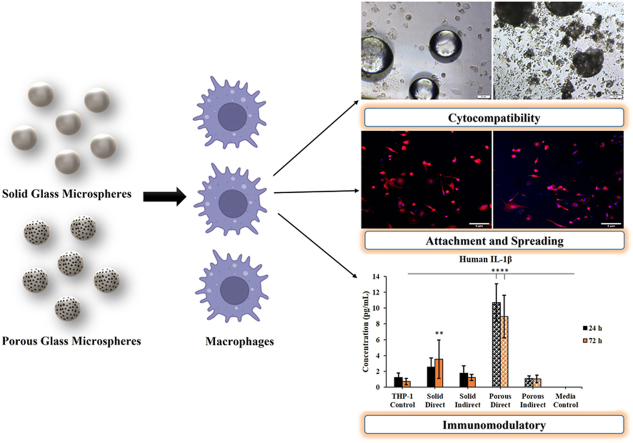
Abstract
This study aimed to investigate the immunomodulatory effect of two different forms of phosphate-based glass microspheres (solid and porous), on human macrophages. Human THP-1 monocytes were converted to M0 macrophages after being treated with 100 ng/mL phorbol 12-myristate 13-acetate for 48 h. The differentiated cells were analysed for the CD14 marker using flow cytometry. The adhesion, spreading, and viability of M0 macrophages grown directly or indirectly (extracts) at varying concentrations of solid and porous glass microspheres (GMs) were analysed via phase contrast microscopy, confocal microscopy, and XTT assay. The expression of IL-8, IL-1β, IL-6, IL-10, TNF-α, and IL-12p70 cytokines was investigated using flow cytometry. The conversion to M0 macrophages was confirmed by their adherent nature, increased granularity, and CD14 expression. The results showed that both solid and porous GMs or extracts favored the attachment, spreading, and proliferation of macrophages in a comparable manner to cells grown in a normal tissue culture medium. Only the higher concentration of porous GMs (10 mg/mL) changed the morphology of M0 macrophages and increased the expression of IL-1β and IL-8 pro-inflammatory cytokines; this could be related to the fast degradation nature of porous GMs. Of the six cytokines analysed, M0 macrophages grown directly or indirectly with GMs only expressed IL-1β, IL-10, and IL-8. Accordingly, solid microspheres may have advantages as regenerative agents due to their controlled degradation.
Keywords: THP-1, Phosphate-based glass microspheres, macrophages, Cytokines, Immunomodulatory
Graphical abstract
1. Introduction
Phosphate-based glasses (PBGs) are biocompatible [[1], [2], [3]] and osteoconductive [4] materials that can be made in a variety of forms and compositions to fit the required applications. They have been shown to have antibacterial properties [1]; they are fully degradable and when they degrade they release calcium and phosphorus ions that could aid remineralisation of dentin [5] and bone [4].
Recently, PBGs have been prepared as solid and porous microspheres [6]. Both microspheres were spherical in shape and can be prepared with different diameters ranging from 50 μm up to 200 μm using flame spheroidization methods. The composition of microspheres varies according to the metal oxides included. Depending upon the processing parameters and composition, different porosity levels with controlled degradation rates could be achieved for these glass microspheres. Previous studies proved that the porous microspheres of ∼76% porosity showed a higher degradation rate over solid ones with a porosity of ∼37% [6]. In particular, the porous microspheres could be exploited to promote the uptake and controlled release of biological moieties such as antibodies, modified single-chain antibody variable fragments, or internalised short interfering RNA [7]. These microspheres could also be prepared as an injectable paste that could be delivered using a 14 or 18G needle without damage or dispersion [8]. The flow of this paste could be adjusted by controlling the microsphere size, surface area, and microsphere/liquid ratio. Furthermore, several excipients such as xanthan gum, methyl cellulose, and carboxyl methyl cellulose could be used as viscosity modifiers [8]. These microspheres are cytocompatible [9] and have shown regenerative potential when used in an Ovine bone defect model [10]. Recently, our research group has demonstrated that these glass microspheres could potentially be used for dental pulp regeneration. The PBG microsphere formulations tested showed good osteogenic potential and could be used as an alternative to commercially available mineral trioxide aggregate (MTA) [11]. Likewise, a wide variety of applications is possible with these microspheres whether it is in regenerative medicine, stem cell research, protein/drug delivery [[12], [13], [14]] or radiotherapy for tumor management [15]. However, exploring the response of immune cells to any experimental material is highly important for successful tissue regeneration. Although many studies were reported on the immunomodulatory effects of bioactive silica-based glasses, the response of immune cells to these phosphate-based glass microspheres, however, has not been studied before. The novelty of this study is to investigate the response of immune cells to these PBG microspheres particularly as these glasses are highly degradable, and the degradation can take few weeks up to years according to the composition. On the contrary, the degradation of silica-based glasses takes a long time that might reach several years according to the composition [5]. A clear understanding of the impact of these biomaterials and their degradation products when encountering the recipient immune system is crucial. Sometimes it can even evoke unpleasant immune responses that could eventually result in the failure of implanted devices. Proper balance between pro- and anti-inflammatory responses displayed by the immune cells especially macrophages is vital for constructive tissue regeneration. Prolonged secretion of pro-inflammatory cytokines and chemokines by the activated macrophages could activate other immune cells and would ultimately result in delayed wound healing and tissue repair [16]. Therefore, with a degradable material such as the PBG microspheres investigated in this study, it is highly important to study the effect of the degradation products on the immune cells. Hence, this study aimed to investigate the immunomodulatory effects of solid and porous phosphate-based glass microspheres using THP-1 derived M0 macrophages. Accordingly, the impact of these microspheres on the viability of macrophages was tested using both direct contact and extract methods. The rational of using both direct contact and the extract method was to study if there is any negative effect of microspheres or their degradation products on the response of macrophages. In the direct contact method, macrophages were seeded in the presence of glass microspheres while in the extract method, macrophages were grown in conditioned medium, prepared by soaking the microspheres in the cell culture medium for 24 h as explained below in the method section. The cell attachment and cytokine (IL-8, IL-1β, IL-6, IL-10, TNF-α, and IL-12p70) expression were also investigated using four different concentrations of glass microspheres and their extracts.
