Abstract
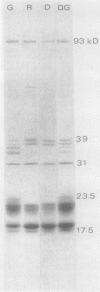
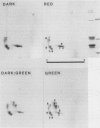
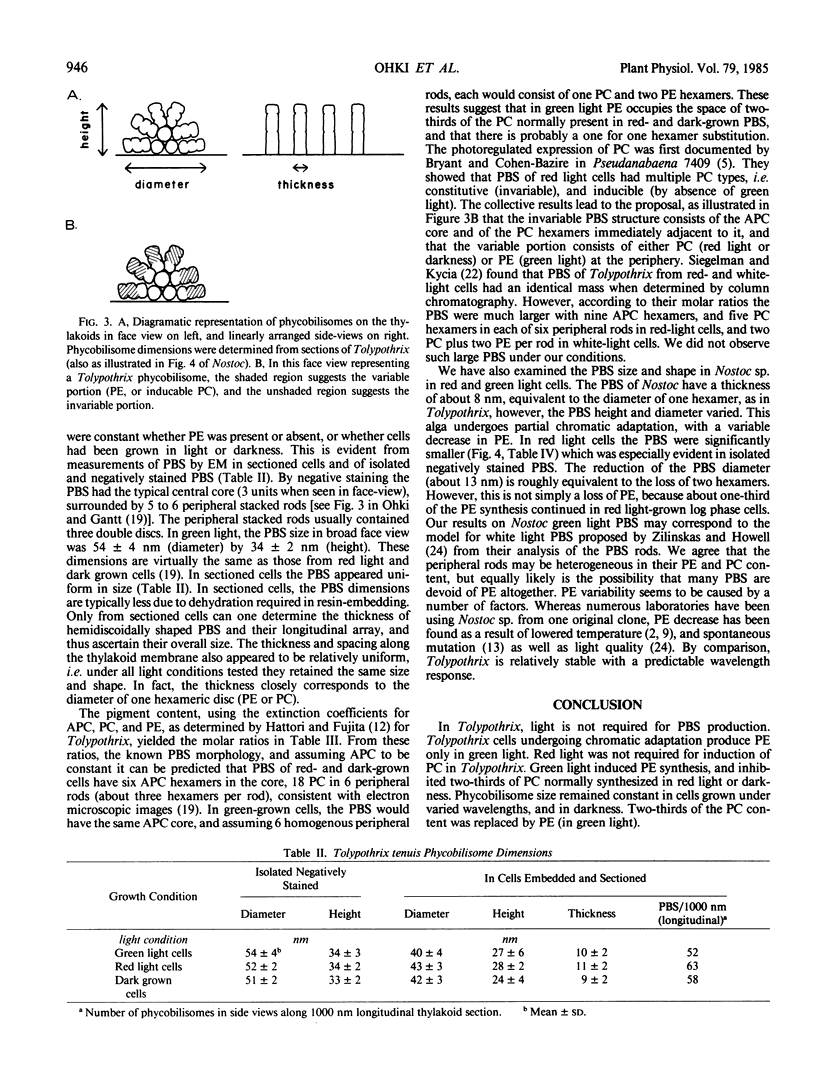
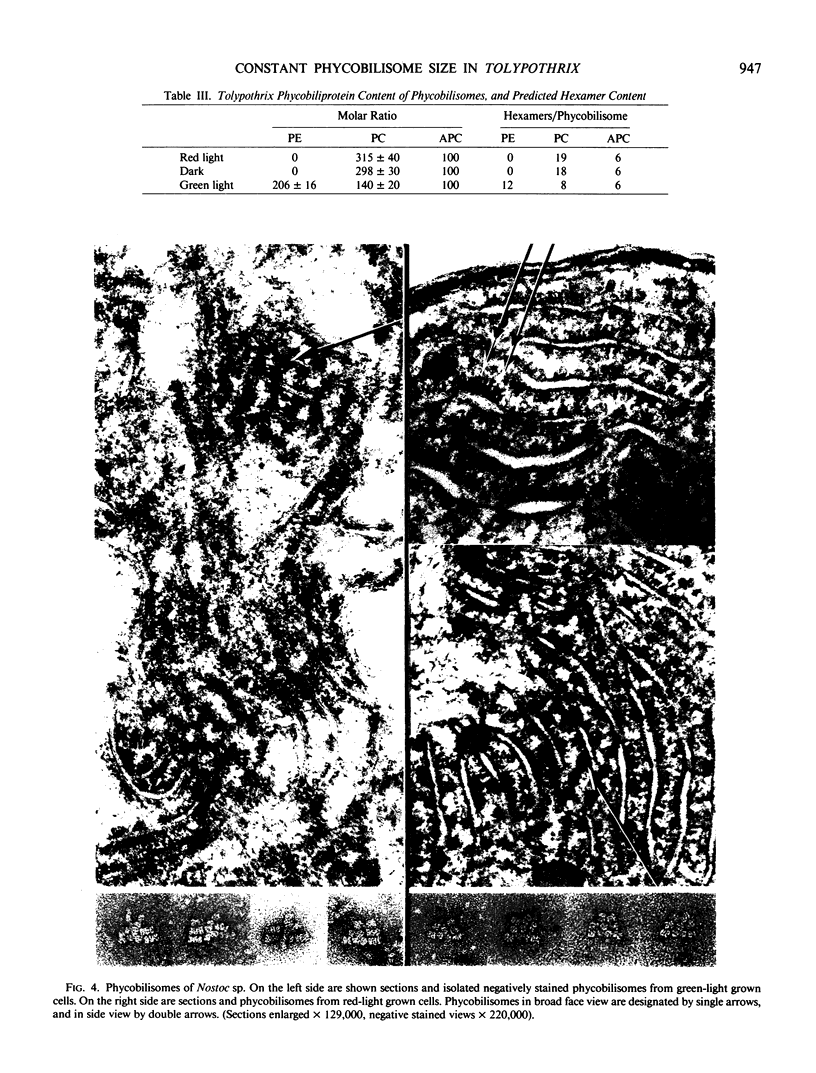
Phycobilisomes of Tolypothrix tenuis, a cyanobacterium capable of complete chromatic adaptation, were studied from cells grown in red and green light, and in darkness. The phycobilisome size remained constant irrespective of the light quality. The hemidiscoidal phycobilisomes had an average diameter of about 52 nanometers and height of about 33 nanometers, by negative staining. The thickness was equivalent to a phycocyanin molecule (about 10 nanometers). The molar ratio of allophycocyanin, relative to other phycobiliproteins always remained at about 1:3. Phycobilisomes from red light grown cells and cells grown heterotrophically in darkness were indistinguishable in their pigment composition, polypeptide pattern, and size. Eight polypeptides were resolved in the phycobilin region (17.5 to 23.5 kilodaltons) by isoelectric focusing followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Half of these were invariable, while others were variable in green and red light. It is inferred that phycoerythrin synthesis in green light resulted in a one for one substitution of phycocyanin, thus retaining a constant phycobilisome size. Tolypothrix appears to be one of the best examples of phycobiliprotein regulation with wavelength. By contrast, in Nostoc sp., the decrease in phycoerythrin in red light cells was accompanied by a decrease in phycobilisome size but not a regulated substitution.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. K., Rayner M. C., Sweet R. M., Eiserling F. A. Regulation of Nostoc sp. phycobilisome structure by light and temperature. J Bacteriol. 1983 Sep;155(3):1407–1416. doi: 10.1128/jb.155.3.1407-1416.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D. A., Cohen-Bazire G. Effects of chromatic illumination on cyanobacterial phycobilisomes. Evidence for the specific induction of a second pair of phycocyanin subunits in Pseudanabaena 7409 grown in red light. Eur J Biochem. 1981 Oct;119(2):415–424. doi: 10.1111/j.1432-1033.1981.tb05624.x. [DOI] [PubMed] [Google Scholar]
