Abstract
Background
Coronary artery calcification (CAC), a surrogate of atherosclerosis, is related to stent underexpansion and adverse cardiac events. However, the effect of CAC on plaque stability is still controversial and the morphological significance of CAC has yet to be elucidated.
Methods
A retrospective series of 419 patients with acute coronary syndrome (ACS) who underwent optical coherence tomography (OCT) were enrolled. Patients were classified into three groups based on the calcification size in culprit plaques and the features of the culprit and non-culprit plaques among these groups were compared. Logistic regression was used to analyze independent risk factors for culprit plaque rupture and the nonlinear relationship between calcification parameters and culprit plaque rupture. Furthermore, we compared the detailed calcification parameters of different kinds of plaques.
Results
A total of 419 culprit plaques and 364 non-culprit plaques were identified. The incidence of calcification was 53.9 % in culprit plaques and 50.3 % in non-culprit plaques. Compared with culprit plaques without calcification, plaque rupture, macrophages and cholesterol crystals were more frequently observed in the spotty calcification group, and the lipid length was longer; the incidence of macrophages and cholesterol crystals was higher in the macrocalcification group. Calcification tended to be smaller in ruptured plaques than in non-ruptured plaques. Moreover, the arc and length of calcification were greater in culprit plaques than in non-culprit plaques.
Conclusions
Vulnerable features were more frequently observed in culprit plaques with spotty calcification, whereas the presence of macrocalcification calcifications did not significantly increase plaque vulnerability. Calcification tends to be larger in culprit plaques than in non-culprit plaques.
Keywords: Coronary artery calcification, Optical coherence tomography, Acute coronary syndrome
1. Introduction
Coronary artery calcification (CAC), a surrogate of atherosclerosis, is related to stent underexpansion [1] and has long been known as an established predictor of adverse cardiac events [2,3]. The clinical practice guidelines of Europe [4] recommend measuring CAC based on CT to assess the general cardiovascular disease risk in asymptomatic populations or as evidence to initiate or delay preventive statin therapy. Although the extent of CAC represents an effective method to identify vulnerable patients [2,5], the effect of CAC on plaque stability is not fully clarified. In early CAC studies, researchers concluded that calcification may stabilize plaques [6,7]. An autopsy study also found larger calcification in stable plaques than in ruptured plaques [8]. In recent years, with the popularity of high-resolution optical coherence tomography (OCT) technology, it has been found that spotty calcification not only increases the vulnerability of plaques but also accelerates the progression of atherosclerosis [9,10]. Macrocalcification can stabilize plaques and plaque rupture is negatively correlated with the number of macrocalcifications [11,12]. The reason for the controversies may be that earlier studies were just based on qualification of calcification and did not classify calcification in detail. This implied that the detailed three-dimensional morphology of CAC has incremental value beyond qualification when analyzing plaque stability. In the present study, we divided calcifications into spotty calcifications and macrocalcifications by morphology and size using OCT to identify and compare specific morphological features of calcium-containing lesions in acute coronary syndrome (ACS) patients. In addition, we compared the morphology of CAC in culprit and non-culprit plaques of ACS patients and investigated the nonlinear relationship between calcification parameters and plaque rupture for the first time. We aim to focus on human CAC from the extent and pattern of calcification observed on OCT and explore its relationship with plaque vulnerability.
2. Methods
2.1. Population
We retrospectively identified 487 patients with ACS who underwent OCT imaging at the Second Affiliated Hospital of Harbin Medical University (Harbin, China) between January 2018 and February 2019. ACS includes ST segment elevation myocardial infarction (STEMI) and non-ST-segment elevation acute coronary syndromes (NSTE-ACS). In this study, NSTE-ACS patients were NSTEMI patients. STEMI and NSTE-ACS are defined in detail in the supplementary material.
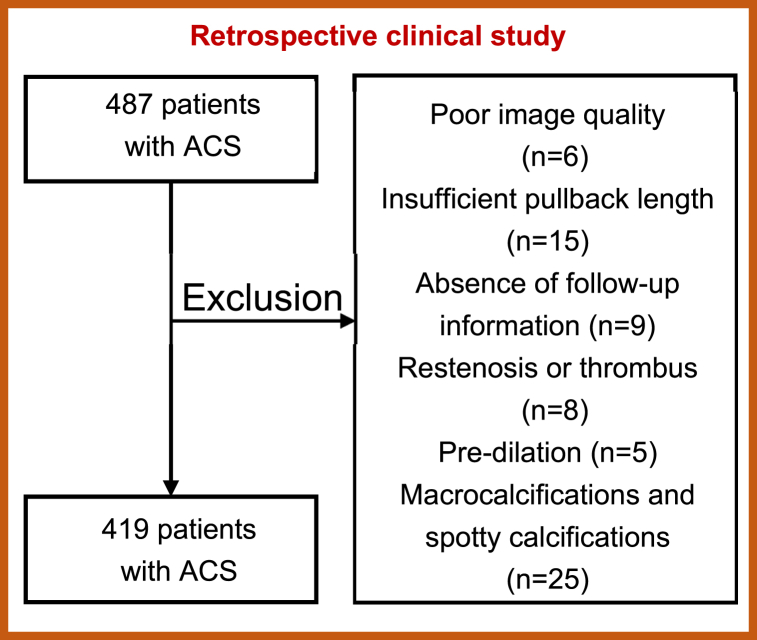
Sixty-eight patients were excluded for the following reasons: (1) poor image quality (n = 6); (2) insufficient retraction length (retraction length <40 mm, n = 15); (3) lack of clinical information (n = 9); (4) in-stent restenosis or thrombosis (n = 8); (5) predilation (n = 5); and (6) patients with both spotty calcification and macrocalcification within one plaque (n = 25). Finally, 419 patients were enrolled in this study and patients were divided into 3 groups according to the size of calcification in the culprit plaque. Fig. 1 shows the exclusion criteria. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Second Hospital of Harbin Medical University. All patients provided written informed consent.
Fig. 1.
Exclusion criteria.
2.2. Angiographic analysis
Quantitative coronary angiography (QCA) analysis was performed using offline software (QAngio XA 7.3, Leiden, The Netherlands). The reference vessel diameter (RVD), minimum lumen diameter (MLD) and diameter stenosis (DS) were measured by an experienced physician who was blinded to the patient's clinical information and OCT findings. The percentage of diameter stenosis was calculated as follows: (reference vessel diameter - minimum lumen diameter)/reference vessel diameter) × 100 %. All lesions were defined according to the AHA/ACC guidelines [13].
