Abstract
Background:
Rural populations face many health disadvantages compared to urban areas. There is a critical need to better understand the current lung cancer screening landscape in these communities to identify targeted areas to improve the impact of this proven tool.
Methods:
Data from the County Health Rankings of New Hampshire and Vermont was reviewed for population density, distribution of adult smokers, and level of education compared to the distribution of Lung Cancer Screening Facilities throughout these two states.
Results:
Screening programs in southern counties of Vermont with lower levels of education have decreased access. In New Hampshire, there are no programs within 30 miles of the areas with the largest distribution of smokers, and decreased access in some areas with the lowest levels of education.
Conclusions:
Improving equitable access to high-quality screening services in rural regions and the creation of targeted interventions to address decreased access in areas of high tobacco use and low education is vital to decreasing the incidence of latestage presentations of lung cancer within these populations.
Keywords: Lung cancer, Lung cancer screening, Rural, Patient education levels, Low-dose computed tomography, Smoking
Summary for the table of contents
Rural populations face many health disadvantages compared to urban areas. Improving equitable access to high-quality screening services in rural regions and the creation of targeted interventions to address decreased access in areas of high tobacco use and low education is vital to decreasing the incidence of late stage presentations of lung cancer within these groups.
Introduction
Lung cancer persists as the leading cause of cancer death among men and women in the United States,1 and mortality rates in rural populations are even higher when compared to their urban counterparts.2,3 Despite recent advances in medical therapy,4,5 and surgical technique,6,7 treatment efforts at the earliest stage have proven most promising.8,9 While those diagnosed at an early stage have an estimated 5-year survival of 46%,10 survival of all patients diagnosed with lung cancer remains poor at 22%.11 In 2011, data from the National Lung Screening Trial (NLST), a multi-institutional randomized controlled trial of 53,454 patients, formally demonstrated the utility of lung cancer screening. It reported a 20% lung specific mortality reduction and an overall mortality reduction of 6.7% in a “high-risk” cohort that underwent low-dose computed tomography (LDCT).12,12 As a result of the findings, many professional organizations published guidelines recommending annual low-dose CT for “high-risk” patients, defined as those (1) 55–80 years of age, (2) with at least a 30 pack-year smoking history, (3) who are current or former smokers who have quit in the last 15 years.13,14,15,16,17 Though LDCT has been shown to decrease both lung-specific and overall mortality through early detection, today, less than 4% of eligible high-risk patients participate in screening.18 It is estimated that this number is much lower in rural populations,19 thus, the need to identify the factors that effectively promote successful participation in early detection efforts in this cohort is essential.20
Estimates show rural areas have nearly twice the lung cancer incidence of the largest metropolitan areas.2 While only accounting for 15% of the U.S. population,21 rural cohorts represent a disproportionately high percentage of the screening-eligible population (23%). Two key challenges to rural lung cancer screening include a higher rate of tobacco use and lower educational achievement; influences known to be associated with decreased participation in screening efforts and increased rates of lung cancer.2
Rural smokers are an important group to engage with screening since their risk for lung cancer is thought to be amplified by younger age at start of use, greater cumulative tobacco smoke inhalation compared to their urban peers, and higher rates of nicotine dependence and second-hand smoke exposure.22 Efforts to engage rural smokers have proven challenging, and research has suggested smokers were less likely to perceive a survival benefit and/or believe that surgery could help their screen detected cancer.22 Other studies have demonstrated that fatalistic concerns and anxiety about having a CT scan predicted lower screening intentions and follow-through.23 In contrast, higher education level relates to early detection efforts, as it is a crucial predictor of participation in cancer screenings.24 People with higher educational achievement are more likely to participate in screenings for cervical, breast, and colorectal cancer.25,26 Screening interventions must target smokers and less educated patients to counteract these findings. Additional efforts to survey the current geographic distribution of accredited screening facilities in relation to these factors are necessary to understand barriers to screening utilization.