2. Experimental section
2.1. Preparation of phosphate-based glass and microspheres
Solid and porous glass microspheres (GMs) with 40 P2O5·16 CaO· 24 MgO· 20 Na2O (in mol%) were prepared as described previously [6,17]. Briefly, phosphate glass was prepared by a process of melt-quenching at 1150 °C for 1.5 h using NaH2PO4, CaHPO4, MgHPO4, and P2O5 (Sigma-Aldrich, St. Louis, Missouri, USA). The purity grade of all the chemicals used was 99.9%. After cooling, the glass was ground into powder using a Retsch PM100 milling machine. Then a flame spheroidisation process employing oxy-acetylene flame (MK 74, Metallisation Ltd, UK) was used to prepare both solid and porous GMs. For porous microspheres, calcium-carbonate was used as a porogen. The microspheres produced were collected and washed for 2 min by stirring in 5 M acetic acid to remove any residual porogen remnants followed by 5 min in deionised water and then dried at 50 °C for 24 h. The prepared microspheres were in the range of 80–180 μm [11].
2.2. THP-I derived M0 macrophage phenotypic characterisation
THP-1 monocytic cells were converted into M0 macrophages before proceeding with the cell culture studies using 100 ng/mL phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich, St. Louis, Missouri, USA) for 48 h [18]. Consequently, the cells were maintained in a PMA-free medium (RPMI basal medium with 10% FBS and 1% Pencillin-Streptomycin, Sigma-Aldrich, St. Louis, Missouri, USA) for 72 h. The conversion of THP-1 monocytes into M0 macrophages was confirmed by the adherent nature of macrophages and via the expression of CD14 marker using flow cytometry after staining with FITC Anti-CD14 antibody [MEM-15] (ab28061, Abcam (Cambridge, UK) for 30 min. Stained cells were acquired with approximately 30,000 events for each sample on the FACS Aria III flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). FlowJo software version 10 (Becton Dickinson) was used for interpreting the data.
2.3. Cell culture analyses
Solid and porous GMs of different concentrations (10, 0.1, 0.01, and 0.001 mg/mL) or their extracts (at 100, 10, 0.01, and 0.001 mg/mL) treated cells were used as the experimental groups. The cells in a normal cell culture medium served as the positive control while the cells treated with dimethyl sulfoxide (DMSO) were considered as negative control.
The GMs extract was prepared by incubating GMs in a tissue culture medium (RPMI supplemented with 10% FBS and 1% Penicillin-Streptomycin) at 0.1 g/mL after sterilization under UV light for 1 h. The medium was kept under shaking for 24 h at 37 °C in a mini shaking incubator (Incu-Shaker, Benchmark Scientific, USA). The conditioned medium containing the ions released from GMs was then used for cell culture extract experiments. From the 100 mg/mL stock conditioned medium, the rest of the test concentrations such as 10, 0.01 and 0.001 mg/mL were prepared.
For the viability study, cells were used at a density of 3x104 cells/cm2 using 96 well plates whilst for attachment and cytokine expression, 1x104 cells/cm2 using 12 well tissue culture plates were used. Three samples were used for each group and the entire experiment was repeated three times. The results were taken as the average and standard deviation of the three repeats.
2.4. Cell attachment
The attachment of M0 macrophages grown for 48 h at varying concentrations of solid or porous GMs or their respective extracts was analysed using phase contrast microscopy (Olympus IX73). Confocal laser scanning microscopy (CLSM, Nikon Eclipse Ti Elements, Nikon Instruments Inc., Japan) was also used to study the morphology of M0 macrophages treated with 10 mg/mL solid or porous GMs as an example.
For confocal microscopy, the cells were first fixed with 4% paraformaldehyde for about 20 min followed by permeabilization for 5 min at room temperature with 0.1% Triton X-100 solution (Sigma Aldrich, St. Louis, Missouri, USA). Subsequently, the cells were stained for another 20 min at room temperature with Texas Red™-X Phalloidin (Thermo Fisher Scientific, Waltham, Massachusetts, USA). The nucleus was further counterstained with DAPI (Fluoroshield Mounting Medium with DAPI (Ab104139), Abcam, Cambridge, UK), and the images were acquired using confocal microscopy [19].
2.5. % cell viability
The viability of M0 macrophages treated with varying concentrations of GMs or their extracts for 72 h was determined using the Cell Proliferation kit (XTT assay, Sigma-Aldrich, St. Louis, Missouri, USA) Briefly, after each incubation period (24, 48, and 72 h), XTT was added to the respective wells and the cells were incubated for a minimum of 4 h at 37 °C as per the manufacturer's instructions. The absorbance of the reduced formazan product formed was then measured at wavelengths of 450 nm and 630 nm using a microplate reader (Synergy H1 microplate reader, Biotek Instruments, USA) and the % viability was calculated using the following equation:
2.6. Pro- and anti-inflammatory cytokine expression
For this study, cells were treated with 10 mg/mL solid or porous GMs or their extracts. A panel of six cytokines namely IL-1β, IL-8, IL-6, TNF-α, IL-10, and IL-12p70 was analysed using a FACS Aria III flow cytometer and their respective concentrations were determined, using the BD CBA Human Inflammatory Cytokines Kit (BD Biosciences, Cat# 551811). The acquired events were later analysed using FCAP Array V3 software (BD Biosciences)
2.7. Statistical analysis
Statistical analysis was done using GraphPad Prism version 9.0.0 (121) (GraphPad Software, Inc., San Diego, CA). Statistical significance between samples was determined by Two-way ANOVA followed by Tukey's multiple comparisons test. Each experiment was repeated three times with n = 3 samples. All data were represented as mean ± standard deviation with a confidence interval of 95% and an alpha value of 0.05.
