Abstract
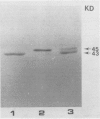
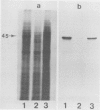
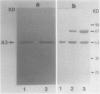
In vitro translation of RNA extracted from Vigna mungo cotyledons showed that α-amylase is synthesized as a polypeptide with a molecular mass of 45,000, while cotyledons contain a form of α-amylase with a molecular mass of 43,000. To find out whether the 45,000 molecular mass polypeptide is a precursor to the 43,000 found in vivo, the cell free translation systems were supplemented with canine microsomal membrane; when mRNA was translated in the wheat germ system supplemented with canine microsomes, the 45,000 molecular mass form was not processed to a smaller form but the precursor form was partly processed in the membrane-supplemented reticulocyte lysate system. When V. mungo RNA was translated in Xenopus oocyte system, only the smaller form (molecular mass 43,000) was detected. Involvement of contranslational glycosylation in the maturating process of the α-amylase was ruled out because there was no effect of tunicamycin, and the polypeptide was resistant to endo-β-H or endo-β-D digestion. We interpret these results to mean that the 45,000 molecular mass form is a precursor with a signal peptide or transit sequence, and that the 43,000 molecular mass is the mature form of the protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boston R. S., Miller T. J., Mertz J. E., Burgess R. R. In Vitro Synthesis and Processing of Wheat alpha-Amylase : TRANSLATION OF GIBBERELLIC ACID-INDUCED WHEAT ALEURONE LAYER RNA BY WHEAT GERM AND XENOPUS LAEVIS OOCYTE SYSTEMS. Plant Physiol. 1982 Jan;69(1):150–154. doi: 10.1104/pp.69.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B. Injected nuclei in frog oocytes: fate, enlargement, and chromatin dispersal. J Embryol Exp Morphol. 1976 Dec;36(3):523–540. [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba T., Minamikawa T. In Vivo Synthesis and Turnover of alpha-Amylase in Attached and Detached Cotyledons of Vigna mungo Seeds. Plant Physiol. 1983 Jan;71(1):173–176. doi: 10.1104/pp.71.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marcu K., Dudock B. Characterization of a highly efficient protein synthesizing system derived from commercial wheat germ. Nucleic Acids Res. 1974 Nov;1(11):1385–1397. doi: 10.1093/nar/1.11.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S., Akazawa T. Biosynthesis of rice seed alpha-amylase: proteolytic processing and glycosylation of precursor polypeptides by microsomes. J Cell Biol. 1983 Mar;96(3):802–806. doi: 10.1083/jcb.96.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S., Akazawa T. alpha-Amylase biosynthesis: evidence for temporal sequence of NH2-terminal peptide cleavage and protein glycosylation. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6566–6568. doi: 10.1073/pnas.79.21.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S., Okamoto K., Watanabe A., Akazawa T. Enzymic Mechanism of Starch Breakdown in Germinating Rice Seeds: 10. IN VIVO AND IN VITRO SYNTHESIS OF alpha-AMYLASE IN RICE SEED SCUTELLUM. Plant Physiol. 1981 Dec;68(6):1314–1318. doi: 10.1104/pp.68.6.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita T. W., Decaleya R., Rappaport L. Synthesis of a possible precursor of alpha-amylase in wheat aleurone cells. Plant Physiol. 1979 Jan;63(1):195–200. doi: 10.1104/pp.63.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. C., Milliman C. Isolation and sequence analysis of a barley alpha-amylase cDNA clone. J Biol Chem. 1983 Jul 10;258(13):8169–8174. [PubMed] [Google Scholar]
- Tomura H., Koshiba T., Minamikawa T. Imunohistochemical Localization of alpha-Amylase in Cotyledons of Vigna mungo Seedlings. Plant Physiol. 1985 Dec;79(4):935–938. doi: 10.1104/pp.79.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]