2.3. OCT imaging and analysis
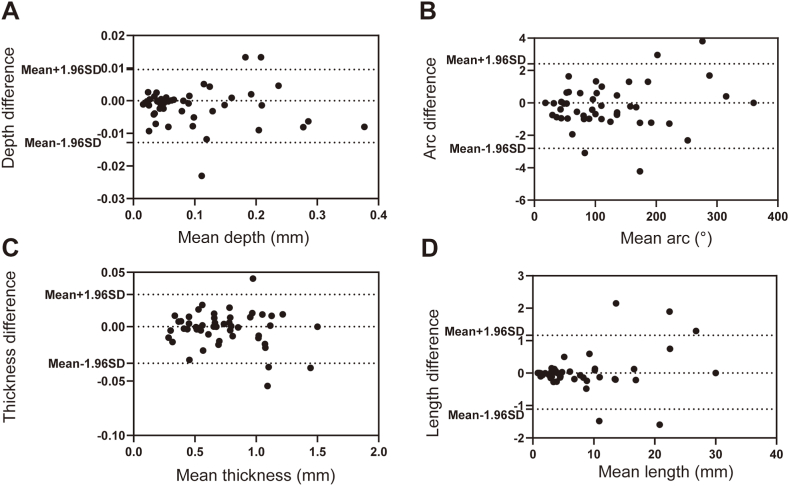
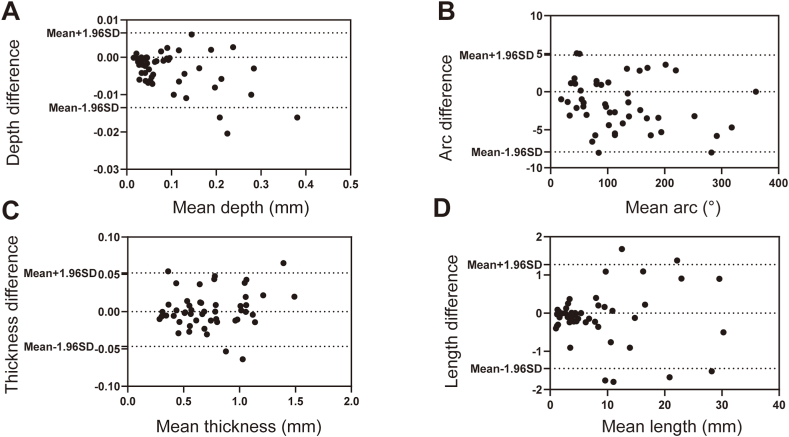
OCT imaging was performed using a commercially available C7-XR/ILUMIEN OCT system (Abbott Vascular, Santa Clara, California) in the present study. OCT of the culprit vessel was performed before intervention of the culprit lesion. OCT imaging of non-culprit lesions was performed after treatment. Representative OCT images are shown in Supplementary Fig. 1. The culprit lesion was identified by echocardiogram, electrocardiogram (ECG), and coronary angiogram. OCT analyses were carried out by 2 investigators independently. We randomly selected 50 frames of OCT images for the two assigned OCT investigators to determine the presence or absence of calcification. The κ coefficient of agreement for the identification of calcification was 0.956 for interobserver agreement and 1.000 for intra-observer agreement. Then, we randomly selected another 50 pullbacks of OCT images with calcification to test the consistency of the quantitative analysis. The results of intra-observer and interobserver variability analyses are shown in Fig. 2, Fig. 3, respectively.
Fig. 2.
Intra-observer variability analysis for CAC measurements. Intra-observer variation in mean depth (A), mean arc (B), mean thickness (C), and mean length (D) of calcification.
Fig. 3.
Inter-observer variability analysis for CAC measurements. Inter-observer variation in mean depth (A), mean arc (B), mean thickness (C), and mean length (D) of calcification.
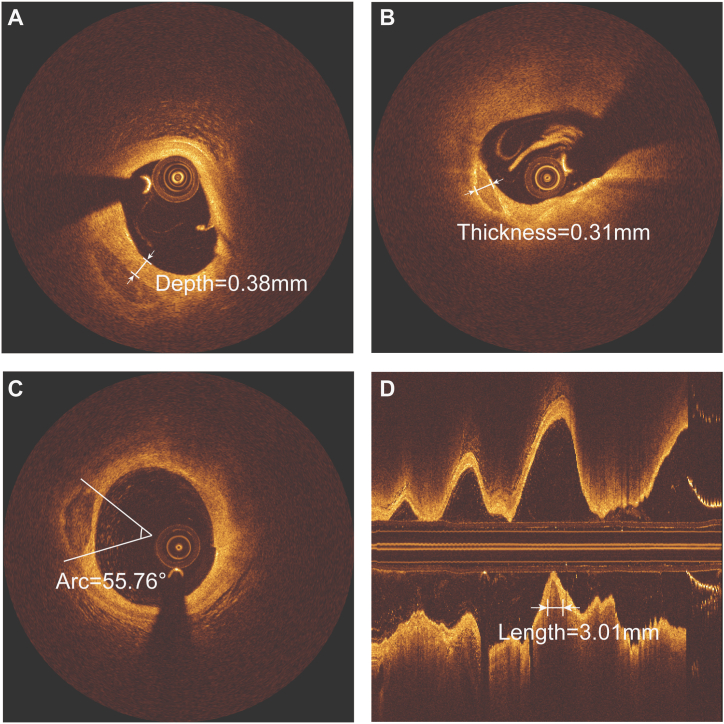
In patients with multiple stenoses, the most severe stenoses and/or lesions with evidence of plaque rupture and associated thrombosis were identified as culprit lesions and the remainder were identified as non-culprit lesions. Plaque rupture was defined as the presence of fibrous cap discontinuity and cavity formation within the plaque [14]. Lipid-rich plaque was defined as a lipid arc >90° [15]. Lipid arcs were measured multiple times at every 1 mm interval on OCT cross-sectional images, and their maximum values were recorded. The lipid length was measured on the longitudinal view. Three measurements were taken at the thinnest part of the fibrous cap of the lipid-rich plaque, and the average value was recorded as the minimum fibrous cap thickness (FCT) [16]. Thin-cap fibroatheroma (TCFA) was defined as a lipid plaque with a necrotic core with a lipid angle greater than 90° and a minimum fibrous cap thickness of less than 65 μm [17]. Macrophage accumulation on the OCT images was defined as an increased signal intensity within the plaque, accompanied by heterogeneous backward shadows [18]. A microchannel was defined as a black hole with a diameter of 50–300 mm within a plaque that was present on at least 3 consecutive frames [19]. The existence of cholesterol crystals was defined by the presence of linear and highly reflective structures within the plaques [20]. Calcification was identified by the presence of well delineated, low back-scattering heterogeneous regions in the OCT images [21]. Spotty calcification was defined as calcification with an arc of less than 90° and a length of less than 4 mm [22]. Macrocalcifications were defined as calcifications with an arc of more than 90° or a length greater than 4 mm [12]. In this study, we measured specific CAC parameters. Considering that a plaque may contain multiple CACs, we measured the maximum length, arc, thickness, and depth of each CAC in the same plaque. The calcification length was measured on the longitudinal view, and the remaining calcification parameters were measured on the cross-sectional view. A schematic diagram of the measured calcification is shown in Fig. 4 (the depth (A), thickness (B), arc (C) and length (D) of CACs). The depth of calcification was defined as the shortest measurable distance between the leading edge of the calcified plaque and the lumen boundary. Finally, the maximum length, maximum thickness, maximum arc and minimum depth of multiple CACs within a plaque were recorded. Additional definitions of OCT plaque morphologies were obtained from previous consensus documents and major OCT studies (Supplementary Material). If two separate plaques within the same vessel were to be identified, a reference segment of ≥5 mm between them was needed. Excellent interobserver consistency was observed in the identification of calcification (κ = 0.956). The interobserver and intraobserver agreement results for quantitative analysis of calcification are presented in the Supplementary Material.