3. Results
3.1. THP-I derived M0 macrophage phenotypic characterization
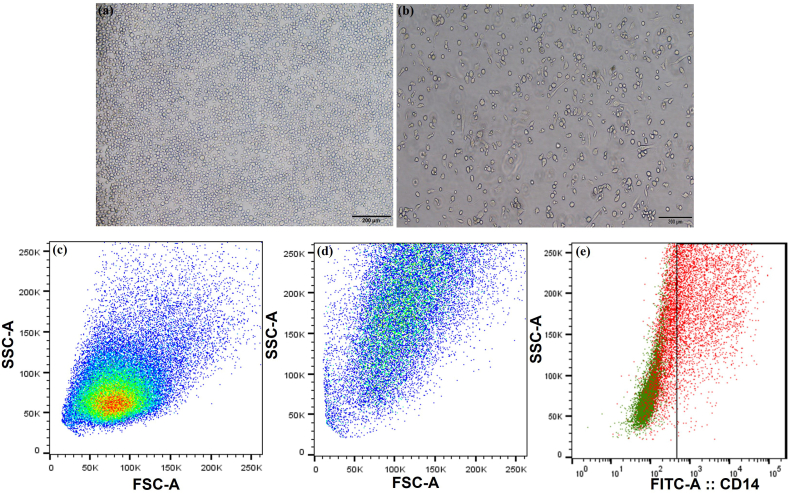
The conversion of THP-1 monocytes to M0 macrophage phenotype was confirmed by morphological evaluation of the cells and by analysis using the CD14 marker expression by flow cytometry. Compared to the floating monocytes (Fig. 1a), the differentiated M0 cells were found to be tissue culture flask adherent and displayed a well-spread morphology (Fig. 1b). The monocytes were showing less granularity whereas, the PMA treatment aided in the increased granularity of the differentiated M0 macrophages (Fig. 1d). Flow cytometric evaluation of the CD14 marker was found to be positive for the differentiated M0 macrophages Fig. 1e, and negative for the THP-1 monocytes.
Fig. 1.
Phenotypic characterization of THP-1 derived M0 macrophages. Phase contrast microscopic images of THP-1 cells (a) and differentiated M0 macrophages (b). The flow cytometry dot plot showing the FSC and SSC pattern of THP-1 derived M0 macrophages (c-unstained and (d)-M0 macrophages). THP-1 derived macrophages expressing the CD14 marker detected by flow cytometry (e).
3.2. Cell attachment
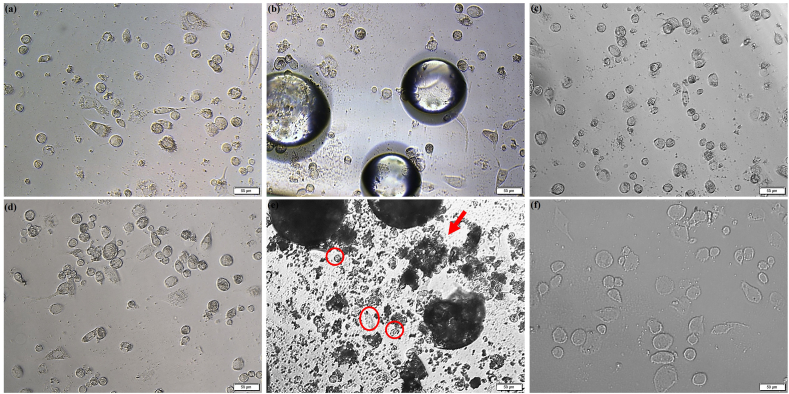
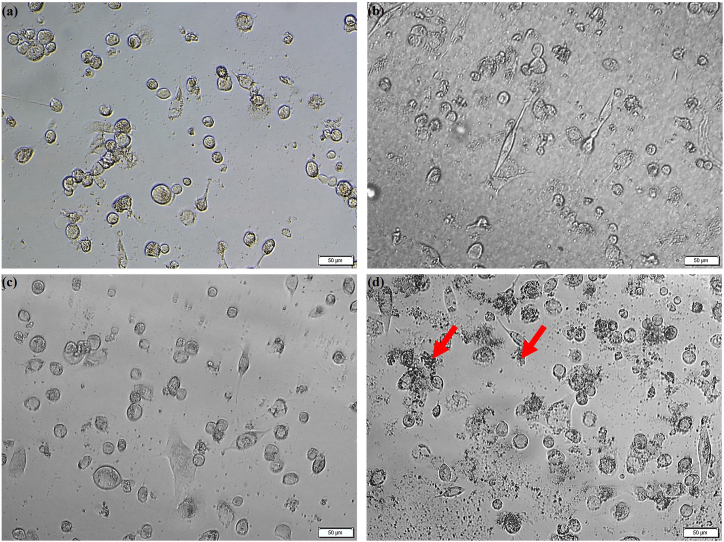
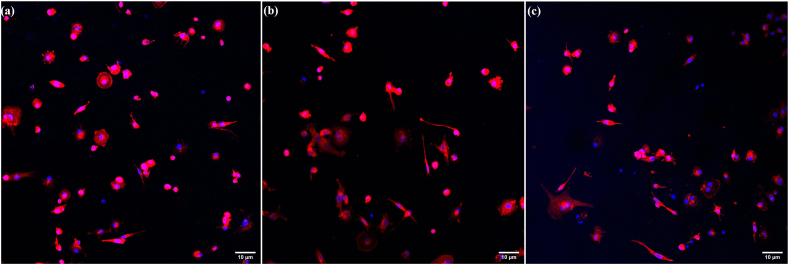
The effect of the GMs and their extract media on the M0 macrophage morphology is shown in Fig. 2. It was observed that only the higher concentration of porous GMs (10 mg/mL) changed the morphology of M0 macrophages; the cells were globular in structure rather than revealing a spread morphology (Red circles). Moreover, the porous GMs seemed to distort or break more easily than the solid GMs due to the centrifugation force used during the extract preparation, and the remnants of the distorted or broken microspheres covered the surface of the cells (Red arrows). Compared to 24 h, the residues of porous GMs were more in the 48 h samples. However, these remnants were not observed for the solid GMs. The morphology of the cells grown in different concentrations of solid or porous GMs extracts was found to be similar to the positive control cells (see Fig. 3). The confocal microscopic images (Fig. 4) also showed that the GMs (Fig. 4b and c) were cytocompatible and did not affect the attachment and spreading behavior of THP-1 derived M0 macrophages as the case with positive control cells (Fig. 4a).