Quiafe et al. noted, “the effectiveness of any lung cancer screening program depends in part, upon uptake, and any inequalities in participation ultimately have the potential to exacerbate inequalities in lung cancer survival”.27 This highlights the critical need to better understand the current screening landscape in rural populations to create targeted interventions that will increase uptake and potentially improve survival. The intent of this study was to evaluate population density, distribution of adult smokers and education level compared to the location of lung cancer screening facilities throughout Vermont and New Hampshire. Gaining a better understanding of the geographic accessibility to accredited screening facilities of these “high-risk” groups is vital to enable the development of evidenced-based engagement strategies to ensure that the reach of future screening initiatives is equitable.
Materials and methods
Modeled after America’s Health Rankings, which has ranked states on certain health indicators since 1990, County Health Rankings provide reports at the county level regarding health differences by location and race-ethnicity. This offers a deeper understanding of health at the community level. We surveyed these rankings with respect to county population, percentage of adult smokers, high school graduation rate, and percentage of adults with “some” post-secondary education for both New Hampshire and Vermont as these two states comprise our central catchment areas. Choropleths were obtained from the County Health Rankings and then superimposed with the locations of accredited and non-accredited Lung Cancer Screening Facilities in both states using the American College of Radiology screening statistics. We defined “accredited lung cancer screening centers” as facilities that meet the basic criteria as specified by the American College of Radiology (ACR) to be considered “accredited”.28 This includes (1) specific accreditation of the low-dose CT scanner through the ACR CT Accreditation Program, (2) a screening protocol that meets minimum technical specifications, (3) and participation in the ACR Lung Cancer Screening Registry.
Decreased access to a screening facility was initially defined a priori as counties located more than 30 miles away, or greater than a 30-min drive from the Dartmouth-Hitchcock Medical Center (DHMC) Lung Cancer Screening program in Lebanon, New Hampshire. This distance was chosen as the average RUCA scores increase from 7 to 9 at approximately 30 miles, there are few main highways, and the travel time increases to greater than 1 h for this commute. The choropleths were charted using the sum population for population distribution in Fig.1A. The percent of population was used for distribution of adult smokers, and both levels of education: high school, and some college, for Fig. 1B, C, and 1D. Statistical averages were performed by the County Health Rankings using their Statistical Model. For more information, please refer to https://www.countyhealthrankings.org/explore-health-rankings/measures-data-sources/county-health-rankings-model?componentType=health-factor&componentId=10.
