Abstract
E-waste processing sites abound with potentially toxic elements (PTE) that negatively affect the environment and human health. The study determined the presence of selected PTE (Cu, Zn, Pb, Hg, and Al) and their spatial distribution in an e-waste processing site in a developing country setting. pH, moisture, organic matter/carbon, and particle size were determined in 30 soil samples. The spatial position of each sampling point was picked with a GPS device, and the area was mapped in a GIS environment. The concentrations of PTE were determined with an atomic absorption spectrophotometer. Findings from the study indicate that the soil is polluted with PTE, rich in organic carbon/matter but has low pH. The Geoaccumulation Indices ranged from unpolluted (Al) to strongly/extremely polluted (Cu). Pollution Load Index showed about 77 % of the samples as extremely/heavily polluted, 10 % as heavily polluted, and 13 % as moderately polluted. Contamination Factors of Zn, Pb, and Cu were very high but considerably low for Hg and Al. Regular monitoring and remediation are required for the soil to be restored and put into productive use.
Keywords: E-waste pollution, Spatial distribution, Potentially toxic elements, Pollution indices, Geographic information system
1. Introduction
The use of electronic and electrical equipment (EEE) in this era of technological advancement has resulted in the production of electronic waste (e-waste), which is a major source of pollution. E-waste poses a health and environmental hazard because of its constituent toxic substances, such as mercury, which can cause brain damage in humans and adversely affect coordination. Globally, it is the domestic waste stream with the highest production rate and is predicted to increase from 53.6 metric tons in 2019 to 74 metric tons by 2030, showing a 21 % and double increment in 5 and 16 years, respectively [1]. This growth rate is due to high consumption rates, short life cycles, and few repair options for EEE. Globally, the descending order of e-waste generation in metric tons is as follows: Asia (24.9), America (13.1), Europe (12.0), Africa (2.9), and Oceania (0.7) [1]. West Africa is the primary route of importation in Africa, with Ghana and Nigeria leading [2]. In 2009 alone, Ghana imported 215,000 tons of new/used EEE and generated 129,000 tons of e-waste [3]. This shows a hike in the generation of e-waste in Ghana.
EEE consists of chemicals that are toxic to humans and are termed PTE [4]. Examples are mercury, lead, cadmium, arsenic, nickel, chromium, thallium, beryllium, indium, etc. Potentially toxic elements can be defined as chemical elements whose density is at least five times greater than that of water and poisonous in low concentrations [4,5]. Naturally, they occur in low concentrations in soils [6], though their levels can be increased by anthropogenic activities during which levels their effects are exhibited. Potentially toxic elements affect organisms’ survival, growth, and reproduction, and depending on the duration of exposure, they can be carcinogenic, mutagenic, and teratogenic [7]. When exposed to PTE, plants undergo oxidative stress, leading to cellular damage and disturbance of cellular ionic homeostasis. However, some beneficial ones like Zn and Cu aid in human metabolism in trace quantities; though they have adverse effects beyond recommended levels [8]. Additionally, Zn and Pb have been identified as non-biodegradable, with the latter and Hg identified as very poisonous with no function in organisms, and together with Al, are known to be carcinogenic [[8], [9], [10], [11]]. Awareness of the toxicities of Cu, Hg, Pb, Zn, and Al renders them a focus for investigations.
Improper e-waste management negatively impacts the air, soil, and water. Common disposal and management practices include burning wire cables, open-air melting of motherboards to extract metals and valuable chips, and rigorous dismantling to obtain copper and aluminium [12]. Such practices cause environmental pollution and endanger human health. Burning and melting of e-waste to extract metals and chips by scrap dealers produce toxic organic emissions like polychlorinated biphenyls, polybrominated diphenyl ethers, and polycyclic aromatic hydrocarbons; while crushing and beating contaminate the soil with heavy metals, which can drain into nearby water bodies or farmlands, absorbed by plants and get bioaccumulated along the food chain [13]. Soils of e-waste sites, thus, have high concentrations of PTE, posing health and ecological risks [12,14,15]. Soil serves as the primary sink of PTE released from e-waste despite being an integral part of life and acting as a support system. They provide an anchorage for roots, accommodate a myriad of beneficial organisms, and serve as a reservoir of water and nutrients.
Analysis of PTE’s source, concentration, and spatial distribution characteristics is essential for controlling PTE pollution. However, such knowledge has not received much attention in Ghana, though there are major e-waste sites like the Akwatia-Line at Kumasi, where tons of e-waste are disposed of, and scavengers retrieve their valuable components, causing the release of PTE into the environment. Significant accomplishments in assessing PTE pollution within sectors such as the mining industry have been made, but not in areas greatly influenced by activities of the informal sector such as e-waste processing, particularly in developing countries. Some informal economic activities release PTE that pollute environmental matrices like soil and endanger human health [16]. The possibility of diverse concentrations and characteristic spatial distribution of PTE is related to different sources of soil PTE pollution in such areas [17]. Investigating PTE in the soil at highly dense and commercial zones has become essential since such sites have several metal extraction enterprises, residential areas, and roads; thus, the pollution of soil by PTE in Akwatia-Line cannot be ignored.
Different pollution indices such as pollution load, contamination factor, geoaccumulation, and potential ecological risk have been employed in assessing the PTE composition of soils. In contemporary times, geostatistical methods such as “Kriging” have been used to explain and forecast spatial variability of specific soil parameters and values of non-sampled areas. For traditional statistical methods, data must be subject to a series of assumptions like broad and repeated sampling and independent observations [18]. Also, principal component analysis has been employed to study PTE pollution in soils [19]. Their results show relationships between the spatial distribution and the relative importance of each metal. Against this background, this study sought to determine the concentration and analyze the spatial distribution of PTE in the e-waste contaminated site at Akwatia-Line in Kumasi, Ghana.
2. Materials and methods
2.1. Study site
The study was conducted at Akwatia-Line, a suburb in Kumasi, the capital city of the Ashanti Region of Ghana. It is positioned at a latitude of 6° 41′ 56.7″ north and a longitude of 1° 36′ 29.6″ west [20], as indicated in Fig. 1. Kumasi is a tropical city with a relatively constant temperature throughout the year and receives an average annual rainfall of 1448 mm. Akwatia-Line is a slum founded in 1998 and located along the disused railway line in Kumasi. Before its establishment, the area was waterlogged, with only one or two functioning milling enterprises. With time, the area was filled with biomass residue and reclaimed for space as the youth population increased. The commonest economic activity is collecting and retrieving important or reusable parts from scraps by burning and beating certain abandoned equipment and dismantling e-waste for valuable metals such as Cu and Al. This renders portions of the soil surface dark with ashes and residues, while others are covered with piles of e-waste and plastics.
Fig. 1.
Map of Ghana showing the study site within the Ashanti Region.
2.2. Materials and equipment
The materials/equipment used for the study include a soil auger, Ziploc bags, sampling bottles, pH meter (Horiba pH meter D-51), analytical balance (OHAUS Advance AR 3130 Model), Ophenanthrene-ferrous complex (ferroin) indicator solution, digester, atomic absorption spectrophotometer, and ArcGIS 10.3 Software.
