Abstract
Aerated and stirred 10-ml suspensions of mechanically isolated Asparagus sprengeri Regel mesophyll cells were used for simultaneous measurements of net H+ efflux and steady-state ATP levels.
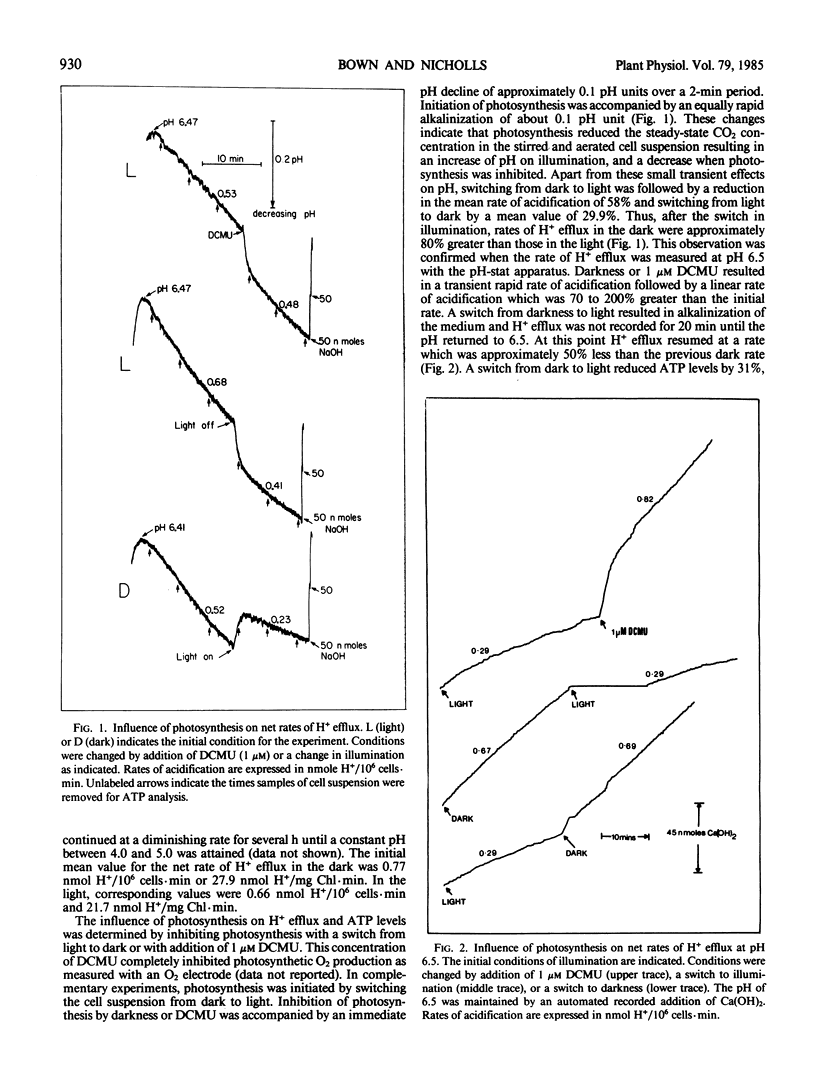
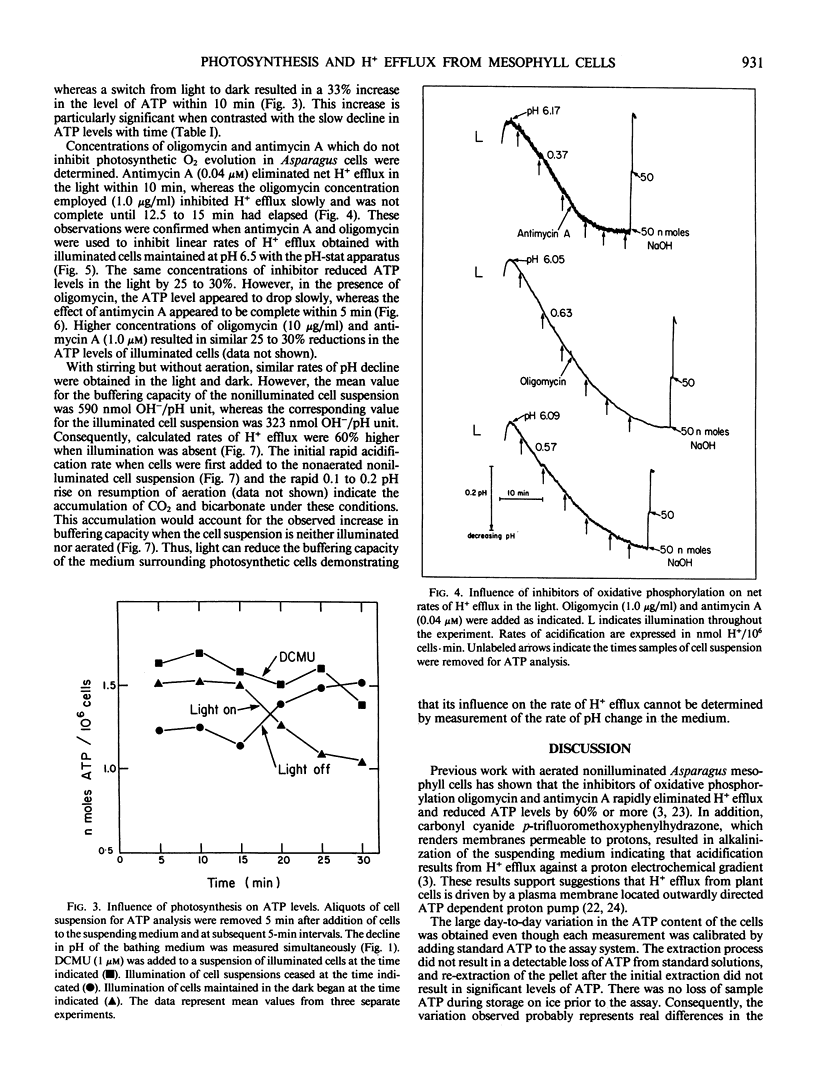
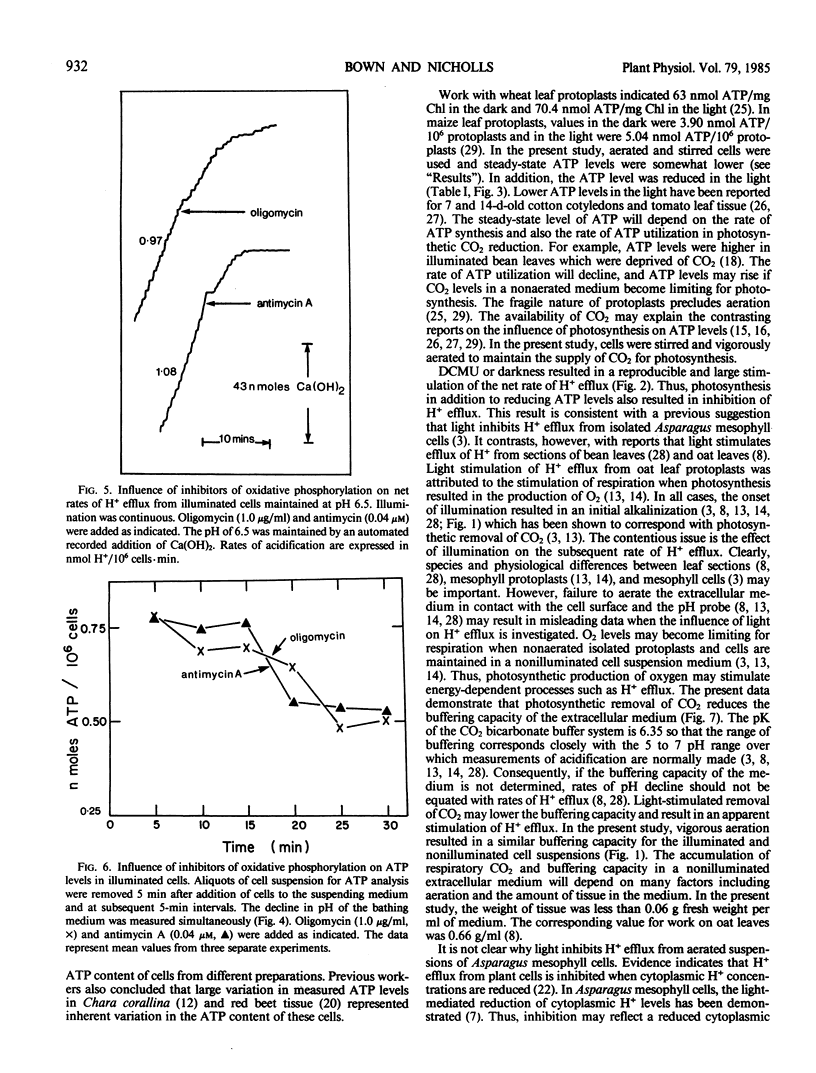
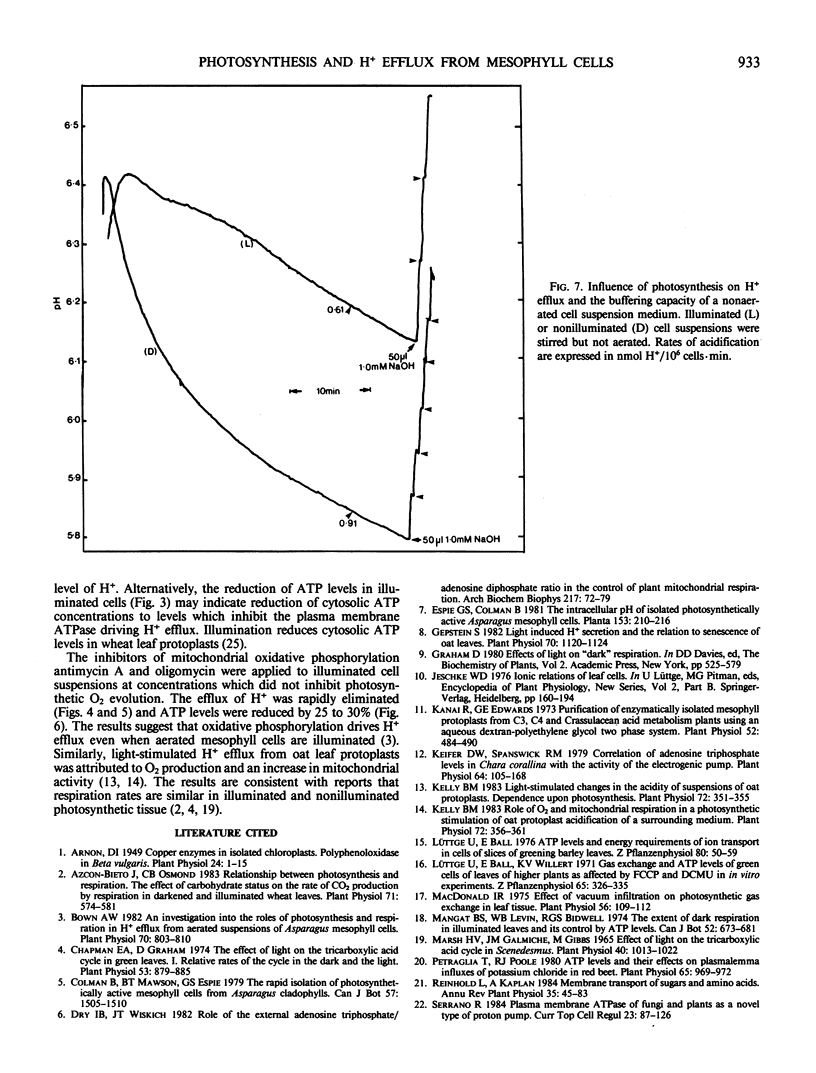
Initial rates of medium acidification indicated values for H+ efflux in the light and dark of 0.66 and 0.77 nanomoles H+/106 cells per minute, respectively. When the medium pH was maintained at 6.5, with a pH-stat apparatus, rates of H+ efflux remained constant. Darkness or DCMU, however, stimulated H+ efflux by 100% or more. Darkness increased ATP levels by 33% and a switch from dark to light reduced ATP levels by 31%. In the absence of aeration, illumination prevented the accumulation of respiratory CO2 and the buffering capacity of the medium was about 50% less than that found in the nonilluminated nonaerated medium. As a result, rates of pH decline were similar even though the dark rate of H+ efflux was approximately 50% greater.