Fig. 4.
Measurement methods for calcification.
Fig. 4 shows how we measured the depth (A), thickness (B), arc (C) and length (D) of CACs. The calcification length was measured on the longitudinal view. CAC = coronary artery calcification.
2.4. Statistical analysis
Normality tests were performed using P–P plots or Q‒Q plots for continuous variables. Normally distributed continuous variables are expressed as the mean (SD) and were compared using Student's t-test or one-way analysis of variance (ANOVA), while nonnormally distributed continuous variables are expressed as the median (interquartile range) and were compared using the Mann‒Whitney U test or Kruskal-Wallis H test. Categorical variables are expressed as frequencies and percentages and were compared using the χ2 test or Fisher's exact test. Univariate and multivariate logistic regression were used to determine predictors of culprit plaque rupture. The nonlinear relationships between calcification features and culprit plaque rupture are modeled using restricted cubic splines (RCS). Considering the cluster effect of non-culprit plaques in the same patient, the plaque characteristics were compared using generalized estimating equations (GEEs). Similarly, GEE was used to compare the difference between culprit plaque calcification and non-culprit plaque calcification. A two-tailed P value < 0.05 was considered statistically significant. The analysis was performed using R v.4.0.5 (R Foundation for Statistical Computing, Vienna, Austria) and SPSS version 25.0 (SPSS, IBM, Armonk, NY, USA).
3. Results
3.1. Baseline characteristics
A total of 419 culprit plaques and 364 non-culprit plaques were identified by OCT. The incidence of calcification was 53.9 % in culprit lesions and 50.3 % in non-culprit lesions. Patients were divided into a group without calcification, a group with spotty calcification and a group with macrocalcification according to the level of calcification in the culprit plaque. The clinical characteristics of all patients are shown in Table 1. The average age of the patients was 59.2 years (SD 11.8). There were significant differences in age (P < 0.001) and hypertension (P < 0.006) between the three groups of patients. Patients with spotty calcification within the culprit plaque had a higher rate of aspirin use (P = 0.047).
Table 1.
Baseline clinical characteristics.
| Characteristics | Patients with spotty calcification (n = 70) | Patients with macrocalcification (n = 156) | Patients without culprit CAC (n = 193) | P value |
|---|---|---|---|---|
| Male, n (%) | 44 (62.0) | 112 (72.3) | 150 (77.7) | 0.051 |
| Age, years | 63.4 ± 10.8 | 65.6 ± 9.5 | 59.2 ± 11.8 | <0.001 |
| BMI, (kg/m2) | 25.31 ± 3.69 | 25.34 ± 3.95 | 24.96 ± 3.62 | 0.628 |
| Risk factors | ||||
| Current smoker, n (%) | 37 (52.9) | 90 (57.7) | 108 (56.0) | 0.794 |
| Hypertension, n (%) | 34 (48.6) | 89 (57.1) | 77 (39.9) | 0.006 |
| Diabetes, n (%) | 17 (23.9) | 35 (22.6) | 31 (16.1) | 0.195 |
| Prior PCI/CABG, n (%) | 2 (2.9) | 4 (2.6) | 10 (5.2) | 0.412 |
| Laboratory data | ||||
| LDL-C, mg/dL | 124.7 ± 40.7 | 118.1 ± 38.2 | 121.2 ± 40.7 | 0.468 |
| HDL-C, mg/dL | 51.7 ± 12.2 | 52.1 ± 11.4 | 51.1 ± 11.4 | 0.737 |
| TC, mg/dL | 193.5 ± 39.0 | 186.1 ± 45.5 | 192.8 ± 48.7 | 0.342 |
| TG, mg/dL | 144.6 ± 68.7 | 146.1 ± 101.0 | 160.7 ± 142.6 | 0.434 |
| hs-CRP, mg/dL | 6.8 ± 4.6 | 6.5 ± 4.7 | 5.7 ± 4.3 | 0.153 |
| eGFR, mL/min/1.732 | 80.6 ± 31.2 | 77.1 ± 26.0 | 83.5 ± 26.6 | 0.090 |
| HbA1c, % | 6.64 ± 1.56 | 6.31 ± 1.21 | 6.32 ± 1.48 | 0.232 |
| Diagnosis | 0.595 | |||
| STEMI, n (%) | 54 (77.1) | 129 (82.7) | 158 (81.9) | |
| NSTE-ACS, n (%) | 16 (22.9) | 27 (17.3) | 35 (18.1) | |
| Medications history | ||||
| Aspirin, n (%) | 16 (22.9) | 18 (11.5) | 23 (11.9) | 0.047 |
| Statin, n (%) | 18 (25.7) | 47 (30.1) | 54 (28.0) | 0.781 |
| ACEI/ARB, n (%) | 21 (30.0) | 46 (29.5) | 52 (26.9) | 0.827 |
| Values n (%), mean ± SD; CAC = coronary artery calcification; eGFR = estimated glomerular filtration rate; HDL-C = high-density lipoprotein cholesterol; hs-CRP = hypersensitive C-reactive protein; LDL-C = low-density lipoprotein cholesterol; TC = total cholesterol; TG = triglyceride. | ||||
3.2. Angiographic findings of calcified plaque
An angiographic analysis of culprit plaques is summarized in Table 2. Both spotty and macrocalcifications are most common in the anterior descending branch (LAD), followed by the right coronary artery (RCA) and finally the left circumflex branch (LCX). No significant differences were observed in MLD, RVD and DS% among the three groups.