2.3. Sample collection and preparation
Stratified random sampling was used to select the 30 sampling points. A composite sample was made at each point from soil samples taken at 5, 10, and 15 cm depths using a 15 cm soil auger. The 30 samples were collected from 10 different strata at 60 m intervals. A control sample was collected from an undisturbed area. The samples were packaged and sealed in labeled Ziploc bags and transferred to the laboratory for analysis. The soil profile was determined by filling a transparent bottle with some soil samples, and clean water was added at a volume greater than the amount of soil in the bottle. The bottle was then closed, shaken for some minutes, and allowed to rest. After a day, it was recorded that the particles settled according to their sizes; thus, gravels settled at the bottom, followed by sand, silt, clay, and humus.
2.4. Determination of physico-chemical parameters
2.4.1. pH
The pH of the soil samples was determined with reference to Olayinka et al. [21]. The samples were air-dried. 10 g was then weighed into a 100 mL beaker, after which 20 mL of distilled water was added. The mixture stood for 30 min, with intermittent stirring with a glass rod. The pH meter (Horiba pH meter D-51) was calibrated, its electrode inserted into the suspension, and the pH of the soil was measured.
2.4.2. Moisture content
The moisture content of the samples was determined with reference to Baillie et al. [22]. 1 g of a representative sample was placed in a clean, dry crucible of known mass. The mass of the container and soil were determined () using an analytical balance (OHAUS Advance AR 3130 Model). The crucible was placed in an oven maintained at 110 ± 5 for 4 h to obtain a constant weight (). Moisture content (%) was determined using Equation (1), where W1 and W2 represent the weight of the crucible and sample before and after oven drying, respectively.
| 1 |
2.4.3. Particle size analysis
The samples' particle size was analyzed by modifying the Bouyoucos Method as indicated by Beretta et al. [23]. 50 g of the soil sample was soaked with 50 mL of Calgon solution, and the mixture stood overnight. The mixture was then transferred into a 1000 mL measuring cylinder and was made up to the mark. The mixture was then shaken and left for 40 s. The hydrometer was inserted into the mixture to determine the particle size (firstly, sand content). The clay and silt were determined after 3 h through the same process. The temperatures were recorded simultaneously, and %sand, %clay, and %silt were determined.
2.4.4. Organic matter
The Walkey-Black Method was used to determine the organic carbon/matter content of the soil samples [22]. One gram of the sample was placed into a block digester tube, and 5 mL of K₂Cr₂O₇ solution and 7.5 mL of concentrated H2SO4 were added. The tube was placed in a pre-heated block at 145–155 for 30 min, then removed and allowed to cool. The digest was quantitatively transferred into a 100 mL conical flask. 0.3 mL of Ophenanthrene-ferrous complex (ferroin) indicator solution was added and stirred using a magnetic stirrer. The digest was then titrated with (NH₄)₂Fe(SO₄)₂·6H₂O solution with an endpoint indicating a change from greenish to brown coloration.
2.5. Determination of PTE
HNO3 and HCl were added to 1 g of each sample in a 100 mL flask in the ratio of 1:3. Each of the mixtures was loaded onto a microwave carousel for heating until the soil turned colorless, then cooled by adding water and filtered. An atomic absorption spectrophotometer (Agilent 55 AA G8430A) was then used to determine the PTE concentrations.
2.6. Mapping and spatial distribution
Coordinates of the sampling points, physico-chemical parameters, and concentrations of PTE were exported into MS Excel to obtain data on the GPS coordinates against the physico-chemical parameters and concentrations of heavy metals. Spatial distribution maps of the physico-chemical parameters and heavy metals were created using the quantities technique of graduated symbols method with the help of ArcGIS 10.3 Software. The GPS data obtained were in Keyhole Markup Language (KML) file format. A spatial database was created from the exported data using the Arc Toolbox in the ArcMap, and with the help of the conversion tools, the GPS data in KML file format was converted to layer. The spatial database was enhanced to store and access spatial data or data that defines geometric space. Through this method, the shapefile for Akwatia-Line was created. The layer created contained a geodatabase of the coordinates taken during the fieldwork. The coordinates within the geodatabase were saved as points in the layer. The points in the layer were added to an Open Street base map of Akwatia-Line and processed to generate a shapefile of the area. This was performed to gain an insight into each parameter's pollution level and their comparison with each other.
The spatial interpolation was done using the Inverse Distance Weighted (IDW) technique. Using values from nearby weighted locations, the IDW technique computes an average value for sampled and unsampled locations. The weights are proportional to the proximity of the sampled points to the unsampled location and can be specified by the IDW power coefficient. The larger the power coefficient, the stronger the weight of nearby points, as shown in Equation (2), which estimates the value z at an unsampled location j [24].
| 2 |
The carat ^ above z indicates that the value is estimated at j. The parameter n is the weight parameter applied as an exponent to the distance, thus amplifying the irrelevance of a point at location ii as the distance to j increases. A large n results in nearby points exerting a much greater influence on the unsampled location than a point further away, resulting in an interpolated output looking like a Thiessen interpolation. On the other hand, a minimal value of n will give all points within the search radius equal weight such that all unsampled locations will represent nothing more than the mean values of all sampled points within the search radius.
2.7. Pollution indices
2.7.1. Geoaccumulation Index
Geoaccumulation Index (Igeo) was used in assessing PTE contamination in the soil. This was done by comparing heavy metals concentration in the soil to the background concentration of undisturbed land areas as applied by Nweke & Ukpai [25]. Igeo was computed using Equation (3), where Cn denotes the measured concentration, and Bn is the geochemical background value in the soil of undisturbed land. A factor of 1.5 is used because of the possible variations in background values for a given metal in the environment. The classification of metals’ pollution status based on Igeo is presented in Table 1.
| 3 |
Table 1.
Classification of geoaccumulation index [26].
| Igeo | Igeo Class | Soil quality |
|---|---|---|
| <0 | 0 | Practically unpolluted |
| 0–1 | 1 | Unpolluted to moderately polluted |
| 1–2 | 2 | Moderately polluted |
| 2–3 | 3 | Moderately to strongly polluted |
| 3–4 | 4 | Strongly polluted |
| 4–5 | 5 | Strongly to extremely polluted |
| >5 | 6 | Extremely polluted |
2.7.2. Contamination Factor
Contamination Factor (CF) was used to assess the quantities of the element in the samples normalized over that of the pre-industrial baseline value for the element. This was computed using Equation (4), where CPTE is the concentration of the metal of interest, and Cbackground is the baseline value. The contamination levels of the metals were classified based on their intensities on a scale of 1–6: ≤0 (not polluted), 0–1 (not polluted to medium pollution), 1–2 (moderately polluted), 2–3 (moderate to strong pollution), 4 (strongly polluted), 4–5 (strong to very strong pollution), and 6 (very strong pollution).
| 4 |
2.7.3. Pollution Load Index
Pollution Load Index (PLI) was used to comparatively assess the level of PTE pollution using Equation (5), where n is the number of assessed metals and Cf is the contamination factor of the individual metals. The PLI levels were classified as no pollution (PLI<1), moderate pollution (1< PLI<2), heavy pollution (2<PLI<3), and extremely heavy pollution (3<PLI) as applied by previous studies [27,28].
| 5 |
2.8. Data analysis
Descriptive statistics such as mean, standard error, and minimum and maximum values at a confidence level of 95 % were used to summarize the data. A correlational analysis was performed to determine the relationship between the physico-chemical and PTE parameters. Significance for the analysis was accepted at 5 %.