Fig. 1.
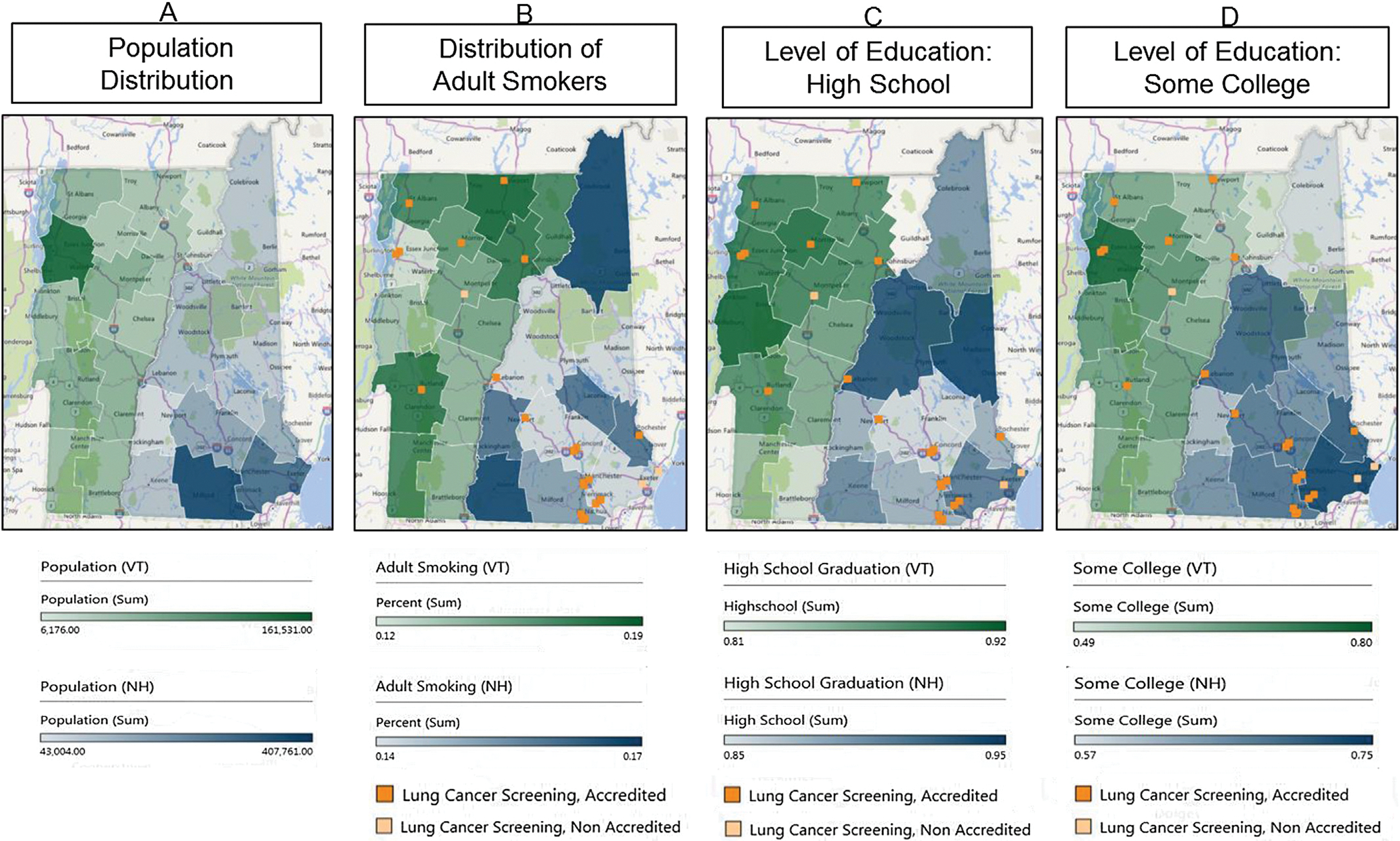
Choropleths demonstrating population estimates (1A), tobacco use (1B), and education levels (1C & 1D), in Vermont and New Hampshire. Regarding population, the color spectrum assigned to Vermont was green while the color spectrum applied to New Hampshire was blue. Lighter color denoted decreasing frequency, while darker colors denoted increasing frequency. A light and dark shade of orange was used to represent the non-accredited and accredited lung cancer screening centers in Vermont and New Hampshire, respectively.
Results
According to the County Health Rankings, Vermont has a population of 624,594 people with 18.1% aged 65 and older.29 Chittenden is the highest populated county (Fig. 1A). New Hampshire has a population of 1,334,795 with 17% of the population age 65 and older.33 Hillsborough, Rockingham, and Merrimack are the highest populated counties (Fig. 1A). Approximately 17% of the population in Vermont are classified as “current adult smokers” The counties with the highest smoking prevalence include Rutland and Bennington in the Southwest corner, and Orleans and Caledonia counties in the Northeast corner. 18% of New Hampshire residents were classified as “current everyday smokers” with the highest smoking counties including Cheshire county in the Southwest and Coos county in the Northeast.
The average distance traveled by patients living in areas of decreased access with either high tobacco use or low education levels was 80.6 miles (range of 37.6–129.3 miles); well above our designated 30 miles, and well above previously reported distances of “long travel times for cancer care” (50 miles) in other studies.30,31,32,33 Windsor and Rutland counties in Vermont were the only regions located less than 50 miles away from DHMC at 37.6 and 48.8 miles respectively.
The accredited lung cancer screening facilities in Vermont appear to be well distributed with centers located both in the highest populated county (Chittenden; Fig. 1A), and the counties with the highest smoking populations (Rutland, Orleans and Caledonia; Fig. 1B). In New Hampshire, screening facilities are also well distributed in regards to population density with locations in Hillsborough, Rockingham and Merrimack counties (Fig. 1A). However, there are no programs available to the areas with the largest distribution of smokers in Cheshire and Coos counties (Fig. 1B).
