNA test) | 26 | 16,069 | 0.82 (0.76, 0.88) | 99%a |
| Step 5: linkage to care at first appointment (among all persons with positive HCV RNA test) | 47 | 31,842 | 0.62 (0.55, 0.70) | 99%a |
| Step 6: treatment initiation (among all persons with positive HCV RNA test) | 42 | 205,163 | 0.41 (0.37, 0.45) | 99%a |
| Step 7: treatment completion (among all persons with positive HCV RNA test) | 22 | 12,762 | 0.38 (0.29, 0.48) | 99%a |
| Step 8: confirmed sustained virologic response (among all persons with positive HCV RNA test) | 34 | 204,015 | 0.29 (0.25, 0.33) | 99%a |
Note: Pooled proportions were separately calculated for each step. Thus, studies included to calculate pooled proportions of one step were not necessarily included when calculating the pooled proportions of subsequent steps.
Q-test indicates p<0.01.
HCV, hepatitis C virus; RNA, ribonucleic acid.
Figure 2A and B shows the proportions obtained at each step of the HCV care cascade by healthcare setting. For Step 1 (Appendix Figure 1A, available online), STD, SUD, and syringe programs had the highest proportions of HCV screening (69%, 95% CI=21%, 99%), whereas prisons or jails and EDs were among the settings with the lowest proportions of HCV screening (43% [95% CI=22%, 66%] and 20% [95% CI=11%, 31%], respectively). The proportions of individuals with positive HCV antibody test results (Step 2) ranged from 10% (95% CI=8%, 12%) to 34% (95% CI=21%, 50%), with individuals diagnosed in STD, SUD, and syringe programs achieving the highest proportions (Appendix Figure 1B, available online). The proportions of individuals with positive HCV RNA test results (Step 3) were similar by healthcare setting, ranging from 52% (95% CI=26%, 77%) to 57% (95% CI=43%, 71%) as shown in Appendix Figure 1C (available online). Successful contact rates of individuals identified with chronic HCV infection (Step 4) ranged from 59% (95% CI=48%, 69%) for those diagnosed in EDs to 94% (95% CI=75%, 100%) for those diagnosed in STD, SUD, and syringe programs (Appendix Figure 1D, available online). Similarly, Appendix Figure 1E (available online) shows that higher proportions of linkage to care (Step 5) were observed for individuals who were diagnosed in STD, SUD, and syringe programs (73%; 95% CI=54%, 87%) than for individuals screened and diagnosed in ED settings (26%; 95% CI=22%, 31%).
Figure 2.
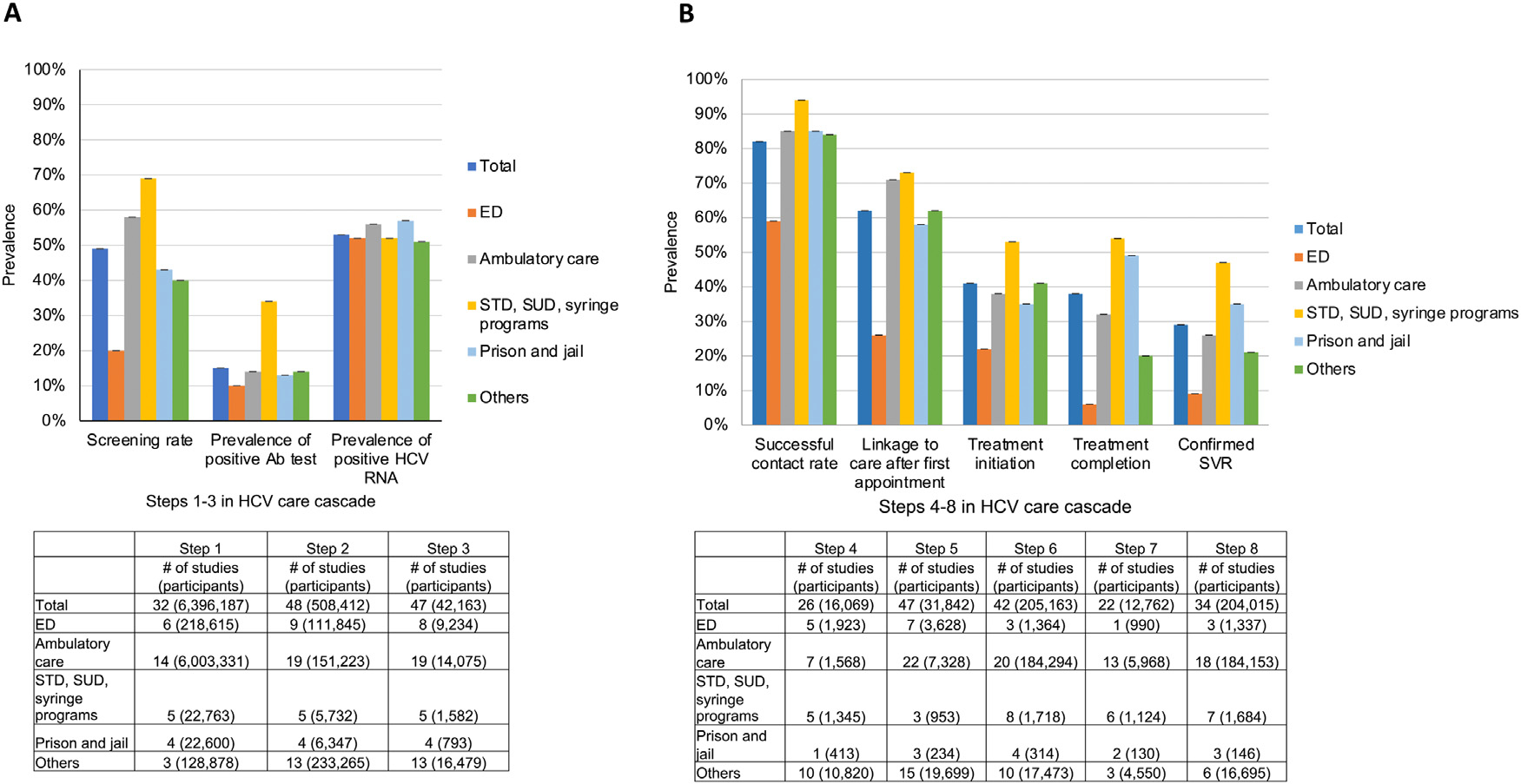
HCV treatment cascade steps by setting.