3. Results and discussion
3.1. Physico-chemical parameters
Table 2 presents summarized findings of the physico-chemical parameters of the soil samples. The organic carbon (OC) contents (%) ranged from 0.32 to 3.43, with a mean value of 2.15 ± 0.17, close to the control sample's value (2.20). The mean organic matter (OM) content (%) was 3.71 ± 0.29, which was also comparable to that of the control sample (3.78). The minimum and maximum OM values were 0.55 and 5.92, respectively. Operations of sawmill and rice mill shops within the area and the eventual decaying sawdust and rice husk might have accounted for the OC and OM contents. Increasing OC content correlated positively with OM content, with a Correlation Coefficient of 0.999. This confirms the report by the Department of Primary Industries and Regional Development of West Australia's Agriculture and Food that 58 % of the mass of soil organic matter content exists as carbon [25].
Table 2.
Physico-chemical parameters in the soil samples.
| Sample ID | Sand (%) | Clay (%) | Silt (%) | Textural class | OC (%) | OM (%) | pH |
|---|---|---|---|---|---|---|---|
| 1 M | 71.08 | 4.08 | 24.84 | Sandy loam | 2.20 | 3.78 | 5.26 |
| 1R | 89.16 | 4.08 | 6.76 | Sand | 2.20 | 3.78 | 5.20 |
| 1L | 59.24 | 16.00 | 24.76 | Sandy loam | 0.68 | 1.17 | 5.13 |
| 2 M | 84.60 | 6.00 | 9.40 | Loamy sand | 1.76 | 3.03 | 5.26 |
| 2R | 74.64 | 4.00 | 21.36 | Sandy loam | 2.20 | 3.78 | 5.23 |
| 2L | 73.00 | 14.12 | 12.88 | Sandy loam | 0.48 | 0.83 | 5.49 |
| 3 M | 84.64 | 4.00 | 11.36 | Loamy sand | 2.20 | 3.78 | 5.40 |
| 3R | 58.72 | 17.92 | 23.36 | Sandy loam | 2.83 | 4.88 | 5.49 |
| 3L | 94.72 | 2.56 | 2.72 | Sand | 3.07 | 5.30 | 5.45 |
| 4 M | 95.68 | 2.56 | 1.76 | Sand | 2.91 | 5.02 | 5.43 |
| 4R | 85.52 | 2.56 | 11.92 | Loamy sand | 3.03 | 5.23 | 5.46 |
| 4L | 87.68 | 4.56 | 7.76 | Sand | 3.31 | 5.71 | 5.49 |
| 5 M | 93.52 | 3.84 | 2.64 | Sand | 2.39 | 4.13 | 5.75 |
| 5R | 84.32 | 8.08 | 7.60 | Loamy sand | 1.36 | 2.34 | 5.64 |
| 5L | 89.72 | 2.56 | 7.72 | Sand | 3.07 | 5.30 | 5.48 |
| 6 M | 87.36 | 5.84 | 6.80 | Loamy sand | 3.43 | 5.92 | 5.61 |
| 6R | 65.52 | 3.84 | 30.64 | Sandy loam | 0.96 | 1.65 | 5.52 |
| 6L | 79.44 | 7.84 | 12.72 | Loamy sand | 2.39 | 4.13 | 5.71 |
| 7 M | 86.80 | 4.08 | 9.12 | Loamy sand | 0.88 | 1.51 | 6.01 |
| 7R | 89.16 | 4.04 | 6.80 | Sand | 0.76 | 1.31 | 5.79 |
| 7L | 95.36 | 2.68 | 1.96 | Sand | 3.43 | 5.92 | 6.02 |
| 8 M | 84.64 | 4.12 | 11.24 | Loamy sand | 2.27 | 3.92 | 5.36 |
| 8R | 84.60 | 5.64 | 9.76 | Loamy sand | 2.67 | 4.61 | 5.11 |
| 8L | 63.32 | 14.00 | 22.68 | Sandy loam | 2.27 | 3.92 | 5.77 |
| 9 M | 82.60 | 5.64 | 11.76 | Loamy sand | 1.86 | 3.23 | 5.36 |
| 9R | 84.76 | 6.04 | 9.20 | Loamy sand | 3.19 | 5.50 | 5.76 |
| 9L | 82.64 | 6.32 | 11.04 | Loamy sand | 0.32 | 0.55 | 5.73 |
| 10 M | 86.72 | 4.00 | 9.28 | Loamy sand | 2.99 | 5.16 | 5.54 |
| 10R | 87.84 | 4.56 | 7.60 | Loamy sand | 2.0 | 3.44 | 5.82 |
| 10L | 79.40 | 6.56 | 14.04 | Loamy sand | 1.48 | 2.55 | 5.51 |
| Minimum Maximum Mean ± SE |
58.72 95.68 82.21 ± 1.85 |
2.56 17.92 6.07 ± 0.74 |
1.76 30.64 11.72 ± 1.35 |
0.32 3.43 2.15 ± 0.17 |
0.55 5.92 3.71 ± 0.29 |
5.11 6.02 5.62 ± 0.04 |
|
| Control | 61.24 | 7.92 | 30.84 | Sandy loam | 2.20 | 3.78 | 5.62 |
Note: 1 M = Point 1 at middle, 1R = Point 1 at right, 1L = point 1 at left, OM= Organic matter, OC= Organic carbon.
Table 2 depicts three classes of soil texture: sand, sandy loam, and loamy sand, all with high sand contents (%). Sandy soils have large coarse aggregates and bear large pores, which allow infiltration, accounting for their low moisture content [26]. Loamy sand was the most dominant class, accounting for about 50 % of the total samples, followed by sand, 26.7 %, and sandy loam, 23.3 %. The texture of the control sample was sandy loam.
The pH of the control samples and the mean pH values were the same (5.62 ± 0.04); almost all the individual pH values revolved around the mean, signifying mild acidic soil within the area. pH showed a weak negative relationship with OM (r = −0.023). Generally, pH is highly influenced by soil texture; thus, soil with low clay and high sand content has a low buffering capacity and high rate of percolation and infiltration, rendering it susceptible to acidification. Thus, the sandy nature of the soil might have caused the leaching of basic cations by rainfall over time due to its high porosity, permeability, and low water-holding capacity [27].