Fig. 2.
Phase contrast microscopy images showing THP-1 derived M0 macrophages grown directly in the presence of 0.001 mg/mL solid and porous GMs (a and d) and 10 mg/mL of solid or porous GMs (b and e) for 48 h as examples in comparison to the positive (c) and negative control cells (f). The arrow refers to the remnants of porous glass microspheres and the affected macrophages are represented by red circles. The scale bar represents 50 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
Phase contrast microscopy images showing THP-1 derived M0 macrophages grown in the presence of 0.001 mg/mL solid and porous GMs (a and c) and 100 mg/mL of solid or porous GMs (b and d) extracts for 48 h as examples. The arrows refer to the porous glass microspheres present in the extract. The scale bar represents 50 μm.
Fig. 4.
Confocal Laser Scanning Microscopy images showing THP-1 derived M0 macrophages grown directly in normal tissue culture medium (a, positive control) in the presence of solid GMs (b) or porous GMs (c) after 48 h of culture. The nucleus was stained with DAPI (blue) while F-actin was stained with Texas Red™-X Phalloidin. The scale bar represents 10 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. % cell viability
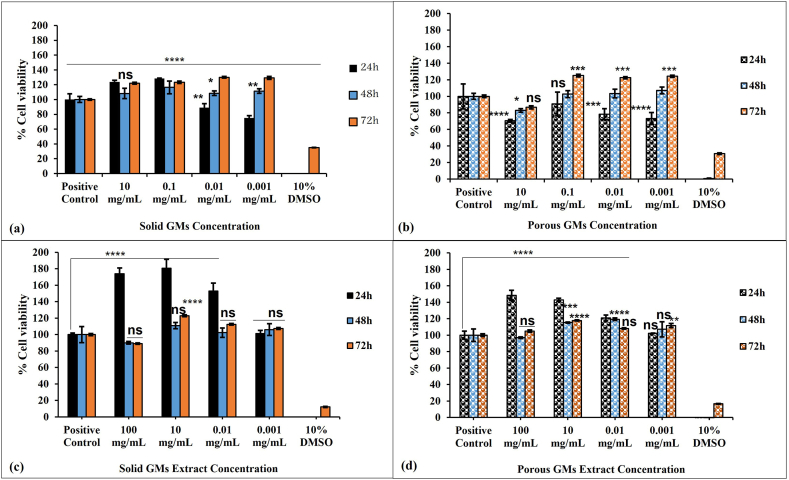
Fig. 5a revealed that the cells grown directly at different concentrations of solid GMs showed higher cell proliferation than the positive control (p < 0.05) except for 0.001 mg/mL at 24 h and 10 mg/mL (p > 0.05) at 48 h. The M0 macrophages treated with the porous GMs group showed reduced viability at 24 h at all tested concentrations in comparison to the positive control (p < 0.001) except for 0.1 mg/mL (p > 0.05) (Fig. 5b). At 48 h, the cells grown directly with different concentrations of porous GMs showed a similar proliferation rate to the positive control cells except for 10 mg/mL (p < 0.05) which showed reduced cell proliferation. A statistically significant increase in cell proliferation was observed at 72 h for all tested concentrations except for 10 mg/mL (p > 0.05).
Fig. 5.
Proliferation of THP-1 cells seeded in the presence of varying concentrations of solid or porous GMs or their extracts for 72 h. Statistical significance between the groups and THP-1 positive control was denoted by asterisks with *, **, *** and **** represented the p values < 0.05, 0.01, 0.001 and 0.0001 respectively. Each bar represents mean ± standard deviation of three independent experiments with triplicates.
The data of GM extracts showed a more favorable response on the viability of M0 macrophages. Even at higher concentrations of both solid and porous GMs extracts, the viability is significantly (p < 0.0001) higher than the positive control at 24 h - Fig. 5c and d. However, at 48 h and 72 h, the rate of proliferation in all tested concentrations was similar to the positive control group with only a few exceptions [10 mg/mL for solid GMs at 72 h (p < 0.0001), 10 mg/mL and 0.01 mg/mL for porous GMs at 48 h (p < 0.001) and 10 mg/mL and 0.001 mg/mL for porous GMs at 72 h (p < 0.01)] that showed significantly higher viability than positive control cells.
From Fig. 5 and regardless of the gap in concentration used, it is very difficult to see the dose-effect relationship with solid microspheres however it is more obvious with porous microspheres.