Regarding education, in Vermont, the accredited screening centers are located in the areas with the highest percentage of those that have completed high school, while none of the less-educated counties have access to an accredited center (Windham, Bennington, Windsor and Essex; Fig. 1C & D). In New Hampshire, though Merrimack County has facilities in both the East and West, Belknap, a county with the lowest levels of education, does not have a nearby facility (Fig. 1C). In counties with the lowest levels of those that have completed some college, no facilities were located in either Vermont (Essex County) or New Hampshire (Coos County, Fig. 1D).
Discussion
Despite the randomized, controlled National Lung Screening Trial12 and subsequent analyses demonstrating both significant disease-specific and overall mortality reductions,34 it is estimated that screening is underutilized with less than 3.7% of qualifying high-risk patients participating.18 Recruitment and enrollment numbers in rural populations are even more discouraging,19 and the reasons for this are likely multifactorial. Systemic barriers have included required shared decision making, a tool unique to lung cancer screening which can be impacted by provider knowledge and patient health literacy.35 Reduced radiologist capacity has limited the ability to process the number of screens relative to the number at risk.36 Furthermore, provider concerns regarding LDCT false positive rates, potential complications of follow-up biopsies, and feasibility of implementing LDCT in their respective health care settings represent a significant challenge.37 The need to better address risk for low participation is imperative to address this public health dilemma.
Rural areas are estimated to cover 97% of the nation’s land area, but contain less than 20% of the population according to U.S. Census Data.38 Compared to urban populations, rural inhabitants are more likely to own their home, have served in the military, have an older median age (51 vs 45), have a lower median income, and are less likely to have obtained a bachelor’s degree or higher.38 Historically, rural populations were composed of non-Hispanic Whites, which is similar to the current composition of New Hampshire and Vermont. However, over the last 70 years, the face of rural and small-town America has slowly evolved resulting in increasing racial and ethnic diversity.39 Hispanic and Asian populations are the fastest growing minority groups in both urban and rural areas, while Black/African-American (AA) populations have remained the largest minority group in rural cities.39 This influx has not uniformly distributed across the United States with specific racial and ethnic groups settling in specific regions of the US.39 While these demographic inconsistencies can limit some analytic evaluations between rural populations located in different geographic areas, comparisons are still possible.
Because the non-majority race and ethnic groups in rural communities are often underrepresented, these inhabitants have a higher rate of being impoverished and/or medically disenfranchised, and cancer diagnoses are disproportionally present.40 Given their location and distance from academic tertiary and quaternary hospitals, it is often challenging to provide appropriate and evidence-based oncologic care.41 Specifically regarding lung cancer care, we know that rural populations are thought to have an increased risk for developing lung cancer due to higher rates of smoking and lower educational achievement.2 Furthermore, these cohorts have been shown to have less geographic access to screening facilities overall, which may affect enrollment into and utilization of lung cancer screening programs as investigated in our study.42 Both Vermont and New Hampshire have an above average incidence of lung cancer compared to the national incidence of 63 cases/100,000 persons (66.7 cases and 68.1 cases/100,000, respectively).33 However, distribution of lung cancer screening programs is also above the national average of 4.8 centers/million in both Vermont (8.0 centers/million people), and New Hampshire (9.0 centers/million people).33 Nevertheless, these populations lag significantly in the number of eligible patients enrolled in screening programs when compared to the distribution of “high-risk” individuals residing within these states.33
The County Health Rankings were designed to determine why a state that appears to have adequate resources would still have deficiencies in health-promoting behaviors by examining the landscape at the county level. We found that while lung cancer screening facilities in New Hampshire and Vermont were fairly well distributed in regards to the most populated areas, the regions with the highest tobacco use had decreased access to accredited facilities. Moreover, the accredited centers were located in areas with the greatest number of high school graduates, and those that had completed “some college”, leaving the less educated areas with decreased access.
Attitudes expressed among smokers towards lung cancer screening are complex. They have varied between those that perceive screening as too much effort or unnecessary due to the lack of respiratory symptoms,43 those that are crippled by emotional barriers developed secondary to the stigma associated with smoking,44 and more specifically, those that that are reluctant to participate secondary to worry, fatalism, avoidance, and believing oneself too old to gain benefit.45 Hence, this is a population that is less likely to participate in screening and is at the highest risk.