(A) Steps 1–3. The proportion of individuals who were screened was calculated among individuals who were eligible for HCV screening (Step 1). The proportion of individuals with positive Ab test results was calculated among screened individuals (Step 2), and the proportion of individuals with positive HCV RNA test results was calculated among individuals with positive Ab test results (Step 3). (B) Steps 4–8. Using the pooled estimates of the 8 steps and the number of participants from Steps 1 to 3, the proportion of HCV-infected individuals completing each step of the HCV care cascade was calculated by dividing the number of individuals who completed each step (Steps 4–8) by the number of individuals with positive HCV RNA test results.
Note: Calner et al. (2019) included 3 settings (ED, ambulatory care, and others); Ford et al. (2017) included 2 settings (ambulatory care and STD, SUD, and syringe service programs). The sum of the numbers for each individual setting may differ from the total for some of the steps of the HCV care cascade.
Ab, antibody; ED, emergency department; HCV, hepatitis C virus; RNA, ribonucleic acid; STD, sexually transmitted disease; SUD, substance use disorder; SVR, sustained virologic response.
The proportions of individuals initiating treatment (Step 6) ranged from 22% (95% CI=6%, 45%) among individuals diagnosed in ED settings to 53% (95% CI=39%, 67%) among individuals diagnosed in STD, SUD, and syringe programs (Appendix Figure 1F, available online). Whereas the proportions of individuals completing treatment (Step 7) were highest for incarcerated individuals (49%; 95% CI=41%, 58%) and for individuals diagnosed in STD, SUD, and syringe programs (54%; 95% CI=36%, 71%), this proportion was lowest for individuals diagnosed in EDs (6%; 95% CI=4%, 8%) (Appendix Figure 1G, available online). Similarly, the proportions of individuals achieving SVR (Step 8) were highest for incarcerated individuals (35%; 95% CI=3%, 78%) and individuals diagnosed in STD, SUD, and syringe programs (47%; 95% CI=35%, 59%), whereas this proportion was lowest for individuals screened and diagnosed in EDs (9%; 95% CI=3%, 18%) (Appendix Figure 1H, available online).
Figure 3A shows the proportion obtained at Steps 1–3 of the HCV care cascade by key subgroup population. Among eligible individuals, the proportion of individuals screened for HCV (Step 1) was highest for those with HIV (88%; 95% CI=87%, 90%), followed by those experiencing homelessness (78%; 95% CI=78%, 78%) and those with SUD (69%; 95% CI=21%, 99%) (Appendix Figure 2A, available online). The proportion of individuals with positive HCV antibody test results (Step 2) ranged from 11% (95% CI=15%, 46%) for populations with ages ranging from 59 years to 77 years as of 2023 (born in 1946–1964 or baby boomers) to 28% (95% CI=15%, 44%) for those with SUD (Appendix Figure 2B, available online). Among individuals with positive HCV antibody test results, the proportion of individuals with positive HCV RNA tests (Step 3) varied from 30% (95% CI=3%, 18%) for those with SUD to 75% (95% CI=49%, 94%) for individuals with HIV (Appendix Figure 2C, available online).