Table 2.
Angiographic findings of culprit plaques with versus without CAC.
| Patients with spotty calcification (n = 70) | Patients with macrocalcification (n = 156) | Patients without culprit CAC (n = 193) | P value | |
|---|---|---|---|---|
| Location | 0.056 | |||
| LAD | 31 (44.3) | 88 (56.4) | 79 (40.9) | |
| RCA | 29 (41.4) | 55 (35.3) | 90 (46.6) | |
| LCX | 10 (14.3) | 13 (8.3) | 24 (12.4) | |
| MLD, mm | 0.73 (0.00,1.09) | 0.77 (0.00,1.09) | 0.76 (0.00,1.20) | 0.561 |
| RVD, mm | 2.69 ± 0.71 | 2.70 ± 0.61 | 2.78 ± 0.62 | 0.441 |
| DS, % | 74.2 ± 22.7 | 75.3 ± 22.9 | 73.8 ± 23.1 | 0.834 |
| Values n (%), mean ± SD or median (25th-75th percentile), CAC = coronary artery calcification; DS = diameter stenosis; LAD = left anterior descending artery; LCX = left circumflex artery; MLD = minimal lumen diameter; RCA = right coronary artery; RVD = reference vessel diameter. | ||||
3.3. OCT findings
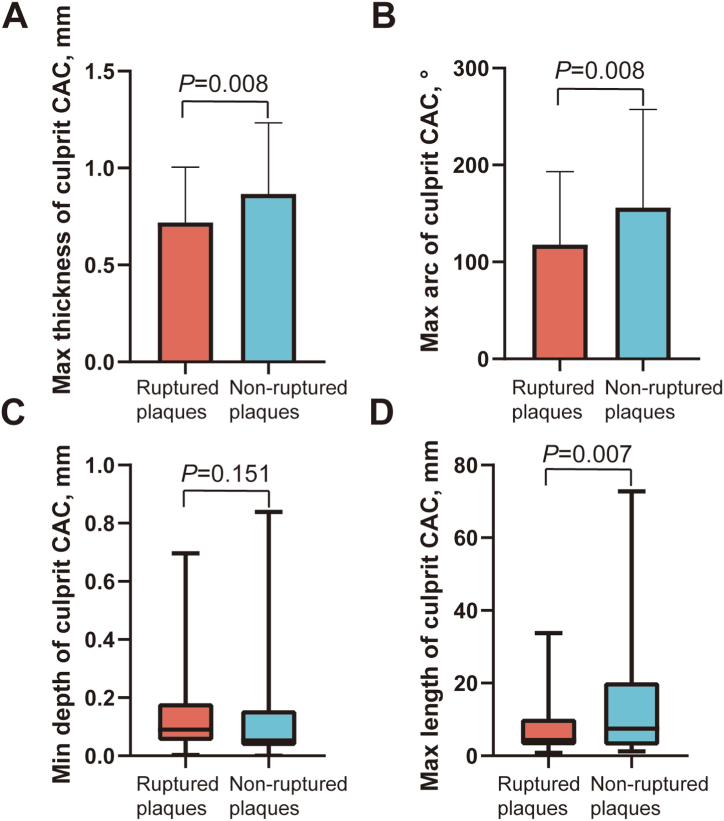
Table 3 demonstrates the OCT findings of the culprit lesions. The incidence of plaque rupture was higher in the spotty calcification group than in the non-calcification group (P = 0.015). The lipid length was longer in the macrocalcification group than in the non-calcification group (P = 0.020). Macrophages and cholesterol crystals were more often observed in plaques with calcification than in plaques without calcification (spotty calcification group vs. non-calcification group, P = 0.001; macrocalcification group vs. non-calcification group, P = 0.004). We further divided the culprit plaques into ruptured and non-ruptured groups. The calcification thickness (P = 0.008), arc (P = 0.008), and length (P = 0.007) in ruptured plaques were smaller than those in non-ruptured plaques (Fig. 5).
Table 3.
OCT findings of different degrees of CAC in culprit lesions.
| Plaques with spotty calcification (n = 70) | Plaques with macrocalcification (n = 156) | Plaques without culprit CAC (n = 193) | Group A vs. Group B |
Group B vs. Group C |
Group A vs. Group C |
|
|---|---|---|---|---|---|---|
| Plaque rupture | 56 (80.0) | 106 (67.9) | 124 (64.2) | 0.063 | 0.468 | 0.015 |
| Lipid-rich plaque | 61 (87.1) | 130 (83.3) | 148 (76.7) | 0.464 | 0.125 | 0.063 |
| Lipid length, mm | 14.5 ± 6.8 | 15.4 ± 7.7 | 13.4 ± 6.6 | 0.438 | 0.020 | 0.293 |
| FCT, μm | 73.0 ± 23.5 | 79.9 ± 28.4 | 78.1 ± 26.9 | 0.098 | 0.579 | 0.211 |
| Max lipid arc, ° | 324.6 ± 60.0 | 324.9 ± 61.7 | 314.5 ± 66.0 | 0.975 | 0.174 | 0.297 |
| TCFA | 33 (47.1) | 62 (39.7) | 76 (39.4) | 0.297 | 0.945 | 0.259 |
| Minimal lumen area, mm2 | 1.44 ± 0.77 | 1.52 ± 1.00 | 1.72 ± 1.22 | 0.832 | 0.203 | 0.082 |
| Macrophage | 64 (91.4) | 134 (85.9) | 141 (73.1) | 0.243 | 0.004 | 0.001 |
| Microchannel | 33 (47.1) | 65 (41.7) | 77 (39.9) | 0.442 | 0.738 | 0.292 |
| Cholesterol crystal | 46 (65.7) | 89 (57.1) | 78 (40.4) | 0.220 | 0.002 | <0.001 |
| Value n (%), mean ± SD, CAC = coronary artery calcification; FCT = fibrous cap thickness; TCFA = thin-cap fibroatheroma. Group A: plaques with spotty calcification; Group B: plaques with macrocalcification; Group C: plaques without culprit CAC. | ||||||
Fig. 5.
Comparisons of calcification features between ruptured and non-ruptured coronary culprit plaques. Comparisons of max thickness (A), max arc (B), min depth (C), and max length (D) of calcification between ruptured and non-ruptured coronary culprit plaques. Quantitative analysis of culprit plaque CACs showed a smaller thickness, arc, and length of calcification in plaques with rupture than in those without rupture. CAC = coronary artery calcification.