3.2. PTE in soil samples
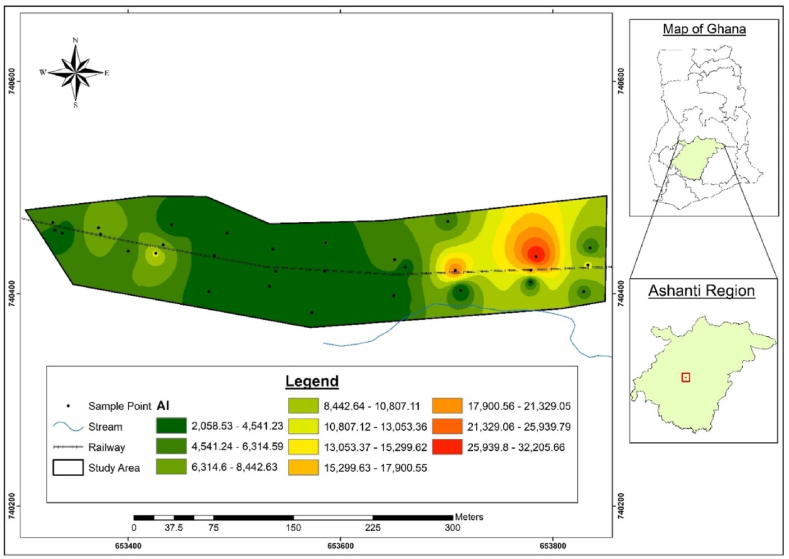
The PTE concentrations of the samples are presented in Table 3. The low concentration of the heavy metals in the control samples shows that their abundance in the soil at Akwatia-Line might have resulted from the processing of e-waste by scrap dealers. Generally, the order of increasing concentrations of the heavy metals was Al > Cu > Zn > Pb > Hg. The levels of aluminium, the most abundant metal ranged from 2057.00 to 32215.22 mg/kg, with a mean value of 6546.13 ± 1168.70 mg/kg, which was greater than the concentration of the control. This could be a result of dismantling the e-waste since Al is used as a casing for different kinds of electronic devices and most computer parts, and in power lines and electric motors. It could also be a result of the low pH recorded in the area because low soil pH makes Al soluble and increases its concentration in the soil solution [28]. The concentration of Al in the control was comparably high (5151.37 mg/kg). This indicates that Al is the most abundant metal in the earth's crust [29]; as such, the abundance of Al in the soil can partly be attributed to nature.
Table 3.
PTE concentrations in the soil samples (mg/kg).
| Sample ID | Cu | Hg | Pb | Zn | Al |
|---|---|---|---|---|---|
| 1 M | 10860.49 | 0.17 | 507.57 | 682.62 | 3492.47 |
| 1R | 246.27 | 0.11 | 110.79 | 360.27 | 2856.59 |
| 1L | 33.79 | 0.11 | 23.21 | 90.89 | 6188.80 |
| 2 M | 4932.80 | 2.23 | 1314.87 | 3771.71 | 7520.04 |
| 2R | 7520.04 | 0.07 | 63.25 | 376.69 | 4758.35 |
| 2L | 37.64 | 0.19 | 78.00 | 152.01 | 8129.63 |
| 3 M | 597.78 | 0.13 | 227.40 | 779.27 | 6289.39 |
| 3R | 155.12 | 0.06 | 109.08 | 420.93 | 11395.21 |
| 3L | 67.91 | 0.09 | 43.53 | 261.30 | 2057.00 |
| 4 M | 512.74 | 0.12 | 293.79 | 414.36 | 2197.17 |
| 4R | 137.75 | 0.21 | 100.15 | 881.16 | 3132.44 |
| 4L | 68.85 | 0.06 | 34.38 | 162.15 | 3912.62 |
| 5 M | 1299.74 | 0.15 | 200.70 | 725.84 | 4029.54 |
| 5R | 92.76 | 0.20 | 77.28 | 540.01 | 3759.47 |
| 5L | 206.41 | 0.06 | 100.56 | 488.88 | 4186.88 |
| 6 M | 166.81 | 0.07 | 41.31 | 325.14 | 2891.71 |
| 6R | 105.28 | 0.09 | 59.13 | 9634.54 | 2413.15 |
| 6L | 44.86 | 0.13 | 83.07 | 1171.60 | 4553.05 |
| 7 M | 856.79 | 0.14 | 563.42 | 650.91 | 3832.99 |
| 7R | 1861.16 | 0.38 | 316.12 | 1113.42 | 2521.85 |
| 7L | 5086.52 | 0.22 | 729.10 | 1286.45 | 5141.75 |
| 8 M | 34430.07 | 0.52 | 14666.96 | 5320.87 | 19869.84 |
| 8R | 2476.12 | 2.53 | 669.18 | 1110.93 | 2156.17 |
| 8L | 174.93 | 0.08 | 176.74 | 78.66 | 5514.44 |
| 9 M | 27788.37 | 1.10 | 11774.09 | 7427.48 | 16740.03 |
| 9R | 54.93 | 0.05 | 19.51 | 51.92 | 3065.81 |
| 9L | 1175.30 | 0.13 | 371.06 | 651.42 | 32215.22 |
| 10 M | 10003.41 | 1.18 | 2852.86 | 2105.78 | 11139.92 |
| 10R | 468.17 | 0.63 | 475.51 | 754.76 | 5588.26 |
| 10L | 275.74 | 0.24 | 299.88 | 236.74 | 4834.02 |
| Minimum | 33.79 | 0.05 | 19.51 | 51.92 | 2057.00 |
| Maximum | 34430.07 | 2.53 | 14666.96 | 9634.54 | 32215.22 |
| Mean ± SE | 3724.62 ± 1470.31 | 0.38 ± 0.11 | 1212.75 ± 608.75 | 1400.13 ± 410.41 | 6546.13 ± 1168.70 |
| Control sample | 17.86 | 0.009 | 25.79 | 37.85 | 5151.37 |
| WHO guideline | 36.00 | 2.00 | 85.00 | 50.00 | – |
Note: 1 M = Point 1 at middle, 1R = Point 1 at right, 1L = point 1 at left.
The concentration of Cu in the samples ranged from 33.79 to 34430.07 mg/kg with a mean value of 3724.62 ± 1470.31 mg/kg. Copper is the most predominant heavy metal found in all electronic gadgets. It is the electrical conductor found in most electrical wirings for the purpose of power generation, transmission, distribution, telecommunications, electronics circuitry, etc. The recovery of Cu through open burning at the e-waste site in Akwatia-Line could account for the high concentration of Cu. Copper was the second most abundant PTE and all the concentrations exceeded the control value of 17.86 mg/kg and the WHO guideline value of 36.00 mg/kg.
Zinc, the third most abundant PTE, recorded a mean concentration of 1400.13 ± 410.41 mg/kg with respective minimum and maximum values of 51.92 mg/kg and 9634.54 mg/kg. All the Zn concentrations in the samples exceeded the WHO guideline limit of 50.00 mg/kg. The mean Zn concentration was higher than the WHO guideline value of 50.00 mg/kg and the control value of 37.85 mg/kg. This could be attributed to the burning of e-waste materials because zinc is usually found in the interior of cathode ray tube screens as zinc sulphide. Zinc is a vital microelement that facilitates enzyme processes. However, the amount of zinc in the soil varies depending on the kind of soil [30].
The concentration of Pb ranged from 19.51 to 14666.96 mg/kg. The mean Pb concentration in the samples (1212.75 ± 608.28 mg/kg) exceeded the WHO guideline limit (85.00 mg/kg) and the control value of 25.79 mg/kg. The abundance of Pb in the samples from the study area could result from burning e-waste (e.g., used computers, refrigerators, printers, photocopy machines, cables, automobile batteries, tires, and air conditioners) as it is used to provide electric connection. The mean value observed, however, was lower than the mean value of 2645.31 mg/kg in soil from an e-waste recycling site reported by Pradhan & Kumar [31].