- Bryant D. A. The photoregulated expression of multiple phycocyanin species. A general mechanism for the control of phycocyanin synthesis in chromatically adapting cyanobacteria. Eur J Biochem. 1981 Oct;119(2):425–429. doi: 10.1111/j.1432-1033.1981.tb05625.x. [DOI] [PubMed] [Google Scholar]
- Gantt E., Lipschultz C. A., Grabowski J., Zimmerman B. K. Phycobilisomes from blue-green and red algae: isolation criteria and dissociation characteristics. Plant Physiol. 1979 Apr;63(4):615–620. doi: 10.1104/pp.63.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N., Lundell D. J., Yamanaka G., Williams R. C. The structure of a "simple" phycobilisome. Ann Microbiol (Paris) 1983 Jul-Aug;134B(1):159–180. doi: 10.1016/s0769-2609(83)80103-3. [DOI] [PubMed] [Google Scholar]
- Kipe-Nolt J. A., Stevens S. E., Bryant D. A. Growth and Chromatic Adaptation of Nostoc sp. Strain MAC and the Pigment Mutant R-MAC. Plant Physiol. 1982 Nov;70(5):1549–1553. doi: 10.1104/pp.70.5.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller K. P., Wehrmeyer W., Schneider H. Isolation and characterization of disc-shaped phycobilisomes from the red alga Rhodella violacea. Arch Microbiol. 1977 Feb 4;112(1):61–67. doi: 10.1007/BF00446655. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Righetti P. G., Drysdale J. W. Isoelectric focusing in gels. J Chromatogr. 1974 Sep 25;98(2):271–321. doi: 10.1016/s0021-9673(00)92076-4. [DOI] [PubMed] [Google Scholar]
- Siegelman H. W., Kycia J. H. Molecular morphology of cyanobacterial phycobilisomes. Plant Physiol. 1982 Sep;70(3):887–897. doi: 10.1104/pp.70.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilinskas B. A., Howell D. A. Role of the Colorless Polypeptides in Phycobilisome Assembly in Nostoc sp. Plant Physiol. 1983 Feb;71(2):379–387. doi: 10.1104/pp.71.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]