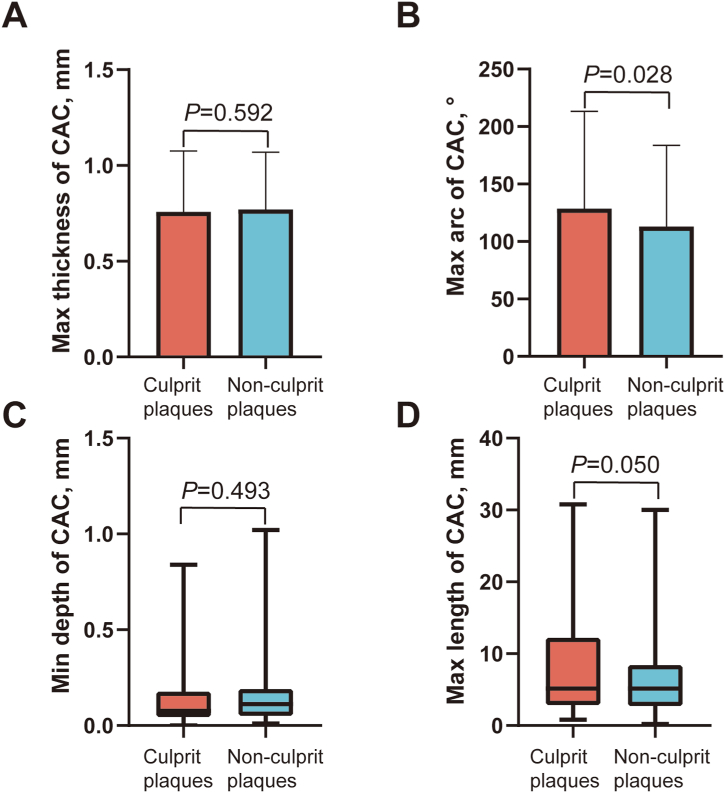
Table 4 shows the OCT findings for non-culprit lesions. The minimum lumen area of non-culprit plaques with calcification was smaller than that of non-culprit plaques without calcification (spotty calcification group vs. non-calcification group, P = 0.049; macrocalcification group vs. non-calcification group, P = 0.027). The incidence of macrophages in the spotty calcification group was the highest among the three groups (spotty calcification group vs. non-calcification group, P = 0.026; spotty calcification group vs. macrocalcification group, P = 0.020). In addition, we compared calcification parameters in culprit plaques and non-culprit plaques. We found that the arc of calcification in culprit plaques was greater than that in non-culprit plaques (P = 0.028) (Fig. 6).
Table 4.
OCT findings of different degrees of CAC in non-culprit lesions.
| Plaques with spotty calcification (n = 50) | Plaques with macrocalcification (n = 133) | Plaques without culprit CAC (n = 181) | Group A vs. Group B |
Group B vs. Group C |
Group A vs. Group C |
|
|---|---|---|---|---|---|---|
| Plaque rupture | 5 (10.0) | 13 (9.8) | 13 (7.2) | 0.981 | 0.377 | 0.430 |
| Lipid-rich plaque | 29 (58.0) | 61 (45.9) | 92 (50.8) | 0.116 | 0.374 | 0.353 |
| Lipid length, mm | 11.1 ± 5.7 | 11.2 ± 6.2 | 9.7 ± 5.2 | 0.911 | 0.093 | 0.134 |
| FCT, μm | 97.6 ± 36.4 | 91.0 ± 27.6 | 98.0 ± 30.7 | 0.400 | 0.230 | 0.887 |
| Max lipid arc, ° | 252.6 ± 72.5 | 260.5 ± 76.2 | 234.2 ± 75.9 | 0.945 | 0.052 | 0.234 |
| TCFA | 6 (12.0) | 17 (12.8) | 18 (9.9) | 0.914 | 0.423 | 0.646 |
| Minimal lumen area, mm2 | 3.05 ± 1.72 | 3.20 ± 1.44 | 3.67 ± 2.02 | 0.617 | 0.027 | 0.049 |
| Macrophage | 39 (78.0) | 78 (58.6) | 107 (59.1) | 0.020 | 0.866 | 0.026 |
| Microchannel | 28 (56.0) | 64 (48.1) | 95 (52.5) | 0.413 | 0.385 | 0.576 |
| Cholesterol crystal | 15 (30.0) | 49 (36.8) | 48 (26.5) | 0.532 | 0.054 | 0.642 |
| Values n (%), mean ± SD, CAC = coronary artery calcification; FCT = fibrous cap thickness; TCFA = thin-cap fibroatheroma. Group A: plaques with spotty calcification; Group B: plaques with macrocalcification; Group C: plaques without culprit CAC. | ||||||
Fig. 6.
Comparisons of OCT findings of calcification between culprit plaques and non-culprit plaques. Comparisons of max thickness (A), max arc (B), min depth (C), and max length (D) of calcification between culprit plaques and non-culprit plaques. Quantitative analysis showed a larger arc of calcification in culprit plaques than in non-culprit plaques. OCT = optical coherence tomography.
3.4. Risk factors associated with culprit plaque rupture
In the univariate logistic regression with culprit lesion rupture as the outcome, we found that maximal lipid arc, fibrous cap thickness (FCT), macrophages, and cholesterol crystals were associated with plaque rupture (Table 5). Next, we performed multivariate regression analysis for variables with P values < 0.1 in the univariate regression. The multivariate analysis revealed that FCT was an independent predictive factor of culprit plaque rupture (P = 0.045). In addition, we did not find nonlinear relationship between calcification parameters and culprit plaque rupture (Supplementary Fig. 2).
Table 5.