There was comparatively least abundance of Hg; its mean value was 0.38 ± 0.11 mg/kg, which was below the WHO guideline of 2.00 mg/kg. Its minimum and maximum values were 0.05 and 2.53 mg/kg, respectively. Generally, the occurrence of Hg was the least among the PTE, with almost all the concentrations below the WHO guideline. The control value was also less than all the concentrations recorded. This low concentration might be caused by the fast evaporation of Hg into organo-mercury compounds [32].
3.3. Pollution indices
3.3.1. Geoaccumulation Index
Table 4 presents the Geoaccumulation Indices of the PTE analyzed. The mean value for Cu was 4.37 ± 0.54, suggesting strongly to extremely polluted soil with Cu. The minimum and maximum values were 0.34 and 10.33, respectively. Mercury had a mean value of 0.65 ± 0.28, which indicates unpolluted to moderately polluted soil with Hg. This might be due to the low mercury content in the e-waste as well as its evaporation from the soil over time, as reported by Forti et al. [1]. Its minimum and maximum values were respectively −1.22 and 4.40. For Pb, the mean value was 2.52 ± 0.43, an indication of moderately to strongly polluted condition that emanates from e-waste activities at the site. A similar reason pertains to Zn with a mean value of 3.46 ± 0.33, indicating strong pollution. Its minimum value was −0.13 and the maximum was 7.41. Unlike the other metals, Al showed no pollution at the site, and Igeo values ranged from −1.91-2.06, with a mean value of −0.63 ± 0.18. Using the mean values of the various heavy metals; the site is strongly to extremely polluted with Cu, Hg showed unpolluted to moderately polluted condition, and Pb showed moderately to strongly polluted condition. The site was strongly polluted with Zn but it was practically unpolluted with Al. It can, therefore be said that Cu > Zn > Pb > Hg > Al in terms of pollution, and this could be attributed to the scrap activities in the area [24].
Table 4.
Geoaccumulation indices.
| Sample ID | Cu | Hg | Pb | Zn | Al |
|---|---|---|---|---|---|
| 1 M | 8.66 | 0.53 | 3.71 | 3.59 | −1.15 |
| 1R | 3.20 | −0.07 | 1.52 | 2.67 | −1.44 |
| 1L | 0.34 | −0.11 | −0.74 | 0.68 | −0.32 |
| 2 M | 7.53 | 4.22 | 5.09 | 6.05 | −0.04 |
| 2R | 8.13 | −0.83 | 0.71 | 2.73 | −0.70 |
| 2L | 0.49 | 0.70 | 1.01 | 1.42 | 0.07 |
| 3 M | 4.48 | 0.12 | 2.56 | 3.78 | −0.30 |
| 3R | 2.53 | −0.97 | 1.50 | 2.89 | 0.56 |
| 3L | 1.34 | −0.38 | 0.17 | 2.2 | −1.91 |
| 4 M | 4.26 | −0.06 | 2.93 | 2.87 | −1.81 |
| 4R | 2.36 | 0.83 | 1.37 | 3.96 | −1.30 |
| 4L | 1.36 | −1.10 | −0.17 | 1.51 | −0.98 |
| 5 M | 5.60 | 0.33 | 2.38 | 3.68 | −0.94 |
| 5R | 1.79 | 0.76 | 1.00 | 3.25 | −1.04 |
| 5L | 2.95 | −0.94 | 1.38 | 3.11 | −0.88 |
| 6 M | 2.64 | −0.79 | 0.1 | 2.52 | −1.42 |
| 6R | 1.98 | −0.35 | 0.61 | 7.41 | −1.68 |
| 6L | 0.74 | 0.11 | 1.10 | 4.37 | −0.76 |
| 7 M | 5.00 | 0.24 | 3.87 | 3.52 | −1.01 |
| 7R | 6.12 | 1.65 | 3.03 | 4.29 | −1.62 |
| 7L | 7.57 | 0.91 | 4.24 | 4.5 | −0.59 |
| 8 M | 10.33 | 2.13 | 8.57 | 6.55 | 1.36 |
| 8R | 6.53 | 4.40 | 4.11 | 4.29 | −1.84 |
| 8L | 2.71 | −0.54 | 2.19 | 0.47 | −0.49 |
| 9 M | 10.02 | 3.20 | 8.25 | 7.03 | 1.12 |
| 9R | 1.04 | −1.22 | −0.99 | −0.13 | −1.33 |
| 9L | 5.46 | 0.1 | 3.26 | 3.52 | 2.06 |
| 10 M | 8.55 | 3.3 | 6.21 | 5.21 | 0.53 |
| 10R | 4.13 | 2.38 | 3.62 | 3.73 | −0.47 |
| 10L | 3.36 | 0.98 | 2.96 | 2.06 | −0.68 |
| Minimum Maximum Mean ± SE |
0.34 10.33 4.37 ± 0.54 |
−1.22 4.40 0.65 ± 0.28 |
−0.99 8.57 2.52 ± 0.43 |
−0.13 7.41 3.46 ± 0.33 |
−1.91 2.06 −0.63 ± 0.18 |
| Pollution Status |
Strongly to extremely polluted | Unpolluted to moderately polluted | Moderately to strongly polluted | Strongly polluted | Practically unpolluted |
Note: 1 M = Point 1 at middle, 1R= Point 1 at right, 1L = Point 1 at left.
3.3.2. Contamination Factor
Based on the findings, the CFs were classified as no pollution to very high pollution. Cu recorded the highest CF value which ranged from 1.90 to 1927.78, with a mean value of 208.55 mg/kg, suggesting very strong pollution. With the mean CF values indicated in Table 5, Cu (208.55), Pb (47.04), and Zn (37.01) had very high mean contaminations; followed by Hg (4.78) and Al (1.27) with moderate contamination. The high CF values of Cu, Pb, and Zn in the soil samples can be attributed to the influence of industrial activities, the proximity of mechanic workshops, and other anthropogenic inputs as suggested by Ololade [33]. However, CF values for Hg and Al were relatively lower (most values < 1) because at the e-waste contaminated site, most of their activities lead to less extraction of Hg and Al.
Table 5.