Figure 3.
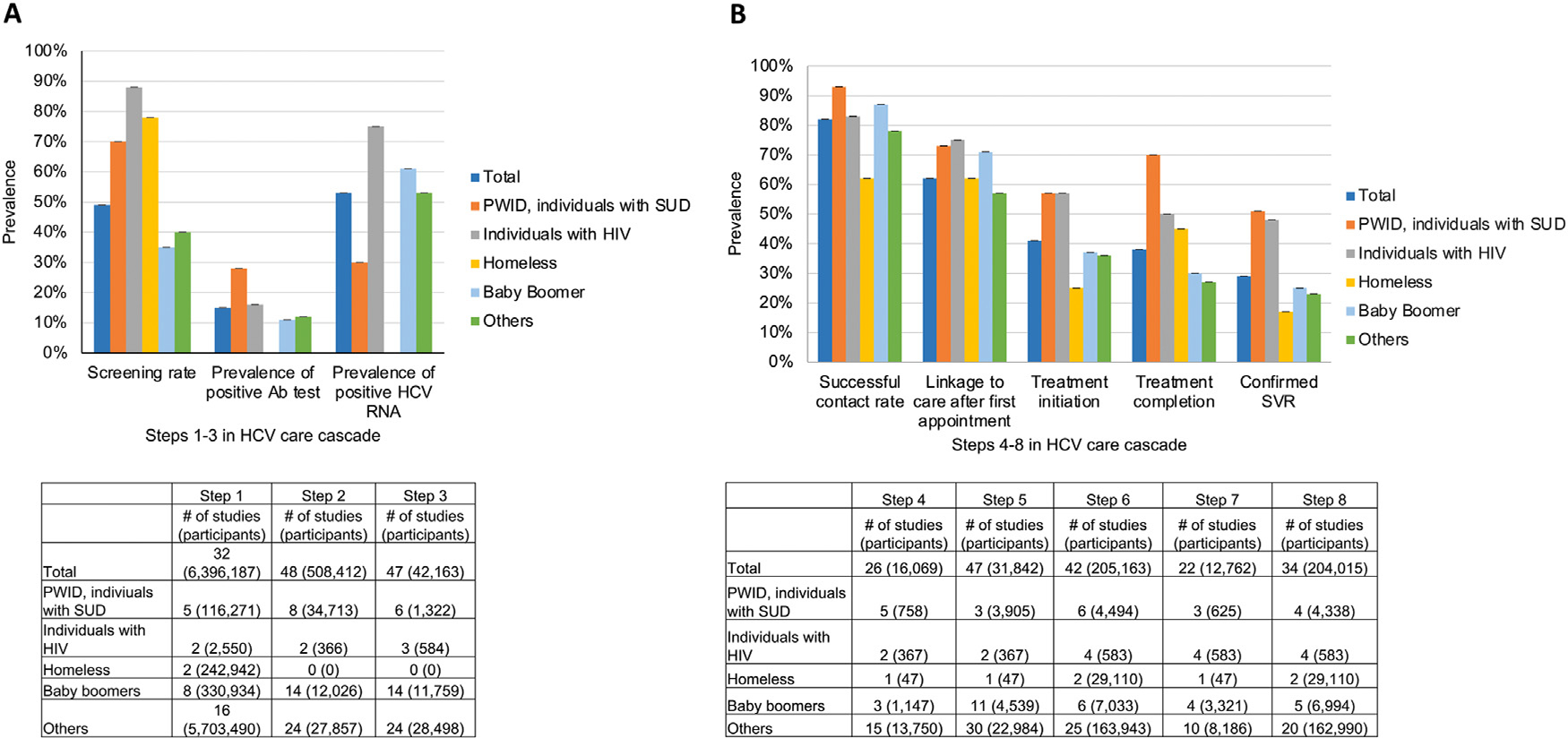
HCV treatment cascade steps by population.
(A) Steps 1–3. The proportion of individuals who were screened was calculated among individuals who were eligible for HCV screening (Step 1). The proportion of individuals with positive Ab test results was calculated among screened individuals (Step 2), and the proportion of individuals with positive HCV RNA test results was calculated among individuals with positive Ab test results (Step 3). (B) Steps 4–8. Using the pooled estimates of the 8 steps and the numbers of participants from Steps 1 to 3, the proportion of HCV-infected individuals completing each HCV cascade step was calculated by dividing the number of individuals who completed each step (Steps 4–8) by the number of individuals with positive HCV RNA test results.
Note: Noska et al. (2017) included 2 subpopulations of U.S. veterans: homeless and not homeless. The sum of the numbers for each individual subpopulation may differ from the total for some of the steps of the HCV care cascade.