Univariate and multivariate logistic regression of culprit plaque rupture.
| Variable | Univariate Analysis |
Multivariate Analysis |
||
|---|---|---|---|---|
| OR (95 % CI) | P value | OR (95 % CI) | P value | |
| Max lipid arc, ° | 1.007 (1.003–1.011) | 0.002 | 1.002 (0.995–1.009) | 0.528 |
| FCT, μm | 0.974 (0.964–0.984) | <0.001 | 0.985 (0.970–1.000) | 0.045 |
| Minimal lumen area, mm2 | 0.855 (0.712–1.026) | 0.092 | 1.488 (0.693–3.194) | 0.307 |
| Macrophage | 30.667 (14.974–62.806) | <0.001 | 2.837 (0.175–45.849) | 0.463 |
| Microchannel | 1.289 (0.846–1.965) | 0.238 | ||
| Spotty calcification | 1.887 (0.960–3.707) | 0.065 | 2.332 (0.819–6.639) | 0.113 |
| Macrocalcification | 0.978 (0.639–1.495) | 0.917 | ||
| Cholesterol crystal | 1.600 (1.057–2.423) | 0.026 | 1.100 (0.470–2.575) | 0.826 |
| FCT = fibrous cap thickness. | ||||
4. Discussion
The major findings of the present study are as follows: (1) vulnerability features were more frequently observed in culprit plaques with spotty calcification, whereas the presence of large calcifications did not significantly increase plaque susceptibility; (2) less calcification content was observed in ruptured culprit plaques than in non-ruptured culprit plaques; (3) calcification tended to be larger in culprit plaques than in non-culprit plaques. In this study, we compared the plaque characteristics between plaques with spotty calcification and those with macrocalcification for the first time. Furthermore, a preliminary exploration of the nonlinear relationship between calcification parameters and plaque rupture was conducted, aiming to further elucidate the impact of calcification on plaque stability.
CAC has long been considered a marker of atherosclerosis. Ectopic bone production is the basis for CAC [23] and is regulated by various cytokines, proteins, and many other complex cellular pathways. Dynamic regulation of osteogenic and antiosteogenic factors is critical to the development of CAC. Several studies have demonstrated that some osteogenic factors, such as bone morphogenic protein (BMP)-2 and BMP-4, are present in CAC [24,25]. Matrix Gla protein (MGP), an inhibitor of BMP, is highly expressed in the intima and media of non-diseased aortas as well as in advanced plaques [24]. Inflammation is also the driving force for the formation of CAC. The apoptosis of smooth muscle cells (SMCs) generates apoptotic bodies that act as nucleating foci of microcalcifications (5–15 μm) [26]. Microcalcifications grow into larger masses with macrophage infiltration into the lipid pool, where they undergo apoptosis and release matrix vesicles [27]. Inflammation increases when the presence of excess cellular debris in the necrotic core exceeds the capacity of apoptotic cell clearance mechanisms [28]. Further progression of calcification with inflammatory stimuli leads to calcified sheets or plates involving collagenous matrix and fibrin deposition. Calcified sheets or plates may fracture and form nodular calcification, which leads to disruption of the intact fibrous cap and acute luminal thrombosis. Furthermore, inflammation induces the differentiation of SMCs into osteogenic cells [29].
In addition to the pathological mechanisms described above, several cardiovascular risk factors may contribute to calcification formation. In the present study, we found that ACS patients with calcification within the culprit plaque were older, had a higher proportion of hypertension, and had worse renal function, which is consistent with the findings of previous studies. It is clear that the prevalence and severity of CAC increases progressively with age [30]. Hypertension may promote calcification formation by damaging the arterial wall. It not only predicts the presence of calcification but also further predicts the extent of calcification [31]. Chronic kidney disease, another well-established risk factor for the progression of CAC, leads to calcification formation through elevated calcium and phosphate levels caused by dysregulated mineral metabolism [32].
In this study, the effect of calcification on plaque stability was investigated in detail using OCT. By comparing the plaque features with different degrees of calcification, plaques with spotty calcification were the most vulnerable among the three groups, which is consistent with the results of several previous studies [33,34]. We also found that the degree of calcification in the ruptured plaques was milder than that in the non-ruptured plaques, which may be because there were more spotty calcifications in the ruptured plaques. A possible mechanism for plaque instability due to spotty calcification is a compliance mismatch between noncompliant calcified plaques and compliant normal vascular tissue [35]. In this case, the plaques become more unstable with an increase in the surface area of the spotty calcification. In addition, spotty calcification alters the structural stresses within the plaque [36], such as peak mechanical stresses, thus increasing the risk of plaque rupture. Moreover, spotty calcification is also associated with plaque progression [10]. This may explain why the MLA of plaques with spotty calcification is smaller than that of others. The definition of spotty calcification used in this study was originally derived from IVUS and just refers to the calcification length and arc. We believe that adding the calcification thickness to the definition of spotty calcification may make the study conclusions more rigorous and more in line with our impression of macrocalcification.
The effect of macrocalcification on plaque stability is complex. Macrocalcification is generally considered a stabilizing factor for plaques, which is consistent with our results [37]. It has been shown that the extent of calcification in unstable plaques does not change significantly over decades, whereas the area of calcification in stable plaques increases progressively with age [8]. However, the changes in lipid length, macrophages and cholesterol crystals in plaques with macrocalcification were significantly different from those in noncalcified plaques. We think the possible reason for this phenomenon is that, regardless of the type of calcification, calcification first appears in the lipid core of the plaque and juxtaposes with the inflammatory cells within the plaque [38]. Based on the pathophysiological process of calcification formation, spotty calcification may represent an early stage of the vascular calcification cascade, with a progressive increase in calcification volume as atherosclerosis progresses [39,40]. At a later stage, M2-type macrophages promote plaque calcification by promoting osteogenic differentiation and maturation of vascular smooth muscle cells (VSMCs) to form macrocalcifications [41]. As a result, macrocalcifications have more lipid components and macrophages than spotty calcifications. For plaque rupture to occur, the plaque structure requires an extremely thin fibrous cap. Therefore, TCFAs and FCT are considered crucial determinants of plaque vulnerability [42,43]. However, these factors were similar between the macrocalcification group and the non-calcification group. Thus, we believe that although the presence of macrocalcifications may mean that the plaque is in an advanced stage, accompanied by many macrophages and cellular debris, it does not truly increase the probability of plaque rupture. In other words, these components may simply appear as a result of the gradual formation of macrocalcification and may not have specific significance. This is a novel finding about macrocalcification and is not contradictory to the previous notion that macrocalcification is considered a stabilizing factor [37].
In non-culprit plaques, we found no significant differences in plaque features between the three calcification subgroups, and even slightly more lipid content in the macrocalcification group than in the spotty calcification group. An IVUS study showed similar results [33]. The reason for this phenomenon may be the limited severity of non-culprit plaques, so that the effect of spotty calcification on the plaques was undermined. Therefore, there was no significant difference in the vulnerability of non-culprit plaques with different degrees of calcification. Moreover, in non-culprit lesions, the difference below the threshold level of plaque rupture is modest and hardly has a significant impact on plaque stability. We suggest that the effect of spotty calcification on plaque vulnerability in culprit plaques exceeds that in non-culprit plaques. However, spotty calcification was not identified as an independent risk factor for plaque rupture in the multivariate analysis and needs to be determined in combination with FCT, lipid load, and microstructure. In addition, we further compared the differences in calcification parameters between culprit and non-culprit plaques and found that calcification arc and lengths were greater in culprit plaques than in non-culprit plaques. A previous autopsy study confirmed that patients who died of acute myocardial infarction (AMI) had a larger area of CAC than those who died of noncardiac disease [5]. Another study found that CAC was correlated with plaque burden [44]. In addition, there was a higher proportion of calcified nodules in culprit plaques. The above factors may be responsible for the more severe degree of calcification in culprit lesions.