Education level and a participants’ willingness to participate in screening are closely related. In addition to having an increased overall desire to participate in screening efforts, previous studies have indicated that people with higher levels of education tend to have better decision-making abilities, leading to greater engagement in risk-control behaviors, while people with lower levels of education tend to have more fatalistic beliefs about cancer.46 These beliefs reduce the likelihood that they will take the initiative themselves to use offered opportunities for early detection.47,48 Lower education levels, worse socioeconomic status, increased smoking rates, higher incidence of lung cancer and a fatalistic attitude are all factors in the lower survival associated with lung cancer in rural regions.
It is imperative that screening interventions geographically target the areas where those that carry this risk are most heavily populated. The implementation of mobile screening units to reach more patients or organizing rides or carpooling for those with transportation challenges has been proposed to overcome some of these issues.49 While there are benefits to centralized screening facilities including standardization and efficiency, this must be weighed against the risk of lower participation because of a longer distance to screening sites. Second, engagement of local general practitioners and providers to promote screening appears to be key to improving utilization rates. General practitioners have strong persuasive power in convincing their patients to undergo cancer screening,50,51 and people with lower levels of education are more likely to participate in cancer screening at the urging of a provider or a screening program than at their own initiative.24 Furthermore, patients’ perceptions of their physicians’ confidence in cancer screening is an important predictor of compliance with recommendations, as provider confidence communicates the importance of screening to the patient.24 As those with less education need more guidance, advice, or persuasion from these third parties (providers or invitation letters from screening programs) to participate in screening, arming those at the front line with a local screening facility will be key, even if it means potentially sacrificing some of the efficiency and standardization of centralized screening.
Taking this a step further, if we are successful with these efforts, it is expected that more early stage lung cases will be identified that require thoracic surgical intervention.52 Previous examinations of the availability of the thoracic workforce has demonstrated a declining density of surgeons nationwide and in lesser populated states such as rural areas due to an aging and retiring workforce.52 Comparing urban versus rural areas, the difference in thoracic density is slowly widening, with the most pronounced differences appreciated in areas located between small adjacent rural communities.52 The declining thoracic surgeon population combined with the higher prevalence of lung cancer in rural communities and recent changes to increase reimbursement policy for screening mandate critical attention be paid to ensure readiness for the influx of patients needing care should screening efforts be successful. While the Northeast currently holds a disproportionate share of the thoracic surgery workforce given the high density of surgeons,52 it is imperative that we prepare for the increased need in other rural areas and the nation as a whole.
To our knowledge, this is the first study to explore the location of lung cancer screening in relation to the distribution of “high-risk” health factors in “high-risk” populations. While this study provides valuable insight into the screening landscape in rural populations in Northern New England and its relation to smoking rates and educational inequalities, it is subject to limitations. The reporting of the County Health Rankings somewhat limits the interpretation of the results. The most recent data regarding tobacco use are from 2016. The most recent educational data was obtained over a period from 2013 to 2017. It is possible rates of smoking and education have shifted during this time period and though the shift is probably small, the magnitude cannot be determined. Second, the collection of educational data combines information obtained from multiple sources including state-specific sources, EDFacts (a U.S. Department of Education Initiative to use performance data to guide policy), and the American Community Survey. Continuity of methods among these sources in terms of data-gathering may differ leading to inconsistent data. Third, although lung cancer screening was introduced in 2012, inquiry into why it has not been rigorously adapted is fairly new. The problem of low enrollment in lung cancer screening is a global issue, and research efforts have focused on evaluating low enrollment in all eligible patients rather than the factors involved in specific populations. Consequently, little research has occurred to how best engage specific groups or cohorts. While factors relating to smoking have been studied, the link with education has been developed from prior knowledge regarding patient behaviors towards other cancer screenings, such as those for colorectal, cervical or breast cancer, not specifically for lung, which may lead to incorrect assumptions. Last, as mentioned earlier, due to the changing demographics of rural populations, correlations to other rural populations in different geographic areas must be carefully undertaken with close examination of other features to avoid overgeneralization.