Ab, antibody; HCV, hepatitis C virus; PWID, people who inject drug; RNA, ribonucleic acid; SUD, substance use disorder; SVR, sustained virologic response.
Figure 3B shows the proportions of individuals completing Steps 4–8 of the HCV care cascade. Whereas individuals with SUD achieved the highest—or were among those who achieved the highest—proportions at each step of the HCV care cascade from successful patient contact to confirmed SVR, individuals experiencing homelessness achieved the lowest—or were among those who achieved the lowest—proportions at each of these steps (Appendix Figure 2D–H, available online). Specifically, among individuals with SUD, 73% (95% CI=45%, 94%) were linked to a first appointment for HCV infection (Step 5), 70% (95% CI=57%, 82%) completed HCV treatment (Step 7), and 51% (95% CI=30%, 73%) achieved SVR (Step 8). Conversely, among individuals experiencing homelessness, 62% (95% CI=46%, 75%) were linked to their first HCV infection appointment, 25% (95% CI=25%, 26%) initiated treatment (Step 6), and 17% (95% CI=17%, 17%) achieved SVR.
Overall, there was evidence of publication bias for outcomes obtained at Steps 1, 2, and 7 of the care cascade (Egger’s test p-values=0.004, 0.022, and 0.05, respectively) but not at Steps 3–6 and Step 8 (Egger’s test p-values=0.648, 0.767, 0.700, and 0.919, respectively).
Appendix Figure 3A and B (available online) compare results across some developed countries. The proportion of individuals screened for HCV was higher in Europe (67%; 95% CI=43%, 87%) than in the U.S. (44%; 95% CI=30%, 58%) and Australia or Canada (43%; 95% CI=23%, 63%). The proportion of individuals with positive HCV antibody test results in Europe (23%; 95% CI=13%, 36%) was nearly double the U.S. proportion (13%; 95% CI=9%, 16%). The proportions of individuals with positive HCV RNA test results (56%; 95% CI=50%, 61%) and successfully contacted (84%, 95% CI=77%, 91%) were highest in the U.S. Although the proportions of individuals linked to their first appointment for HCV infection treatment were similar between the U.S. (62%; 95% CI=53%, 70%) and Europe (58%; 95% CI=43%, 73%), treatment initiation (39%; 95% CI=34%, 44%) and completion (32%; 95% CI=23%, 76%) were lower in the U.S. The proportion of individuals attaining SVR was higher in Europe (47%; 95% CI=20%, 76%) than in the U.S. (26%; 95% CI=22%, 31%) and Australia or Canada (29%; 95% CI=16%, 44%).
DISCUSSION
This systematic review and meta-analysis demonstrate persistent gaps in achieved outcomes across the HCV care cascade in the era of all-oral DAA agents8 8 despite the prevalence rates of chronic HCV infection diagnosis, treatment initiation, and achieved SVR being double (or triple) of those attained during the interferon-based therapy era.22 The greater treatment effects, low adverse event occurrence, and short treatment duration for new DAA agents may have increased the number of individuals offered treatment, with subsequent improvement in the proportions of HCV cure.4,10,23 Although overall proportions of individuals treated for HCV have increased in the DAA era,22 this meta-analysis showed that outcomes at each step of the HCV care cascade remain low and are still far from achieving the WHO goal of 80%–90% cure by 2030.9 These results highlight multiple opportunities to improve engagement across the HCV care cascade, particularly for HCV diagnosis, DAA treatment initiation and completion, and SVR attainment.
The proportions of individuals screened for HCV infection and linked to care varied by setting and population. Whereas the proportion of positive HCV antibody test results among individuals visiting ED settings (10%) was comparable with that of most other settings in this study (except for STD, SUD, and syringe programs [34%]), screening proportions in ED settings were lowest among eligible individuals. Although existing evidence has shown that EDs may be ideal venues to identify individuals with undiagnosed HCV infection,4,8 this study found that a large number of eligible individuals remained untested for HCV infection, leaving a substantial proportion of individuals with HCV undetected. Such gaps may result from several barriers in implementing universal screening (e.g., populations with ages ranging from 59 years to 77 years as of 2023 [born in 1946–1964 or baby boomers]) or targeted screening (e.g., PWIDs) in ED settings, including limited patient–clinician relationship,24 patient stigma, clinician work burden, and financial and staffing burden to healthcare systems.10,25 Moreover, in the U.S., the Centers for Medicare and Medicaid Services currently exclude EDs from HCV screening reimbursement,4,23 which may discourage a wide offering of HCV testing in those settings.