Contamination factors.
| Sample ID | Cu | Hg | Pb | Zn | Al |
|---|---|---|---|---|---|
| 1 M | 608.09 | 2.16 | 19.69 | 18.04 | 0.68 |
| 1R | 13.79 | 1.43 | 4.30 | 9.52 | 0.55 |
| 1L | 1.89 | 1.39 | 0.90 | 2.40 | 1.20 |
| 2 M | 276.19 | 27.94 | 51.00 | 99.65 | 1.46 |
| 2R | 421.05 | 0.84 | 2.45 | 9.95 | 0.92 |
| 2L | 2.11 | 2.43 | 3.03 | 4.02 | 1.58 |
| 3 M | 33.47 | 1.63 | 8.82 | 20.59 | 1.22 |
| 3R | 8.69 | 0.76 | 4.23 | 11.12 | 2.21 |
| 3L | 3.80 | 1.15 | 1.69 | 6.90 | 0.40 |
| 4 M | 28.71 | 1.44 | 11.40 | 10.95 | 0.43 |
| 4R 4L |
7.71 3.85 |
2.67 0.70 |
3.88 1.33 |
23.28 4.28 |
0.61 0.76 |
| 5 M | 72.77 | 1.89 | 7.79 | 19.18 | 0.78 |
| 5R | 5.19 | 2.54 | 3.00 | 14.27 | 0.73 |
| 5L | 11.56 | 0.78 | 3.90 | 12.92 | 0.81 |
| 6 M | 9.34 | 0.87 | 1.60 | 8.59 | 0.56 |
| 6R | 5.89 | 1.18 | 2.29 | 254.55 | 0.47 |
| 6L | 2.51 | 1.62 | 3.22 | 30.95 | 0.88 |
| 7 M 7R |
47.97 | 1.78 | 21.85 | 17.20 | 0.74 |
| 104.21 | 4.71 | 12.26 | 29.42 | 0.49 | |
| 7L | 284.80 | 2.81 | 28.28 | 33.99 | 1.00 |
| 8 M | 1927.78 | 6.54 | 568.93 | 140.58 | 3.86 |
| 8R | 138.64 | 31.67 | 25.96 | 29.35 | 0.42 |
| 8L | 9.79 | 1.03 | 6.86 | 2.08 | 1.07 |
| 9 M | 1555.90 | 13.78 | 456.71 | 196.23 | 3.25 |
| 9R | 3.08 | 0.65 | 0.76 | 1.37 | 0.60 |
| 9L | 65.81 | 1.60 | 14.39 | 17.21 | 6.25 |
| 10 M | 560.10 | 14.72 | 110.66 | 55.63 | 2.16 |
| 10R | 26.21 | 7.83 | 18.44 | 19.94 | 1.08 |
| 10L | 15.44 | 2.95 | 11.63 | 6.25 | 0.94 |
| Minimum | 1.90 | 0.65 | 0.76 | 1.37 | 0.40 |
| Maximum | 1927.78 | 31.67 | 568.93 | 254.55 | 6.25 |
| Mean | 208.55 ± 82.32 | 4.78 ± 1.40 | 47.04 ± 23.60 | 37.01 ± 10.85 | 1.27 ± 0.23 |
| Contamination Status |
Very strong pollution | Strong to very strong pollution | Very strong pollution | Very strong pollution | Moderately polluted |
Note: 1 M = Point 1 at middle, 1R = Point 1 at right, 1L = point 1 at left.
3.3.3. Pollution Load Index
Pollution Load Index was used to determine the pollution severity and its variation. This index functions as a quick tool for comparing the status of pollution of different places [34]. Table 6 shows the pollution load indices of the soil samples. All samples recorded mean values greater than 3 which signifies extremely heavy pollution with heavy metals. This finding implies that e-waste contaminated site is polluted with heavy metals, resulting from an increased rate of scrap and other industrial activities.
Table 6.
Pollution load indices.
| Sample | Min | Max | Mean ± SE | Status |
|---|---|---|---|---|
| 1 | 1.47 | 12.59 | 5.82 ± 3.43 | Extremely heavy pollution |
| 2 | 2.5 | 35.61 | 14.71 ± 10.50 | Extremely heavy pollution |
| 3 | 1.83 | 6.55 | 4.03 ± 1.37 | Extremely heavy pollution |
| 4 | 1.64 | 4.66 | 3.46 ± 0.93 | Extremely heavy pollution |
| 5 | 3.26 | 6.94 | 4.51 ± 1.21 | Extremely heavy pollution |
| 6 | 2.29 | 4.53 | 3.35 ± 0.65 | Extremely heavy pollution |
| 7 | 7.51 | 15.03 | 10.75 ± 2.23 | Extremely heavy pollution |
| 8 | 2.74 | 82.8 | 34.16 ± 24.66 | Extremely heavy pollution |
| 9 | 1.04 | 91.03 | 34.37 ± 28.48 | Extremely heavy pollution |
| 10 | 4.50 | 40.57 | 18.23 ± 11.27 | Extremely heavy pollution |
| Overall | 2.88 | 30.03 | 13.34 ± 8.47 | Extremely heavy pollution |
3.4. Spatial distribution
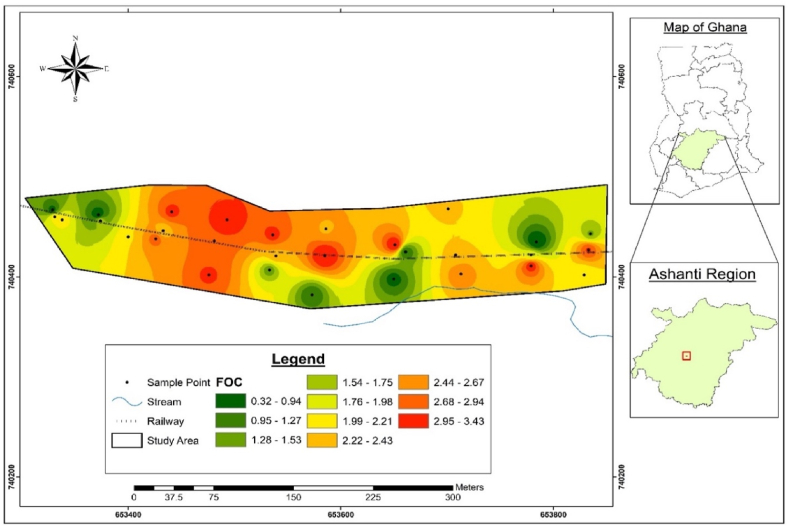
The spatial distribution of the PTE and physico-chemical parameters based on interpolations of the concentrations relative to the geospatial positions of the sampling points are presented in Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9. The lower values which are the green areas indicate lower concentration through to intermediate, represented by the yellow areas to the red areas that have high values, indicating very high concentration areas.
Fig. 2.
Spatial distribution of organic matter.
Fig. 3.
Spatial distribution of organic carbon.
Fig. 4.
Spatial distribution of pH.
Fig. 5.
Spatial distribution of aluminium.
Fig. 6.
Spatial distribution of copper.
Fig. 7.
Spatial distribution of lead.
Fig. 8.
Spatial distribution of zinc.
Fig. 9.
Spatial distribution of mercury.
3.4.1. Physico-chemical parameters
Generally, OM and OC contents were highly concentrated in the study area except for the extreme western, lower part at the center, and southern parts of the study areas, as shown in Fig. 2, Fig. 3. The spatial interpolation of %OC and %OM showed the same distribution pattern, and it was found that the site was rich in OM and OC. The pH of the study area was acidic, with the green areas being more acidic than the red areas. These findings could have resulted from the influence of operations of the sawmill and rice mill shops due to high decomposition as stated in research conducted by the United States Department of Agriculture [35]. In Fig. 4, the western part of the study area was more acidic than the center. Also, some points showed a very red coloration, indicating areas of comparatively high levels and these were the areas where the sawmill and rice mill shops are situated. Few points recorded high pH in areas where the burning of e-waste is done since pH is influenced by the ash. It may also be a result of a low level of leaching due to the topography of the land [36].