5. Study limitations
First, this was a single-centre retrospective study with a limited sample size. Second, to explore the independent effect of spotty calcification and macrocalcification on plaque stability, we excluded patients who had both calcification types within one lesion and those in whom the synergistic effect of both calcification types could not be investigated. Third, we only recorded the morphological parameters of calcifications without analyzing the effect of the calcification number and the anatomical distance of calcifications from the bifurcations on plaque stability. Fourth, because of the lack of sufficient penetration depth of OCT, in the case of thick calcifications and calcifications behind lipid plaque, we can only complete the posterior edge of the calcification manually according to the contour of the calcification. This may result in less accurate calcification thickness measurements. Notably, although IVUS has better penetration, ultrasound will reflect calcification and cannot quantify the extent of calcification. The CT-based calcium score only enables an overall measure of CAC severity at a resolution of 0.25–0.5 mm. Therefore, utilizing OCT imaging for quantifying intraplaque calcification remains a preferred choice. Fifth, although thrombus aspiration was performed prior to imaging, a small residual thrombus may still interfere with calcification quantification. Finally, it has been shown that microcalcifications in the TCFA promote plaque rupture by increasing local tissue stress [45]. However, given the limited resolution of OCT, we were unable to visualize the microcalcifications.
5.1. Ethics declarations
This study was approved by the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University (Approval reference number, KY2017-249). All patients provided written informed consent.
Data Availability
The authors do not have permission to share data.
CRediT authorship contribution statement
Zhifeng Qin: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. Li Yu: Writing – original draft. Yanwen Zhang: Validation, Writing – original draft. Qinglu Xu: Supervision, Writing – review & editing. Chao Li: Software, Visualization. Suhong Zhao: Data curation, Formal analysis. Xiangwen Xi: Formal analysis, Methodology. Yanan Tian: Methodology. Zhao Wang: Funding acquisition, Writing – review & editing. Jinwei Tian: Conceptualization, Funding acquisition, Writing – review & editing. Bo Yu: Conceptualization, Funding acquisition, Writing – review & editing.
Funding statement
This research was supported by the National Natural Science Foundation of China (82370343, U21A20391, 81971715 to JWT), Natural Science Foundation of Heilongjiang Province of China (ZD2023H005 to JWT), Fok Ying-Tong Education Foundation for Young Teachers (171032 to JWT), Heilongjiang Applied Technology Research and Development Plan (GA20C007 to JWT), HMU Marshal Initiative Funding (HMUMIF-21020 to JWT) and Key Laboratory of Emergency and Trauma of Ministry of Education (Hainan Medical University) (Grant. KLET-202117 to JWT).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e23191.
Contributor Information
Zhao Wang, Email: zhaowang92@gmail.com.
Jinwei Tian, Email: tianjinweidr2009@163.com.
Bo Yu, Email: Yubodr@163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Fujino A., et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention. 2018;13:e2182–e2189. doi: 10.4244/eij-d-17-00962. [DOI] [PubMed] [Google Scholar]
- 2.Budoff M.J., et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J. Am. Coll. Cardiol. 2007;49:1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 3.Criqui M.H., et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA. 2014;311:271–278. doi: 10.1001/jama.2013.282535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piepoli M.F., et al. European guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European association for cardiovascular prevention & rehabilitation (EACPR) Eur. Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mauriello A., et al. Coronary calcification identifies the vulnerable patient rather than the vulnerable Plaque. Atherosclerosis. 2013;229:124–129. doi: 10.1016/j.atherosclerosis.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Huang H., et al. The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation. 2001;103:1051–1056. doi: 10.1161/01.cir.103.8.1051. [DOI] [PubMed] [Google Scholar]
- 7.Beckman J.A., Ganz J., Creager M.A., Ganz P., Kinlay S. Relationship of clinical presentation and calcification of culprit coronary artery stenoses. Arterioscler. Thromb. Vasc. Biol. 2001;21:1618–1622. doi: 10.1161/hq0901.095554. [DOI] [PubMed] [Google Scholar]
- 8.Otsuka F., Finn A.V., Virmani R. Do vulnerable and ruptured plaques hide in heavily calcified arteries? Atherosclerosis. 2013;229:34–37. doi: 10.1016/j.atherosclerosis.2012.12.032. [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi M., et al. New insights into spotty calcification and plaque rupture in acute coronary syndrome: an optical coherence tomography study. Heart Ves. 2016;31:1915–1922. doi: 10.1007/s00380-016-0820-3. [DOI] [PubMed] [Google Scholar]
- 10.Kataoka Y., et al. Spotty calcification as a marker of accelerated progression of coronary atherosclerosis: insights from serial intravascular ultrasound. J. Am. Coll. Cardiol. 2012;59:1592–1597. doi: 10.1016/j.jacc.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Shi X., et al. Calcification in atherosclerotic plaque vulnerability: friend or foe? Front. Physiol. 2020;11:56. doi: 10.3389/fphys.2020.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ong D.S., et al. Coronary calcification and plaque vulnerability: an optical coherence tomographic study. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.115.003929. [DOI] [PubMed] [Google Scholar]
- 13.Guidelines for percutaneous transluminal coronary angioplasty. A report of the American college of cardiology/American heart association task force on assessment of diagnostic and therapeutic cardiovascular procedures (subcommittee on percutaneous transluminal coronary angioplasty) J. Am. Coll. Cardiol. 1988;12:529–545. [PubMed] [Google Scholar]
- 14.Virmani R., Burke A.P., Farb A., Kolodgie F.D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 2006;47:C13–C18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 15.Kato K., et al. Nonculprit plaques in patients with acute coronary syndromes have more vulnerable features compared with those with non-acute coronary syndromes: a 3-vessel optical coherence tomography study. Circ Cardiovasc Imaging. 2012;5:433–440. doi: 10.1161/CIRCIMAGING.112.973701. [DOI] [PubMed] [Google Scholar]