Poor outcomes for ED settings were not limited to Step 1 (HCV screening) of the HCV care cascade. Indeed, individuals from ED settings had considerably lower outcome proportions from Step 4 (successful patient contact) to Step 8 (confirmed SVR) than individuals from other settings. Patient retention barriers in the HCV care cascade have been previously described.4,26,27 Given the social vulnerability and complexity of many individuals attending EDs (e.g., PWIDs, individuals experiencing homelessness or unemployment) and their restricted primary care services access,3,26 the transition through each HCV care cascade step may be more challenging for them. Although lack of health insurance has been reported as the main predictor of failure to link individuals to care,4 competing priorities (e.g., securing food, associated comorbidities) and structural barriers (e.g., poor integration of ED services in healthcare systems, strict appointment schedules)10,26,27 are additional difficulties that need to be addressed to keep these individuals in care.
Compared with individuals from other settings, incarcerated individuals were among those with the lowest proportion of HCV screening (43%), despite having the highest proportion of positive HCV RNA test results (57%). Although all 5 studies that assessed screening in jails or prisons included in this meta-analysis implemented an intervention to improve screening in various countries (e.g., opt-out screening, peer workers accompaniment for screening, dried blood spot test), this study’s results revealed that HCV screening remained low among incarcerated individuals. Common barriers for Step 1 of the HCV care cascade are the lack of standardized screening programs worldwide,28 which is likely the result of insufficient engagement and support from various stakeholders and government institutions (e.g., public health, healthcare systems)29 and the complexity of these individuals’ social environments before, during, and after incarceration, including frequent transfers between jails or prisons or overall short stays, unstable housing when released, and lack of or restrictions to health insurance.29,30
Whereas the proportions obtained for linkage to the first appointment and treatment initiation were lower in incarcerated individuals, proportions of treatment completion and SVR were among the highest. Although these results may be an overestimation of true outcomes owing to the implementation of specific interventions that may be absent in standard practice, prisons may indeed offer more stable and controlled conditions to complete the HCV continuum of care than other settings.28,31 This may be more applicable to individuals serving long sentences unless successful transitioning to the community is supported and achieved by a coordinated team.29,31 Finally, this study’s results support previous findings showing that despite some progress, the major bottleneck in the HCV care cascade for incarcerated individuals remains the screening phase (Step 1).32 Thus, efforts should be directed toward increasing HCV screening in incarcerated individuals. Using a combination of strategies instead of a single one would provide better outcomes at this step by addressing time of screening, fear of stigma, and lack of trust in prison or healthcare staff.28,29
This meta-analysis showed that the proportions of treatment initiation and completion and confirmed SVR were among the highest for individuals with SUD (PWIDs included). This study’s findings confirmed existing evidence showing that individuals with SUD attain a proportion of HCV cure similar to that of the general population,33 underscoring that persisting concerns regarding the medication adherence capacity of this population may be unfounded. Although findings from published literature suggest that these individuals face multilevel barriers,34 including personal- and social-related barriers (e.g., competing concerns such as financial limitations, transportation difficulties, comorbidities, and social discrimination),27,35 clinician barriers (e.g., lack of experience in interacting with individuals with SUD contributing to stigmatization and reinfection concerns),33 and system-related barriers (e.g., DAA prescription restrictions based on clinician specialty, current SUD status, lack of insurance) preventing them from seeking and remaining in care,24 this study shows that once screened, these individuals were successfully contacted and linked to a first appointment for HCV care. All except one of the studies looking at outcomes in individuals with SUD implemented an intervention; thus, this study’s results suggest that existing barriers can be overcome by implementing appropriate health policies to identify and retain these individuals in care.