3.4. Pro- and anti-inflammatory cytokine expression
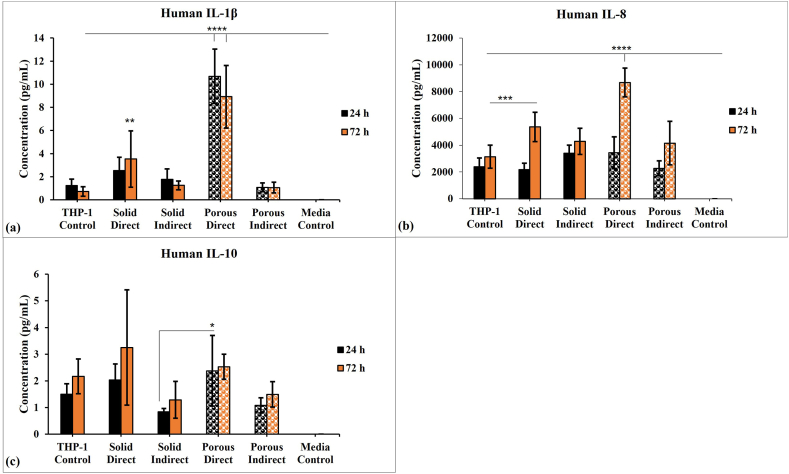
THP-1-derived macrophages grown in the presence of GMs, or their extracts were also analysed for an array of pro-inflammatory and anti-inflammatory cytokines such as IL-1β, TNF-α, IL-6, IL-10, IL-8, and IL-12p70. The IL-1β, IL-8, TNF-α, IL-6 and IL-12p70 are pro-inflammatory cytokines while IL-10 has anti-inflammatory properties. Both types of cytokines were investigated over 24 and 72 h period. The M0 macrophages grown in normal tissue culture medium served as the positive control. Of the six cytokines analysed, M0 macrophages grown in GMs or extracts only expressed IL-1β, IL-10, and IL-8 (Fig. 6). Macrophages cultured with porous GMs showed the highest expression of IL-1β and IL-8 (p < 0.0001); the IL-1β expression did not significantly change with time, but IL-8 expression significantly increased with time. The expression of the anti-inflammatory cytokine IL-10 was found to be similar for all the treated groups along with the positive control except for cells grown directly with porous microspheres at 24 h that showed the highest IL-10 expression. According to the data presented in Fig. 6, solid microspheres mitigate the pro-inflammatory response when compared with porous microspheres.
Fig. 6.
Cytometric bead array showing the concentrations of inflammatory cytokines such as (a) IL-1β, (b) IL-8, and (c) IL-10 released from the THP-1 derived M0 macrophages treated with solid and porous GMs and its extract. Data are represented as mean ± standard deviation of three independent experiments with triplicates and the statistical significance between the groups and THP-1 control was denoted by asterisks with *, **, *** and **** symbolized the p values < 0.05, 0.01, 0.001 and 0.0001 respectively.
4. Discussion
Phosphate-based glasses (PBGs) are a unique group of bioactive materials, which have been widely investigated for bone regeneration studies due to their striking similarity with the inorganic phase of bone [20]. Unlike other bioactive glasses, PBGs are silica-free and can be produced in different forms including discs, microtubes, fibers, and microspheres [21] to fit various potential applications. In this study, microspheres were selected owing to their large surface area, favorable ion release profiles and ease of delivery [6] since they can be easily prepared as an injectable paste. Unlike the solid microspheres, the porous ones have a higher surface area, and this is related to the presence of interconnected porosity of approximately 76%. The presence of such porosity could provide more chance of including drugs, growth factors, or cells into microspheres. The incorporated drugs or biologics could help in enhancing the tissue regeneration process. Therefore, both types of microspheres were investigated in this study to see the difference in cell response that will be reflected later during tissue repair. Our previous study showed that both types of microspheres favored the human dental pulp stem cells attachment and viability. Both types of microspheres encouraged mineralization, but higher mineral content was observed in the presence of porous microspheres than solid ones. This variation could be attributed to the variation in the amount of released ions such as calcium, phosphorus, and magnesium ions [11].
Although there are many studies reported on the cytocompatibility [20] and regeneration potential of PBGs microspheres [10,22], very few studies have investigated the immunomodulatory effect of these microspheres. The immune response is highly important when there is an expected high level of ions being released when the microspheres come into contact with tissue fluids or medium. Understanding the interactions between biomaterials and immune cells is essential as the immune cells play a pivotal role in tissue remodeling and regeneration processes [15,23]. The coordination between biomaterials and immune cells is very crucial in maintaining the homeostasis of the tissues and thereby reducing inflammation [24]. Although there will be an initial inflammatory response when deploying biomaterials of interest in the human body, this initial inflammatory response is desired for tissue regeneration, but prolonged inflammation can hinder the regenerative process [25].
Macrophages are one of the key players which regulate tissue healing and repair during a tissue injury [26]. Any variation in macrophage function could result in altered tissue regeneration and prolonged inflammation [27]. Activated macrophages (M1) play a crucial role in immune defense by activating various immunological pathways and by secreting pro-inflammatory cytokines such as IL-1 β, IL-6, and TNF-α. However, prolonged activation of M1 macrophages can trigger chronic inflammation which can ultimately impede the tissue healing process [28]. As macrophages are very dynamic in nature, they can easily switch between M1 and M2 phenotypes. A balance between M1 and M2 polarization is very crucial for favorable tissue repair and regeneration. Hence, it is crucial to study the behavior of these cells when in contact with biomaterials. Thus, the effect of solid and porous GMs on human macrophages was investigated in this study. Human THP-1 derived macrophages were selected for the study due to their similarity with human macrophages. Several studies have reported on the advantages of using THP-1 derived macrophages over peripheral blood-derived macrophages [29,30]. THP-1 monocytic cells were reported to express low levels of CD14 surface markers [29]. The THP-1 cell lines used in this study showed no CD14 expression compared to ∼60 % expression after treatment with PMA. The expression of the CD14 surface marker confirmed the successful conversion of monocytes to macrophages. Moreover, this conversion was also confirmed microscopically by the adherent nature of macrophages, and subsequently, the effect of solid and porous microspheres on THP-1 derived M0 macrophages was explored. The results indicated that, unlike solid GMs, the porous microspheres break or degrade easily owing to their porous nature [31] which therefore affected the morphology of cells when used at high concentrations [32,33]. Addition of various modifier oxides (e.g., Fe2O3, TiO2, or SrO) can control the PBGs’ degradation [34]. A previous study showed that porous PBG microspheres lost almost 30% of their mass in comparison to 9% for solid microspheres [6]. The faster degradation or breakdown of porous GMs might explain the hindered viability of macrophages seen at high concentrations. Furthermore, the size of the studied microspheres falls in the range of 80–180 μm in diameter; this size is too large to be phagocytosed by macrophages. A single macrophage can phagocytose particles up to 5–10 μm in size [16]. However, the degraded particles seen with porous microspheres might be small in size to be phagocyted by macrophages. This could also account for the variation seen between porous and solid microspheres. On the contrary, in a previous study, owing to their high surface area, the porous GMs allowed more attachment and migration of human bone marrow-derived mesenchymal stem cells inside their pores [6]. This altered variation in viability exhibited by different cells towards porous microspheres could be due to the tolerance levels of different cells towards different sizes and compositions of particles. The dose-dependent macrophage response was also noted in a previous study conducted on bioactive glasses (BG) where at higher concentrations of BG particles, there was an inhibition in the proliferation of macrophages [35]. Macrophages are heterogeneous in nature and can switch to M1 pro-inflammatory and M2 anti-inflammatory phenotypes depending on the microenvironment [36]. M1 polarised macrophages can secrete inflammatory cytokines such as IL-1β, IL-6, IL-8 and TNF-α, whereas M2 macrophages release IL-4, IL-13 and IL-10 [37]. A balance in the ratio of pro and anti-inflammatory cytokine production is crucial in tissue remodeling [38]. The biomaterials intended for tissue regeneration should aid in modulating the immune response to have these desirable outcomes [39]. Since the results of the viability study showed that 10 mg/mL glass microspheres or their extract significantly reduced the proliferation of macrophages, therefore, this concentration was used for the cytokine expression study. This was carried out to see what kind of pro-inflammatory or anti-inflammatory cytokine was upregulated and correlated to reduced viability of macrophages. In the future work however, a step in between the 10 mg/mL and 0.01 mg/mL glass microspheres will be used as a subtoxic level to investigate whether it is mainly an inflammatory response or more related to damaged cells.