Conclusions
Despite the fact that designated low-dose chest CT screening centers have increased by greater than eight times since their inception in 2014, disparities still exist in the distribution of accredited locations. When specifically evaluating distribution in rural populations, this disparity is even more profound. Our data shows these deficiencies are especially true in the rural areas of the Northern New England with high tobacco use and lower levels of education. This gap in access is troubling, given the higher incidence and later presentation of lung cancer in this and other populations compared to more populated settings. Improving access to high-quality screening in rural regions is critical. Furthermore, the concentration of patients and providers limits our utility of “surgeon density” and restricts our ability to compare our rural population with trends in the Northeast. Identifying areas of need, followed by the creation of targeted interventions to address issues of access in areas of high tobacco use and low education is vital to increasing the incidence of early presentation, and decreasing morbidity and mortality within these high-risk, vulnerable rural populations.
Funding
JD Phillips is supported by The Dartmouth-Hitchcock Cancer Research Fellows Program and by the NCI Cancer Center Support Grant 5P30CA023108 to the Dartmouth-Hitchcock Norris Cotton Cancer Center as well as The Dartmouth Clinical and Translational Science Institute, under award number UL1TR001086 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH).
References
- 1.American Cancer Society. Key statistics for lung cancer. Available from: URLhttps://www.cancer.org/cancer/non-small-cell-lung-cancer/about/keystatistics.html. Accessed July 2, 2019.
- 2.Atkins GT, Kim T, Munson J. Residence in rural areas of the United States and lung cancer mortality. Disease incidence, treatment disparities, and stage-specific survival. Ann Am Thorac Soc. 2017. Mar;14(3):403–411. [DOI] [PubMed] [Google Scholar]
- 3..Afshar N, English DR, Milne RL. Rural-urban residence and cancer survival in high-income countries: a systematic review. Cancer. 2019. Apr 1 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 4.Qin H, Wang F, Liu H, et al. New advances in immunotherapy for non-small cell lung cancer. Am J Transl Res. 2018. Aug 15;10(8):2234–2245. [PMC free article] [PubMed] [Google Scholar]
- 5.Herzberg B, Campo MJ, Gainor JF. Immune checkpoint inhibitors in non-small cell lung cancer. Oncol. 2017. Jan;22(1):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen D, Gharagozloo F, Tempesta B, Meyer M, Gruessner A. Long-term results of robotic anatomical segmentectomy for early-stage non-small-cell lung cancer. Eur J Cardio Thorac Surg. 2019;55(3):427–433. [DOI] [PubMed] [Google Scholar]
- 7.Kneuertz PJ, D’Souza DM, Moffatt-Bruce SD, Merritt RE. Robotic lobectomy has the greatest benefit in patients with marginal pulmonary function. J Cardiothorac Surg. 2018. Jun 5;13(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao X, Zhu X, Fei J, et al. Short-term outcomes and clinical efficacy of stereotactic body radiation therapy (SBRT) in treatment of adrenal gland metastases from lung cancer. Radiat Oncol. 2018. Oct 22;13(1):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Q, Xie R, Lin S, You X, Weng X. Anti-PD-1/PD-L1 antibody therapy for pretreated advanced or metastatic non-small cell lung carcinomas and the correlation between PD-L1 expression and treatment effectiveness: an update meta-analysis of randomized clinical trials. BioMed Res Int. 2018;2018, 3820956. Published 2018 Sep 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis JA, Petty WJ, Tooze JA, et al. Low-Dose CT Lung Cancer screening practices and attitudes among primary care providers at an academic medical center. Canc Epidemiol Biomarkers Prev. April 2015;24(4):664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenkins WD, Matthews AK, Bailey AA, et al. Rural areas are disproportionately impacted by smoking and lung cancer. Preventative Medicine Reports. 2018;10: 200–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The National Lung Screening Trial research team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365: 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wedner R, Fontham ETH, Barrera E, et al. American Cancer Society lung cancer screening guidelines. Ca - Cancer J Clin. 2013;63:106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Lung Cancer Screening; 2013. Accessed on October 15, 2018 Version 1.