Although the proportion of HCV screening was among the highest for individuals experiencing homelessness (78%) on the basis of 2 studies, these individuals were less likely to be successfully contacted (62%), to be linked to a first appointment (62%), to receive DAA treatment (25%), and to achieve SVR (17%). Individuals experiencing homelessness may face multilevel barriers to completing the HCV care cascade, from lack of knowledge of HCV acquisition, disease progression, and treatment availability36 to experiencing concurrent alcohol or drug use,37 which may interfere with adherence to appointments and referrals to specialists. Priorities such as housing or employment may outweigh seeking HCV-related treatment.34 Moreover, lack of insurance coverage or clinician stigma may reduce participation in the HCV care cascade for these individuals, who often inject drugs.37
Differences were also found in outcomes across the HCV care cascade among developed countries. Specifically, the proportions of individuals initially screened for HCV infection and the proportions of treatment initiation, treatment completion, and SVR attainment were higher in Europe than in the U.S. These results may reflect the overall difficulties experienced by many people in the U.S. in accessing care owing to a healthcare system that is centered in private insurance with little government regulation and is thus fragmented, expensive, and complex to navigate.38,39 Such limitations have a higher impact on individuals with chronic illnesses and in marginalized populations,39 which describes many individuals affected by HCV infection. Some initiatives such as the HCV surveillance program in the state of Louisiana—which has provided unrestricted access to generic DAAs to Medicaid enrollees and incarcerated individuals since 2019—are a start to overcoming the pervasive structural barriers mentioned earlier.40
Limitations
This systematic review and meta-analysis have limitations. First, outcomes of this study’s HCV care cascade included 8 steps evaluated at one time point, and each study included in this meta-analysis could have reported on some but not all 8 steps. Second, the results of this meta-analysis showed significant heterogeneity owing to the various care settings, countries, and study populations included. Although a random-effects model was used to account for study variability, other factors (e.g., different intervention programs) may have affected the estimates across the HCV care cascade. Third, the analysis revealed the potential for publication bias for Steps 1, 2, and 7 of the HCV care cascade; thus, the results obtained for outcomes representing Steps 1, 2, and 7 should be interpreted with caution. Fourth, meta-analyses of some subgroups were limited by the relatively small number of studies identified, particularly for subgroups of incarcerated individuals and individuals with HIV or experiencing homelessness; thus, those small numbers may underestimate or overestimate the proportions obtained at each step of the HCV care cascade. Fifth, most studies assessing incarcerated individuals used the term prison interchangeably with jail; thus, this study’s results do not differentiate outcomes between jails and prisons. Sixth, the investigators were unable to assess publication bias in this study because most included studies did not have a control group to compare with an intervention group. Finally, most included studies were interventional; thus, the findings of this study may overestimate outcomes and may not be generalizable to real-world clinical settings in which no intervention is implemented.
Despite these limitations, this systematic review and meta-analysis assessed outcomes for each step of the HCV care cascade in the DAA therapy era. The meta-analyses of various subgroups in terms of setting, population, study design, and country/territory provided comprehensive and detailed information. This systematic review and meta-analysis identified gaps at each step of the HCV care cascade and strategies for future studies. This HCV care cascade may be useful in monitoring the impact of new public health initiatives and treatment care models worldwide.
CONCLUSIONS
This systematic review and meta-analysis demonstrated persistent gaps across the HCV care cascade, confirming that the availability of effective all-oral DAA therapies does not guarantee medication access by populations most in need. This study’s findings also suggest that continued efforts are needed to improve the care cascade for individuals with HCV infection. Increasing the number of people completing each HCV care cascade step, especially among individuals diagnosed with HCV in ED settings, individuals experiencing homelessness, incarcerated individuals, and individuals with SUD, may be a strategy to achieve the WHO’s goal of curing HCV infection and reducing the incidence of HCV infection.
Supplementary Material
ACKNOWLEDGMENTS
The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The research reported in this publication was supported by Grant K01DA045618 from the National Institute on Drug Abuse of NIH.
The authors declare that there are no conflicts of interest relevant to this work. No financial disclosures were reported by PHC, HT, IU, MR, XJ, HJS, AJ, LH, RC, and DJ. HP received grant funding from Bristol-Myers Squibb/Pfizer Alliance American Thrombosis Investigator Initiated Research Program. DLW received grant funding from Merck Sharp & Dohme. NO is an employee of Johnson & Johnson. This work was conducted when she was affiliated with the University of Florida.
Footnotes
CREDIT AUTHOR STATEMENT
Pilar Hernandez-Con: Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization, Project administration. Debbie L. Wilson: Investigation, Data curation, Writing – review & editing, Visualization. Huilin Tang: Software, Formal analysis, Writing – review & editing. Ikenna Unigwe: Investigation, Writing – original draft. Munaza Riaz: Investigation, Writing – original draft, Writing – review & editing. Natalie Ourhaan: Investigation, Writing – original draft. Xinyi Jiang: Investigation. Hyun Jin Song: Investigation, Writing – original draft. Amanda Joseph: Investigation. Linda Henry: Writing – review & editing. Robert Cook: Writing – review & editing. Dushyantha Jayaweera: Writing – review & editing. Haesuk Park: Conceptualization, Methodology, Writing – review & editing, Visualization, Supervision, Funding acquisition.
SUPPLEMENTAL MATERIAL
Supplemental materials associated with this article can be found in the online version at https://doi.org/10.1016Zj.amepre.2023.06.016.