The cytokine release data showed that direct culture of cells with porous GMs showed the highest release of IL-1β and IL-8. As observed previously, the increased secretion of IL-1β could result in enhanced Th17-dominant immunopathology and increase the number of M1 macrophages at the site of infection [40]. Furthermore, it has been observed that IL-1β and IL-8 can be used as markers for inflamed dental pulp, and their presence help in recruiting the neutrophils to the infected site [41]. The high neutrophil load can result in significant host tissue damage [42]. This trend of high increase in pro-inflammatory cytokines (IL-6 and IL-1β) was also observed with titanium doped phosphate-based glass (TDPG) microspheres when they were treated with murine macrophage cell line Raw 264.7 [43]. The increased level of pro-inflammatory (IL-1β and IL-8) cytokines’ secretions, seen with direct contact with porous GMs could be related to their faster degradation [6]. Therefore, controlling the degradation properties of porous GMs could result in achieving the desired balance between inflammatory and anti-inflammatory cytokine release profiles.
Porous microspheres are ideally suited for use in dental tissue regeneration as they also provide further advantageous properties offering higher surface area for cells to interact and migrate within them compared to solid ones. Moreover, porous microspheres could also be further exploited via the addition of other biologics, such as drugs and growth factors, etc. Furthermore, this study demonstrates that fine-tuning the degradation properties of PBG microspheres could also aid in modulating the host immune response. Since the biomaterial-immune cell interaction plays a crucial role in deciding the success of implantation, a detailed in vitro study with other immune cells followed by preclinical evaluation is needed to confirm the effective translation of these unique biomaterials.
5. Conclusion
Solid and porous glass microspheres and their extracts were found to be cytocompatible with THP-1 derived macrophages. The higher concentrations of porous GMs, however, showed decreased cell viability and this could be associated with the release of more degradation products that adversely could have affected the cellular action. The porous GMs also demonstrated an increase in the release of the pro-inflammatory IL-1β and IL-8 secretions compared to solid GMs. These increased levels of pro-inflammatory cytokines would lead to delayed wound healing and tissue repair. Accordingly, solid microspheres may have advantages as regenerative agents due to their controlled degradation. Hence, this study points towards the fact that immunomodulation exhibited by biomaterials is very crucial and it determines the success of implantation.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Soumya Sheela: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Fatma Mousa AlGhalban: Methodology, Investigation. Ifty Ahmed: Writing – review & editing, Writing – original draft, Resources. Ensanya A. Abou Neel: Writing – review & editing, Writing – original draft, Validation, Supervision, Resources, Project administration, Funding acquisition, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors acknowledge the funding support by the University of Sharjah (SEED grant no# V. C. R. G./R. 438/2020, 2101100243). This research work was also supported by NAMA Women Advancement Establishment as the strategic sponsor of the 3rd Forum for Women in Research (QUWA): Women Empowerment for Global Impact at the University of Sharjah. The authors also extend their gratitude to Mr. Manju Nidagodu Jayakumar, Research Institute for Medical and Health Sciences for his assistance in the flow cytometry.