Proposals that photosynthesis stimulates H+ efflux are based on changes in the rate of pH decline. The present data indicate that photosynthesis inhibits H+ efflux and that changes in rates of pH decline should not be equated with changes in the rate of H+ efflux.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcón-Bieto J., Osmond C. B. Relationship between Photosynthesis and Respiration: The Effect of Carbohydrate Status on the Rate of CO(2) Production by Respiration in Darkened and Illuminated Wheat Leaves. Plant Physiol. 1983 Mar;71(3):574–581. doi: 10.1104/pp.71.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bown A. W. An investigation into the roles of photosynthesis and respiration in h efflux from aerated suspensions of asparagus mesophyll cells. Plant Physiol. 1982 Sep;70(3):803–810. doi: 10.1104/pp.70.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman E. A., Graham D. The effect of light on the tricarboxylic Acid cycle in green leaves: I. Relative rates of the cycle in the dark and the light. Plant Physiol. 1974 Jun;53(6):879–885. doi: 10.1104/pp.53.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dry I. B., Wiskich J. T. Role of the external adenosine triphosphate/adenosine diphosphate ratio in the control of plant mitochondrial respiration. Arch Biochem Biophys. 1982 Aug;217(1):72–79. doi: 10.1016/0003-9861(82)90480-5. [DOI] [PubMed] [Google Scholar]
- Gepstein S. Light-induced h secretion and the relation to senescence of oat leaves. Plant Physiol. 1982 Oct;70(4):1120–1124. doi: 10.1104/pp.70.4.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Purification of enzymatically isolated mesophyll protoplasts from c(3), c(4), and crassulacean Acid metabolism plants using an aqueous dextran-polyethylene glycol two-phase system. Plant Physiol. 1973 Nov;52(5):484–490. doi: 10.1104/pp.52.5.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keifer D. W., Spanswick R. M. Correlation of Adenosine Triphosphate Levels in Chara corallina with the Activity of the Electrogenic Pump. Plant Physiol. 1979 Aug;64(2):165–168. doi: 10.1104/pp.64.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly B. M. Light-Stimulated Changes in the Acidity of Suspensions of Oat Protoplasts: Dependence upon Photosynthesis. Plant Physiol. 1983 Jun;72(2):351–355. doi: 10.1104/pp.72.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly B. M. Role of o(2) and mitochondrial respiration in a photosynthetic stimulation of oat protoplast acidification of a surrounding medium. Plant Physiol. 1983 Jun;72(2):356–361. doi: 10.1104/pp.72.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald I. R. Effect of vacuum infiltration on photosynthetic gas exchange in leaf tissue. Plant Physiol. 1975 Jul;56(1):109–112. doi: 10.1104/pp.56.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh H. V., Galmiche J. M., Gibbs M. Effect of Light on the Tricarboxylic Acid Cycle in Scenedesmus. Plant Physiol. 1965 Nov;40(6):1013–1022. doi: 10.1104/pp.40.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraglia T., Poole R. J. ATP Levels and their Effects on Plasmalemma Influxes of Potassium Chloride in Red Beet. Plant Physiol. 1980 May;65(5):969–972. doi: 10.1104/pp.65.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R. Plasma membrane ATPase of fungi and plants as a novel type of proton pump. Curr Top Cell Regul. 1984;23:87–126. doi: 10.1016/b978-0-12-152823-2.50007-6. [DOI] [PubMed] [Google Scholar]
- Stitt M., Lilley R. M., Heldt H. W. Adenine nucleotide levels in the cytosol, chloroplasts, and mitochondria of wheat leaf protoplasts. Plant Physiol. 1982 Oct;70(4):971–977. doi: 10.1104/pp.70.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich-Eberius C. I., Novacky A., Ball E. Effect of cyanide in dark and light on the membrane potential and the ATP level of young and mature green tissues of higher plants. Plant Physiol. 1983 May;72(1):7–15. doi: 10.1104/pp.72.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton J. D., Earle E. D., Yoder O. C., Spanswick R. M. Reduction of Adenosine Triphosphate Levels in Susceptible Maize Mesophyll Protoplasts by Helminthosporium maydis Race T Toxin. Plant Physiol. 1979 May;63(5):806–810. doi: 10.1104/pp.63.5.806. [DOI] [PMC free article] [PubMed] [Google Scholar]