REFERENCES
- 1.Morales-Arraez D, Hernandez-Guerra M. Electronic alerts as a simple method for amplifying the yield of hepatitis C virus infection screening and diagnosis. Am J Gastroenterol. 2020;115(1):9–12. 10.14309/ajg.0000000000000487. [DOI] [PubMed] [Google Scholar]
- 2.Konerman MA, Thomson M, Gray K, et al. Impact of an electronic health record alert in primary care on increasing hepatitis C screening and curative treatment for baby boomers. Hepatology. 2017;66(6):1805–1813. 10.1002/hep.29362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackburn NA, Patel RC, Zibbell JE. Improving screening methods for hepatitis C among people who inject drugs: findings from the HepTLC initiative, 2012–2014. Public Health Rep. 2016;131(suppl 2):91–97. 10.1177/00333549161310S214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franco RA, Overton ET, Tamhane AR, et al. Characterizing failure to establish hepatitis C care of baby boomers diagnosed in the emergency department. Open Forum Infect Dis. 2016;3(4):ofW211. 10.1093/ofid/ofw211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkinson AL, van Santen DK, Traeger MW, et al. Hepatitis C incidence among patients attending primary care health services that specialise in the care of people who inject drugs, Victoria, Australia, 2009 to 2020. Int J Drug Policy. 2022;103:103655. 10.1016/j.drugpo.2022.103655. [DOI] [PubMed] [Google Scholar]
- 6.Artenie A, Stone J, Fraser H, et al. Incidence of HIV and hepatitis C virus among people who inject drugs, and associations with age and sex or gender: a global systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2023;8(6):533–552. 10.1016/S2468-1253(23)00018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kish T, Aziz A, Sorio M. Hepatitis C in a New Era: a review of current therapies. P T. 2017;42(5):316–329. [PMC free article] [PubMed] [Google Scholar]
- 8.Radley A, Robinson E, Aspinall EJ, Angus K, Tan L, Dillon JF. A systematic review and meta-analysis of community and primary-care-based hepatitis C testing and treatment services that employ direct acting antiviral drug treatments. BMC Health Serv Res. 2019;19 (1):765. 10.1186/s12913-019-4635-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Global Hepatitis Report: https://www.who.int/publications/i/item/9789241565455. Accessed June 30, 2022. Geneva: WHO; Published 2017. [Google Scholar]
- 10.Ancona RM, Habib D, Faryar KA, Ruffner AH, Hart KW, Lyons MS. Clarifying the volume of estimated need for public health and prevention services within an emergency department population. J Am Coll Emerg Physicians Open. 2020;1(5):845–851. 10.1002/emp2.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bajis S, Dore GJ, Hajarizadeh B, Cunningham EB, Maher L, Grebely J. Interventions to enhance testing, linkage to care and treatment uptake for hepatitis C virus infection among people who inject drugs: A systematic review. Int J Drug Policy. 2017;47:34–46. 10.1016/j.drugpo.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Facente SN, Patel S, Hecht J, et al. Hepatitis C care cascades for 3 populations at high risk: low-income trans women, young people who inject drugs, and men who have sex with men and inject drugs. Clin Infect Dis. 2021;73(6):e1290–e1295. 10.1093/cid/ciab261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):39. 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huedo-Medina TB, Sánchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11(2):193–206. 10.1037/1082-989X1L2.193. [DOI] [PubMed] [Google Scholar]
- 16.Politi J, Regidor E, Donat M, et al. Free access to direct-acting antivirals in Spain: more favorable impact on hepatitis C mortality among highly educated people. Clin Infect Dis. 2023;76(8):1423–1430. 10.1093/cid/ciac928. [DOI] [PubMed] [Google Scholar]
- 17.Pol S, Lair-Mehiri L, Vallet-Pichard A. Is elimination of HCV realistic by 2030: France. Liver Int. 2021;41(suppl 1):45–49. 10.1111/liv.14862. [DOI] [PubMed] [Google Scholar]
- 18.Russo P, Pani L, Staniscia T, Romano F, Marzioni M. Impact of reimbursement limits on patient access to direct-acting antivirals in Italy: analysis of data from national registries. Eur Rev Med Pharmacol Sci. 2020;24(10):5758–5768. 10.26355/eurrev_202005_21368. [DOI] [PubMed] [Google Scholar]
- 19.Jiang N, Bruneau J, Makarenko I, et al. HCV treatment initiation in the era of universal direct acting antiviral coverage - Improvements in access and persistent barriers. Int J Drug Policy. 2023;113:103954. 10.1016/j.drugpo.2023.103954. [DOI] [PubMed] [Google Scholar]