References
- 1.Abou Neel E.A., Ahmed I., Pratten J., Nazhat S.N., Knowles J.C. Characterisation of antibacterial copper releasing degradable phosphate glass fibres. Biomaterials. 2005;26:2247–2254. doi: 10.1016/j.biomaterials.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Abou Neel E.A., Chrzanowski W., Pickup D.M., O'Dell L.A., Mordan N.J., Newport R.J., Smith M.E., Knowles J.C. Structure and properties of strontium-doped phosphate-based glasses. J. R. Soc. Interface. 2009;6:435–446. doi: 10.1098/rsif.2008.0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abou Neel E.A., Roohpour N., Padidar B., Mordan N.J., Bozec L. Biomimetic dentin repair with a dual-analogue phosphate glass-polyacrylate paste: a proof-of-concept. Mater. Chem. Phys. 2021;266 doi: 10.1016/j.matchemphys.2021.124539. [DOI] [Google Scholar]
- 4.Abou Neel E.A., Rani Kg A., Samsudin A.R. Mineralized nodule formation in primary osteoblasts culture in titanium doped phosphate glass and in-house prepared freeze dried demineralized bone extracts. Mater. Chem. Phys. 2022;276 doi: 10.1016/j.matchemphys.2021.125425. [DOI] [Google Scholar]
- 5.Abou Neel E.A., Khalifa N., Awad M., Soumya S., Alawadhi H. Remineralization potential and biocompatibility of titanium dioxide-doped phosphate glasses. Mater. Lett. 2022;309 doi: 10.1016/j.matlet.2021.131456. [DOI] [Google Scholar]
- 6.Hossain K.M.Z., Patel U., Kennedy A.R., Macri-Pellizzeri L., Sottile V., Grant D.M., Scammell B.E., Ahmed I. Porous calcium phosphate glass microspheres for orthobiologic applications. Acta Biomater. 2018;72:396–406. doi: 10.1016/j.actbio.2018.03.040. [DOI] [PubMed] [Google Scholar]
- 7.Li S., Nguyen L., Xiong H., Wang M., Hu T.C.C., She J.X., Serkiz S.M., Wicks G.G., Dynan W.S. Porous-wall hollow glass microspheres as novel potential nanocarriers for biomedical applications. Nanomedicine Nanotechnology, Biol. Med. 2010;6:127–136. doi: 10.1016/j.nano.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matamoros-Veloza A., Hossain K.M.Z., Scammell B.E., Ahmed I., Hall R., Kapur N. Formulating injectable pastes of porous calcium phosphate glass microspheres for bone regeneration applications. J. Mech. Behav. Biomed. Mater. 2020;102 doi: 10.1016/j.jmbbm.2019.103489. [DOI] [PubMed] [Google Scholar]
- 9.Gupta D., Hossain K.M.Z., Ahmed I., Sottile V., Grant D.M. Flame-spheroidized phosphate-based glass particles with Improved characteristics for applications in mesenchymal stem cell culture therapy and tissue engineering. ACS Appl. Mater. Interfaces. 2018 doi: 10.1021/acsami.8b05267. [DOI] [PubMed] [Google Scholar]
- 10.McLaren J.S., Macri-Pellizzeri L., Hossain K.M.Z., Patel U., Grant D.M., Scammell B.E., Ahmed I., Sottile V. Porous phosphate-based glass microspheres show biocompatibility, tissue Infiltration, and osteogenic onset in an ovine bone defect model. ACS Appl. Mater. Interfaces. 2019 doi: 10.1021/acsami.9b04603. [DOI] [PubMed] [Google Scholar]
- 11.Abou Neel E.A., Sheela S., AlGhalban F.M., Arjuna A. Osteogenic potential of solid and porous phosphate glass microspheres as pulp capping materials. J. Non-Cryst. Solids. 2023;611 doi: 10.1016/j.jnoncrysol.2023.122330. [DOI] [Google Scholar]
- 12.Labbaf S., Tsigkou O., Müller K.H., Stevens M.M., Porter A.E., Jones J.R. Spherical bioactive glass particles and their interaction with human mesenchymal stem cells in vitro. Biomaterials. 2011;32:1010–1018. doi: 10.1016/j.biomaterials.2010.08.082. [DOI] [PubMed] [Google Scholar]
- 13.Liu X., Jin X., Ma P.X. Nanofibrous hollow microspheres self-assembled from star-shaped polymers as injectable cell carriers for knee repair. Nat. Mater. 2011;10:398–406. doi: 10.1038/nmat2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pekarek K.J., Jacob J.S., Mathiowitz E. Double-walled polymer microspheres for controlled drug release. Nature. 1994;367:258–260. doi: 10.1038/367258a0. [DOI] [PubMed] [Google Scholar]
- 15.Srinivas S.M., Nasr E.C., Kunam V.K., Bullen J.A., Purysko A.S. Administered activity and outcomes of glass versus resin (90)Y microsphere radioembolization in patients with colorectal liver metastases. J. Gastrointest. Oncol. 2016;7:530–539. doi: 10.21037/jgo.2016.03.09. https://doi:10.21037/jgo.2016.03.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng K., Niu W., Lei B., Boccaccini A.R. Immunomodulatory bioactive glasses for tissue regeneration. Acta Biomater. 2021;133:168–186. doi: 10.1016/j.actbio.2021.08.023. [DOI] [PubMed] [Google Scholar]
- 17.Abou Neel E.A., Kiani A., Valappil S.P., Mordan N.M., Baek S.Y., Zakir Hossain K.M., Felfel R.M., Ahmed I., Divakarl K., Chrzanowski W., Knowles J.C. Glass microparticle- versus microsphere-filled experimental dental adhesives. J. Appl. Polym. Sci. 2019;136 doi: 10.1002/app.47832. [DOI] [Google Scholar]
- 18.Chanput W., Mes J.J., Wichers H.J. THP-1 cell line: an in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014;23:37–45. doi: 10.1016/j.intimp.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Abou Neel E.A., Chrzanowski W., Georgiou G., Dalby M.J., Knowles J.C. In vitro biocompatibility and mechanical performance of titanium doped high calcium oxidemetaphosphate-based glasses. J. Tissue Eng. 2010;1:1–11. doi: 10.4061/2010/390127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel U., Macri-Pellizzeri L., Zakir Hossain K.M., Scammell B.E., Grant D.M., Scotchford C.A., Hannon A.C., Kennedy A.R., Barney E.R., Ahmed I., Sottile V. In vitro cellular testing of strontium/calcium substituted phosphate glass discs and microspheres shows potential for bone regeneration. J. Tissue Eng. Regen. Med. 2019;13:396–405. doi: 10.1002/term.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Islam M.T., Felfel R.M., Abou Neel E.A., Grant D.M., Ahmed I., Hossain K.M.Z. Bioactive calcium phosphate–based glasses and ceramics and their biomedical applications: a review. J. Tissue Eng. 2017;8 doi: 10.1177/2041731417719170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lakhkar N.J., Park J.H., Mordan N.J., Salih V., Wall I.B., Kim H.W., King S.P., Hanna J.V., Martin R.A., Addison O., Mosselmans J.F.W., Knowles J.C. Titanium phosphate glass microspheres for bone tissue engineering. Acta Biomater. 2012 doi: 10.1016/j.actbio.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 23.Michée S., Brignole-Baudouin F., Riancho L., Rostene W., Baudouin C., Labbé A. Effects of benzalkonium chloride on THP-1 differentiated macrophages in vitro. PLoS One. 2013;8 doi: 10.1371/journal.pone.0072459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Julier Z., Park A.J., Briquez P.S., Martino M.M. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017;53:13–28. doi: 10.1016/j.actbio.2017.01.056. [DOI] [PubMed] [Google Scholar]