3.4.2. PTE
The spatial distributions presented in Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9 indicate that the soil at the Akwatia-Line has varying concentrations of Cu, Hg, Pb, Zn, and Al. The distribution pattern of all the PTE shows a lower concentration in the western part of the study area and this is because, the area is filled with buildings interspersed with shops, with little or no activities of e-waste processing. The distribution patterns of Al, Cu, and Pb show high concentration at some minute places in the far east of the study area (Fig. 5, Fig. 6, Fig. 7), where the manual disassembly of the e-waste is heavily carried out. As seen in Fig. 8, Zn was highly concentrated at the lower part in the center and a minute area in the eastern part of the study area, while Fig. 9 showed a less concentration of Hg. Areas of high concentration were at some minute areas in the eastern and western parts where the e-waste was dismantled. This is in line with Bu et al. [37] in their study which indicated that higher concentrations of the heavy metals were proximal to the coalfield and highway in the midwestern area, concluding that industrial emissions and vehicle exhaust caused contamination of the soil.
4. Conclusions
The study showed that the soil samples from the Akwatia-Line, Kumasi were polluted with Cu, Pb, Zn, and Al, which can be attributed to open burning, dumping, and scrap operations at the site. The concentrations of the heavy metals in the soil samples from the site were higher than that of the control samples and exceeded the WHO permissible limits. The Geoaccumulation Indices of the soil samples from the site ranged from practically unpolluted (Al), unpolluted to moderately polluted (Hg), moderately polluted to strongly polluted (Pb), strongly polluted (Zn) to strongly to extremely polluted (Cu). Contamination Factors at the site also ranged from moderately polluted (Al), strong to very strong pollution (Hg) to very strong pollution (Cu, Pb, Zn) as pollution status which can be attributed to the scrap activities in the area.
The spatial distribution showed that the eastern parts of the site recorded higher concentrations of Al, Cu, Pb, and Hg whilst a high concentration of Zn was recorded at the extreme south. The interpolation also showed that almost the entire area has great concentrations of organic matter and organic content as a result of sawmill and rice mill operations in the area. The soil texture was generally found to belong to the loamy sand class due to the greater %sand and lower levels of silt and clay in almost all the samples. pH was generally found to be mild acidic. The findings indicate the pollution status of the e-waste contaminated site, making it harmful to use. Extensive monitoring and remediation activities are required in the area to ensure proper e-waste management and productive use of the soil. Further study should focus on the provision of alternative livelihoods for the scrap dealers as a means to control their polluting activities.
Data Availability
The data that support the findings of this study have been included in the article.
CRediT authorship contribution statement
Alhassan Sulemana: Writing - review & editing, Writing - original draft, Supervision, Resources, Methodology, Investigation, Formal analysis, Conceptualization. Matilda Koduah: Writing - review & editing, Writing - original draft, Resources, Methodology, Investigation, Formal analysis, Conceptualization. Stephen Owiredu: Writing - review & editing, Writing - original draft, Resources, Methodology, Investigation, Formal analysis, Conceptualization. Charles L. Tengan: Writing - review & editing, Writing - original draft, Resources, Methodology, Investigation, Formal analysis, Conceptualization. Ebenezer A. Agyare: Writing - review & editing, Writing - original draft, Resources, Methodology, Investigation, Formal analysis, Conceptualization. Kofi S. Boateng: Writing - review & editing, Writing - original draft, Resources, Methodology, Investigation, Formal analysis, Conceptualization. Emma K. Nsafoah: Writing - review & editing, Writing - original draft, Resources, Methodology, Investigation, Formal analysis, Conceptualization. Jonas B. Beogkina: Writing - review & editing, Writing - original draft, Resources, Methodology, Investigation, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Forti V., Baldé C.P., Kuehr R., Bel G. United Nations University (UNU)/United Nations Institute for Training and Research (UNITAR) – co-hosted SCYCLE Programme, International Telecommunication Union (ITU) & International Solid Waste Association (ISWA); Bonn/Geneva/Rotterdam: 2020. The Global E-Waste Monitor 2020 (3rd Edition, Issue July). http://ewastemonitor.Info/as: Forti V., Baldé C.P., Kuehr R., Bel G. The Global E-Waste Monitor 2020: Quantities, Flows and the Circular Economy Potential. ISBN Digital: 978-92-808-9114-0 ISBN Print: 978-92-808-9115-7. [Google Scholar]
- 2.Daum K., Stoler J., Grant R. Toward a more sustainable trajectory for e-waste policy: a review of a decade of e-waste research in Accra, Ghana. Int. J. Environ. Res. Publ. Health. 2017;14(2):135. doi: 10.3390/ijerph14020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burchard G., Wilhelm M., Till H. High levels of PAH-metabolites in urine of e-waste recycling workers from Agbogbloshie, Ghana. E-Waste Africa Programme, 2011. 2014;1:369–376. doi: 10.1016/j.scitotenv.2013.06.097. [DOI] [PubMed] [Google Scholar]
- 4.Pourret O., Bollinger J., Hursthouse A. Heavy Metal: a misused term? Acta Geochimica, Springer. 2021;40:466–471. [Google Scholar]
- 5.Raychaudhuri S.S., Pramanick P., Talukder P., Basak A. Polyamines, metallothioneins, and phytochelatins—Natural defense of plants to mitigate heavy metals. Stud. Nat. Prod. Chem. 2021;69:227–261. doi: 10.1016/B978-0-12-819487-4.00006-9. (Chapter 6), [DOI] [Google Scholar]
- 6.Picchio V., Cammisotto V., Pagano F., Carnevale R., Chimenti I. Introducing heavy metals. Intechopen, Cell Interaction-Regulation of Immune Responses, Disease Development and Management Strategies. 2020;1–15 [Google Scholar]
- 7.Ali H., Khan E., Ilahi I. (2019). Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019 doi: 10.1155/2019/6730305. [DOI] [Google Scholar]
- 8.Liu P., Zhang Y., Feng N., Zhu M., Tian J. (2020). Potentially toxic element (PTE) levels in maize, soil and irrigation water and risks through maize consumption in northern Ningxia, China. BMC Publ. Health. 2020;20:1729. doi: 10.1186/s12889-020-09845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okoro H.K., Orosun M.M., Oriade F.A., Momoh-Salami T.M., Ogunkunle C.O., Adeniyi A.G., Zvinowanda C., Ngila J.C. Sustainability. 2023;15(8):6974. doi: 10.3390/su15086974. [DOI] [Google Scholar]
- 10.Neider R., Bembi D. Potentially toxic elements in the environment – a review of sources, sinkss, pathways and mitigation measures. Rev. Environ. Health. 2023 doi: 10.1515/reveh-2022-0161. [DOI] [PubMed] [Google Scholar]