- 20.Bardsley M, Heinsbroek E, Harris R, et al. The impact of direct-acting antivirals on hepatitis C viraemia among people who inject drugs in England; real-world data 2011–2018. J Viral Hepat. 2021;28(10):1452–1463. 10.1111/jvh.13575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeung A, Palmateer NE, Dillon JF, et al. Population-level estimates of hepatitis C reinfection post scale-up of direct-acting antivirals among people who inject drugs. J Hepatol. 2022;76(3):549–557. 10.1016/j.jhep.2021.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yehia BR, Schranz AJ, Umscheid CA, Lo Re V 3rd. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLOS ONE. 2014;9(7):e101554. 10.1371/journal.pone.0101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsieh YH, Patel AV, Loevinsohn GS, Thomas DL, Rothman RE. Emergency departments at the crossroads of intersecting epidemics (HIV, HCV, injection drug use and opioid overdose)-Estimating HCV incidence in an urban emergency department population. J Viral Hepat. 2018;25(11):1397–1400. 10.1111/jvh.12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall AD, Grebely J, Dore GJ, Treloar C. Barriers and facilitators to engaging in hepatitis C management and DAA therapy among general practitioners and drug and alcohol specialists-The practitioner experience. Drug Alcohol Depend. 2020;206:107705. 10.1016/j.drugalcdep.2019.107705. [DOI] [PubMed] [Google Scholar]
- 25.Paterson B, Hirsch G, Andres K. Structural factors that promote stigmatization of drug users with hepatitis C in hospital emergency departments. Int J Drug Policy. 2013;24(5):471–478. 10.1016/j.drugpo.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Minassian A, Vilke GM, Wilson MP. Frequent emergency department visits are more prevalent in psychiatric, alcohol abuse, and dual diagnosis conditions than in chronic viral illnesses such as hepatitis and human immunodeficiency virus. J Emerg Med. 2013;45(4):520–525. 10.1016/j.jemermed.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Paisi M, Crombag N, Burns L, et al. Barriers and facilitators to hepatitis C screening and treatment for people with lived experience of homelessness: A mixed-methods systematic review. Health Expect. 2022;25(1):48–60. 10.1111/hex.13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kronfli N, Linthwaite B, Kouyoumdjian F, et al. Interventions to increase testing, linkage to care and treatment of hepatitis C virus (HCV) infection among people in prisons: A systematic review. Int J Drug Policy. 2018;57:95–103. 10.1016/j.drugpo.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Wurcel AG, Reyes J, Zubiago J, et al. “I’m not gonna be able to do anything about it, then what’s the point?”: A broad group of stakeholders identify barriers and facilitators to HCV testing in a Massachusetts jail. PLOS ONE. 2021;16(5):e0250901. 10.1371/journal.pone.0250901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crowley D, Murtagh R, Cullen W, Lambert JS, McHugh T, Van Hout MC. Hepatitis C virus infection in Irish drug users and prisoners - a scoping review. BMC Infect Dis. 2019;19(1):702. 10.1186/s12879-019-4218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.European Centre for Diseases Prevention and Control. Systematic review on active case finding of communicable diseases in prison settings. Stockholm: ECDC; 2017. https://www.ecdc.europa.eu/en/publications-data/systematic-review-active-case-finding-communicable-diseases-prison-settings. [Google Scholar]
- 32.Rumble C, Pevalin DJ, O’Moore É. Routine testing for blood-borne viruses in prisons: a systematic review. Eur J Public Health. 2015;25(6):1078–1088. 10.1093/eurpub/ckv133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trooskin SB, Dore G, Kostman J. We Must Do Better: Addressing HCV treatment barriers in persons who inject drugs in the United States. J Infect Dis. 2020;222(suppl 9):S773–S781. 10.1093/infdis/jiaa574. [DOI] [PubMed] [Google Scholar]
- 34.Gelberg L, Gallagher TC, Andersen RM, Koegel P. Competing priorities as a barrier to medical care among homeless adults in los Angeles. Am J Public Health. 1997;87(2):217–220. 10.2105/ajph.87.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edlin BR, Kresina TF, Raymond DB, et al. Overcoming barriers to prevention, care, and treatment of hepatitis C in illicit drug users. Clin Infect Dis. 2005;40(suppl 5):S276–S285. 10.1086/427441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norton BL, Voils CI, Timberlake SH, et al. Community-based HCV screening: knowledge and attitudes in a high risk urban population. BMC Infect Dis. 2014;14:74. 10.1186/1471-2334-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strehlow AJ, Robertson MJ, Zerger S, et al. Hepatitis C among clients of health care for the homeless primary care clinics. J Health Care Poor Underserved. 2012;23(2):811–833. 10.1353/hpu.2012.0047.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janus K, Minvielle E. Rethinking health care delivery: what European and United States health care systems can learn from one another. Washington, DC: Health Affairs Forefront; Published 2017. 10.1377/forefront.20171214.835155. [DOI] [Google Scholar]
- 39.Schoen C, Osborn R, Squires D, Doty MM. Access, affordability, and insurance complexity are often worse in the United States compared to ten other countries. Health Aff (Millwood). 2013;32(12):2205–2215. 10.1377/hlthaff.2013.0879. [DOI] [PubMed] [Google Scholar]
- 40.Louisiana Department of Health. STD/HIV/Hepatitis Program. Louisiana hepatitis C elimination plan: 2019–2024: https://www.louisianahealthhub.org/wp-content/uploads/2021/03/Eliminate_Hepatitis_C_State_Plan.pdf. Accessed April 28, 2023. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