- 25.Vasconcelos D.P., Águas A.P., Barbosa M.A., Pelegrín P., Barbosa J.N. The inflammasome in host response to biomaterials: bridging inflammation and tissue regeneration. Acta Biomater. 2019;83:1–12. doi: 10.1016/j.actbio.2018.09.056. [DOI] [PubMed] [Google Scholar]
- 26.Brzicova T., Javorkova E., Vrbova K., Zajicova A., Holan V., Pinkas D., Philimonenko V., Sikorova J., Klema J., Topinka J., Rossner P. Molecular responses in THP-1 macrophage-like cells exposed to diverse nanoparticles. Nanomaterials. 2019;9:687. doi: 10.3390/nano9050687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson J.M., Rodriguez A., Chang D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008:86–100. doi: 10.1016/j.smim.2007.11.004. https://doi:10.1016/j.smim.2007.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheikh Z., Brooks P.J., Barzilay O., Fine N., Glogauer M. Macrophages, foreign body giant cells and their response to implantable biomaterials. Materials. 2015:5671–5701. doi: 10.3390/ma8095269. https://doi:10.3390/ma8095269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosshart H., Heinzelmann M. THP-1 cells as a model for human monocytes. Ann. Transl. Med. 2016;4:438. doi: 10.21037/atm.2016.08.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lund M.E., To J., O'Brien B.A., Donnelly S. The choice of phorbol 12-myristate 13-acetate differentiation protocol influences the response of THP-1 macrophages to a pro-inflammatory stimulus. J. Immunol. Methods. 2016;430:64–70. doi: 10.1016/j.jim.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Cai Y., Chen Y., Hong X., Liu Z., Yuan W. Porous microsphere and its applications. Int. J. Nanomedicine. 2013 doi: 10.2147/IJN.S41271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gough J.E., Jones J.R., Hench L.L. Nodule formation and mineralisation of human primary osteoblasts cultured on a porous bioactive glass scaffold. Biomaterials. 2004;25:2039. doi: 10.1016/j.biomaterials.2003.07.001. –2046. [DOI] [PubMed] [Google Scholar]
- 33.Skelton K.L., Glenn J.V., Clarke S.A., Georgiou G., Valappil S.P., Knowles J.C., Nazhat S.N., Jordan G.R. Effect of ternary phosphate-based glass compositions on osteoblast and osteoblast-like proliferation, differentiation and death in vitro. Acta Biomater. 2007 doi: 10.1016/j.actbio.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Gupta D., Hossain K.M.Z., Roe M., Smith E.F., Ahmed I., Sottile V., Grant D.M. Long-term culture of stem cells on phosphate-based glass microspheres: synergistic role of chemical formulation and 3D architecture. ACS Appl. Bio Mater. 2021;4:5987–6004. doi: 10.1021/acsabm.1c00120. [DOI] [PubMed] [Google Scholar]
- 35.Xie W., Fu X., Mo Y., Cheng J., Wang H., et al. Dose-dependent modulation effects of bioactive glass particles on macrophages and diabetic wound healing. J. Mater. Chem. B. 2019:940–952. doi: 10.1039/C8TB02938E. [DOI] [PubMed] [Google Scholar]
- 36.Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sridharan R., Cameron A.R., Kelly D.J., Kearney C.J., O'Brien F.J. Biomaterial based modulation of macrophage polarization: a review and suggested design principles. Mater. Today. 2015;18:313–325. doi: 10.1016/j.mattod.2015.01.019. [DOI] [Google Scholar]
- 38.Sadowska J.M., Ginebra M.P. Inflammation and biomaterials: role of the immune response in bone regeneration by inorganic scaffolds. J. Mater. Chem. B. 2020;8:9404–9427. doi: 10.1039/d0tb01379j. [DOI] [PubMed] [Google Scholar]
- 39.Wang T., Bai J., Lu M., Huang C., Geng D., Chen G., Wang L., Qi J., Cui W., Deng L. Engineering immunomodulatory and osteoinductive implant surfaces via mussel adhesion-mediated ion coordination and molecular clicking. Nat. Commun. 2022;13 doi: 10.1038/s41467-021-27816-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prantner D., Darville T., Sikes J.D., Andrews C.W., Brade H., Rank R.G., Nagarajan U.M. Critical role for interleukin-1β (IL-1β) during Chlamydia muridarum genital infection and bacterial replication-independent secretion of IL-1β in mouse macrophages. Infect. Immun. 2009;77:5334–5346. doi: 10.1128/IAI.00883-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silva A.C.O., Faria M.R., Fontes A., Campos M.S., Cavalcanti B.N. Interleukin-1 beta and interleukin-8 in healthy and inflamed dental pulps. J. Appl. Oral Sci. 2009;17:527–532. doi: 10.1590/S1678-77572009000500031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holder M.J., Wright H.J., Couve E., Milward M.R., Cooper P.R. Neutrophil extracellular traps exert potential cytotoxic and proinflammatory effects in the dental pulp. J. Endod. 2019 doi: 10.1016/j.joen.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 43.Aghila Rani K.G., Samsudin A.R., Abou Neel E.A. Titanium dioxide doped phosphate glasses modulating pro-inflammatory macrophages responses for tissue regeneration application. Mater. Chem. Phys. 2023;304 doi: 10.1016/j.matchemphys.2023.127857. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.