- 11.Lara R., Suárez-Peña B., Megido L., Negral L., Rodríguez-Iglesias J., Fernández-Nava Y., Castrillón L. Health risk assessment od potentially toxic elements in the dry deposition fractionof settleable particulate matter in urban and subsurban locations in the city of Gijón, Spain. J. Environ. Chem. Eng. 2021;9(6) doi: 10.1016/j.jece.2021.106794. [DOI] [Google Scholar]
- 12.Fosu-Mensah B.Y., Addae E., Yirenya-Tawiah D., Nyame F., Fantke P. Heavy metals concentration and distribution in soils and vegetation at Korle Lagoon area in Accra, Ghana. Cogent Environmental Science. 2017;3(1) doi: 10.1080/23311843.2017.1405887. [DOI] [Google Scholar]
- 13.Sonone S.S., Jadhav S.V., Sankhla M.S., Kumar R. Water contamination by heavy metals and their toxic effects on Aquaculture and human health through food chain. Letters in Applied NanoBioScience. 2020;10:2148–2166. [Google Scholar]
- 14.Ankit, Saha L., Kumar V., Tiwari J., Sweta Rawat S., Singh J., Bauddh K. Electronic waste and their leachates impact on human health and environment: Global ecological threat and management. Environmental Technology & Innovation. 2021;24 doi: 10.1016/j.eti.2021.102049. [DOI] [Google Scholar]
- 15.Ouabo R.E., Ogundiran M.B., Sangodoyin A.Y., Babalola B.A. Ecological risk and human health implications of heavy metals contamination of surface soil in e-waste recycling sites in Douala, Cameroun. Journal of health & pollution. 2019;9(21) doi: 10.5696/2156-9614-9.21.190310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appiah-Adjei E.K., Baidu E.E., Adjei K.A., Nkansah M.A. Potential heavy metalspollution from artisanalautomobile workshops: the case of Suame Magazine, Ghana. Environmental Earth Science. 2019;78:1–12. doi: 10.1007/s12665-019-8069-7. [DOI] [Google Scholar]
- 17.Pan C., Yu F., Tao X., Guo J., Yu Y. Contamination, spatial distribution and source analysis of heavy metals in surface soil of Anhui Chaohu Economic Development Zone, China. Sustainability. 2020;12(19):1–15. doi: 10.3390/su12198117. [DOI] [Google Scholar]
- 18.Demková L., Jezný T., Bobulská L. Assessment of soil heavy metal pollution in a former mining area before and after the end of mining activities. Soil Water Res. 2017;12(4):229–236. doi: 10.17221/107/2016-SWR. [DOI] [Google Scholar]
- 19.Santos-Francés F., Martínez-Graña A., Zarza C.Á., Sánchez A.G., Rojo P.A. Spatial distribution of heavy metals and the environmental quality of soil in the Northern Plateau of Spain by geostatistical methods. Int. J. Environ. Res. Publ. Health. 2017;14(6) doi: 10.3390/ijerph14060568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mapcarta . 2020. Location of Akwatia-Line.https://mapcarta.com/N7840566600 [Google Scholar]
- 21.Olayinka O.O., Akande O.O., Bamgbose K., Adetunji M.T. Physicochemical characteristics and heavy metal levels in soil samples obtained from selected anthropogenic sites in Abeokuta, Nigeria. J. Appl. Sci. Environ. Manag. 2017;21(5):883. doi: 10.4314/jasem.v21i5.14. [DOI] [Google Scholar]
- 22.Baillie I.C., Anderson J.M., Ingram J.S.I. Tropical soil Biology and Fertility: a handbook of methods. J. Ecol. 1990;78(2):547. doi: 10.2307/2261129. [DOI] [Google Scholar]
- 23.Beretta A.N., Silbermann A.V., Paladino L., Torres D., Bassahun D., Musselli R., García-Lamohte A. Soil texture analyses using a hydrometer: modification of the Bouyoucos method. Ciencia e Investigación Agraria. 2014;41(2):25–26. doi: 10.4067/s0718-16202014000200013. https://mgimond.github.io/Spatial/index.html [20] Gimond, M. (2021). Introduction to GIS and Spatial Analysis. [DOI] [Google Scholar]
- 24.Gimond M. 2021. Introduction to GIS and Spatial Analysis.https://mgimond.github.io/Spatial/index.html [Google Scholar]
- 25.Nweke M., Ukpai S. Use of enrichment, ecological risk and contamination factors with geoaccumulation indexes to evaluate heavy metal contents in the soils around Ameka Mining Area, South of Abakaliki, Nigeria. Journal of Geography, Environment and Earth Science International. 2016;5(4):1–13. doi: 10.9734/jgeesi/2016/24908. [DOI] [Google Scholar]
- 26.Mueller G. Schwermetalle in den sedimenten des rheins - veranderungen seit 1971. Umsch. Wissensch. Techn. 1979;79(24):778–783. [Google Scholar]
- 27.Wang X., He M., Xie J., Xi J., Lu X. Heavy metal pollution of the world's largest antimony mine-affected agricultural soils in Hunan province (China) J. Soils Sediments. 2010;10(5):827–837. doi: 10.1007/s11368-010-0196-4. [DOI] [Google Scholar]
- 28.Zgłobicki W., Telecka M., Skupiński S., Pasierbińska A., Kozieł M. Assessment of heavy metal contamination levels of street dust in the city of Lublin, E Poland. Environ. Earth Sci. 2018;77(23):1–11. doi: 10.1007/s12665-018-7969-2. [DOI] [Google Scholar]
- 29.Department of Primary Industries, Regional Development . 2021. West Australia's Agriculture and Food.https://www.agric.wa.gov.au [Google Scholar]
- 30.Orgiazzi A., Panagos P. Soil biodiversity and soil erosion: it is time to get married: adding an earthworm factor to soil erosion modelling. Global Ecol. Biogeogr. 2018;27(10):1155–1167. doi: 10.1111/geb.12782. [DOI] [Google Scholar]
- 31.Gazey C. Department of Primary Industries and Regional Development; 2018. Agriculture and Food.https://www.agric.wa.gov.au/soil-acidity/effects-soil-acidity [Google Scholar]
- 32.Bojórquez-Quintal E., Escalante-Magaña C., Echevarría-Machado I., Martínez-Estévez M. Aluminum, a friend or foe of higher plants in acid soils. Front. Plant Sci. 2017;8 doi: 10.3389/fpls.2017.01767. https://www.frontiersin.org/articles/10.3389/fpls.2017.01767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ololade I.A. An Assessment of heavy metal contamination in soils within auto-mechanic workshops using enrichment and contamination factors with geoaccumulation Indexes. J. Environ. Protect. 2014;5(11):970–982. doi: 10.4236/jep.2014.511098. [DOI] [Google Scholar]
- 34.University of New England . 2019. Health and Safety Manual. Innovation for A Healthier Planet, August, 1–219.https://www.une.edu/sites/default/files/safety_manual_aug_2019_new_format_final_0.pdf [Google Scholar]
- 35.United States Department of Agriculture Inherent factors affecting soil pH. Soil Health – Guides for Educators. 2014 https://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs142p2_051574.pdf Accessed on 14/02/2022. [Google Scholar]
- 36.Kassegne A.B., Esho T.B., Okonkwo J.O., Asfaw S.L. Distribution and ecological risk assessment of trace metals in surface sediments from Akaki River catchment and Aba Samuel Reservoir, Central Ethiopia. Environmental Systems Research. 2018;7(1) doi: 10.1186/s40068-018-0127-8. [DOI] [Google Scholar]
- 37.Teye A.K., Tetteh Kow I. Assessment of heavy metal pollution resulting from informal E-wastes recycling in the Greater Accra Region of Ghana. Research. 2021;1–28 doi: 10.21203/rs.3.rs-390412/v1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study have been included in the article.