- 16.Araki M., et al. Optical coherence tomography in coronary atherosclerosis assessment and intervention. Nat. Rev. Cardiol. 2022;19:684–703. doi: 10.1038/s41569-022-00687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prati F., et al. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur. Heart J. 2010;31:401–415. doi: 10.1093/eurheartj/ehp433. [DOI] [PubMed] [Google Scholar]
- 18.Tearney G.J., et al. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation. 2003;107:113–119. doi: 10.1161/01.cir.0000044384.41037.43. [DOI] [PubMed] [Google Scholar]
- 19.Kitabata H., et al. Relation of microchannel structure identified by optical coherence tomography to plaque vulnerability in patients with coronary artery disease. Am. J. Cardiol. 2010;105:1673–1678. doi: 10.1016/j.amjcard.2010.01.346. [DOI] [PubMed] [Google Scholar]
- 20.Qin Z., et al. Cholesterol crystals in non-culprit plaques of STEMI patients: a 3-vessel OCT study. Int. J. Cardiol. 2022;364:162–168. doi: 10.1016/j.ijcard.2022.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Tearney G.J., et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J. Am. Coll. Cardiol. 2012;59:1058–1072. doi: 10.1016/j.jacc.2011.09.079. [DOI] [PubMed] [Google Scholar]
- 22.Zhang S., et al. Non-culprit plaque characteristics in acute coronary syndrome patients with raised hemoglobinA1c: an intravascular optical coherence tomography study. Cardiovasc. Diabetol. 2018;17:90. doi: 10.1186/s12933-018-0729-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tintut Y., et al. Multilineage potential of cells from the artery wall. Circulation. 2003;108:2505–2510. doi: 10.1161/01.CIR.0000096485.64373.C5. [DOI] [PubMed] [Google Scholar]
- 24.Dhore C.R., et al. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 25.Roijers R.B., et al. Microcalcifications in early intimal lesions of atherosclerotic human coronary arteries. Am. J. Pathol. 2011;178:2879–2887. doi: 10.1016/j.ajpath.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milzi A., et al. Type 2 diabetes mellitus is associated with a lower fibrous cap thickness but has no impact on calcification morphology: an intracoronary optical coherence tomography study. Cardiovasc. Diabetol. 2017;16:152. doi: 10.1186/s12933-017-0635-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.New S.E., et al. Macrophage-derived matrix vesicles: an alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ. Res. 2013;113:72–77. doi: 10.1161/CIRCRESAHA.113.301036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Libby P., Tabas I., Fredman G., Fisher E.A. Inflammation and its resolution as determinants of acute coronary syndromes. Circ. Res. 2014;114:1867–1879. doi: 10.1161/CIRCRESAHA.114.302699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abedin M., Tintut Y., Demer L.L. Vascular calcification: mechanisms and clinical ramifications. Arterioscler. Thromb. Vasc. Biol. 2004;24:1161–1170. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 30.Gerke O., et al. Prevalence and extent of coronary artery calcification in the middle-aged and elderly population. Eur J Prev Cardiol. 2022;28:2048–2055. doi: 10.1093/eurjpc/zwab111. [DOI] [PubMed] [Google Scholar]
- 31.Nicoll R., Zhao Y., Ibrahimi P., Olivecrona G., Henein M. Diabetes and hypertension consistently predict the presence and extent of coronary artery calcification in symptomatic patients: a systematic review and meta-analysis. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17091481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shanahan C.M. Mechanisms of vascular calcification in CKD-evidence for premature ageing? Nat. Rev. Nephrol. 2013;9:661–670. doi: 10.1038/nrneph.2013.176. [DOI] [PubMed] [Google Scholar]
- 33.Amano H., et al. Assessment of angiographic coronary calcification and plaque composition in virtual histology intravascular ultrasound. J Interv Cardiol. 2015;28:205–214. doi: 10.1111/joic.12189. [DOI] [PubMed] [Google Scholar]
- 34.Kataoka Y., et al. Spotty calcification and plaque vulnerability in vivo: frequency-domain optical coherence tomography analysis. Cardiovasc. Diagn. Ther. 2014;4:460–469. doi: 10.3978/j.issn.2223-3652.2014.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wada S., et al. Rupture of donor ascending aorta following heart transplantation. Hiroshima J. Med. Sci. 1994;43:73–76. [PubMed] [Google Scholar]
- 36.Hoshino T., et al. Mechanical stress analysis of a rigid inclusion in distensible material: a model of atherosclerotic calcification and plaque vulnerability. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H802–H810. doi: 10.1152/ajpheart.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pugliese G., Iacobini C., Blasetti Fantauzzi C., Menini S. The dark and bright side of atherosclerotic calcification. Atherosclerosis. 2015;238:220–230. doi: 10.1016/j.atherosclerosis.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Aikawa E., et al. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–2850. doi: 10.1161/circulationaha.107.732867. [DOI] [PubMed] [Google Scholar]
- 39.Reith S., Milzi A., Dettori R., Marx N., Burgmaier M. Predictors for target lesion microcalcifications in patients with stable coronary artery disease: an optical coherence tomography study. Clin. Res. Cardiol. 2018;107:763–771. doi: 10.1007/s00392-018-1243-1. [DOI] [PubMed] [Google Scholar]
- 40.Mori H., et al. Coronary artery calcification and its progression: what does it really mean? JACC Cardiovasc Imaging. 2018;11:127–142. doi: 10.1016/j.jcmg.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 41.Shioi A., Ikari Y. Plaque calcification during atherosclerosis progression and regression. J Atheroscler Thromb. 2018;25:294–303. doi: 10.5551/jat.RV17020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Virmani R., Kolodgie F.D., Burke A.P., Farb A., Schwartz S.M. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 43.Zheng G., Chen J., Lin C., Huang X., Lin J. Effect of statin therapy on fibrous cap thickness in coronary plaques using optical coherence tomography: a systematic review and meta-analysis. J Interv Cardiol. 2015;28:514–522. doi: 10.1111/joic.12245. [DOI] [PubMed] [Google Scholar]
- 44.Mintz G.S., et al. Determinants and correlates of target lesion calcium in coronary artery disease: a clinical, angiographic and intravascular ultrasound study. J. Am. Coll. Cardiol. 1997;29:268–274. doi: 10.1016/s0735-1097(96)00479-2. [DOI] [PubMed] [Google Scholar]
- 45.Kelly-Arnold A., et al. Revised microcalcification hypothesis for fibrous cap rupture in human coronary arteries. Proc Natl Acad Sci U S A. 2013;110:10741–10746. doi: 10.1073/pnas.1308814110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.