- 15.American Lung Association. Providing guidance on lung cancer screening. TheAmerican lung association interim report on lung cancer screening. Available from: URL http://www.lung.org/lung-disease/lung-cancer/lung-cancer-screening-guidelines/lung-cancer-screening.pdf. Accessed on June 15, 2019. [Google Scholar]
- 16.Jacklitsch MT, Jacobson Fl, Austin JH, et al. The American Association for Thoracic Surgery Guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high risk groups. J Thorac Cardiovasc Surg. 2012;144:33–38. [DOI] [PubMed] [Google Scholar]
- 17.Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2013;143:e78S–e92S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brenner AT, Malo TL, Margolis M, et al. Evaluating shared decision making for lung cancer screening. JamaInternMed. August 2018;3054:E1–E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tailor TD, Tong BC, Gao J, Choudhury KR, Rubin GD. A geospatial analysis of factors affecting access to CT facilities: implications for lung cancer screening. J Am Coll Radiol. 2019. Jul 11;(19):30751e30753. pii: S1546–1440. [DOI] [PubMed] [Google Scholar]
- 20.Baldwin DR, Callister MEJ. What is the optimum screening strategy for the early detection of lung cancer. Clin Oncol. July 2016;(28):672–681. [DOI] [PubMed] [Google Scholar]
- 21.Odahowski CL, Zahnd WE, Eberth JM. Challenges and opportunities for lung cancer screening in rural America. J Am Coll Radiol. 2019;16:590–595. [DOI] [PubMed] [Google Scholar]
- 22.Silverstri GA, Nietert PJ, Zoller J, Carter C, Bradford D. Attitudes towards screening for lung cancer among smokers and their non-smoking counterparts. Thorax. 2007;62:126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jonnalagadda S, Bergamo C, Lin JJ, et al. Beliefs and attitudes about lung cancer screening among smokers. Lung Canc. 2012;77:526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willems B, Bracke P. Participants, Physicians, or Programmes: participants’ educational level and initiative in cancer screening. Health Pol. 2018;12: 422–430. [DOI] [PubMed] [Google Scholar]
- 25.Damiani G, Federico B, Basso D, et al. Socioeconomic disparities in the uptake of breast and cervical cancer screening in Italy: a cross sectional study. BMC Publ Health. 2012. Feb 3;12:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frederiksen BL, Jørgensen T, Brasso K, Holten I, Osler M. Socioeconomic position and participation in colorectal cancer screening. Br J Canc. 2010. Nov 9;103(10):1496–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quaife SL, Marlow LAV, McEwen A, Janes SM, Wardle J. Attitudes towards lung cancer screening in socioeconomically deprived and heavy smoking communities: informing screening communication. Health Expect. 2017;20:563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ACR® American College of Radiology. ACR Designated Lung Cancer Screening Center. www.acraccredidation.org/lung-cancer-screening-center. (Accessed on April 23, 2020).
- 29.County Health Rankings. https://www.countyhealthrankings.org/explore-health-rankings/measures-data-sources/county-health-rankings-model?componentType=health-factor&componentId=10; 2016. Accessed on April 23, 2020.
- 30.Porter ED, Fay KA, Hasson RM, Millington TM, Finley DJ, Phillips JD. Routine chest X-rays after thoracic surgery are unnecessary. J Surg Res. 2020. Feb 17;250:188–192. [DOI] [PubMed] [Google Scholar]
- 31.Onega T, Duell EJ, Shi X, Wang D, Demidenko E, Goodman D. Geographic access to cancer care in the. U.S. Cancer. 2008;112(4):909–918. [DOI] [PubMed] [Google Scholar]
- 32.Massarweh NN, Chiang Y-J, Xing Y, et al. Association between travel distance and metastatic disease at diagnosis among patients with colon cancer. J Clin Oncol. 2014;32(9):942–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang R, Columbo JA, Trooboff SW, Servos MM, Goodney PP, Wong SL. Receipt of sentinel lymph node biopsy for thin melanoma is associated with distance traveled for care. J Surg Oncol. 2019;119(1):148–155. [DOI] [PubMed] [Google Scholar]
- 34.de Koning HJ, van der Aalst CM, de Jong, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020. Feb 6;382(6):503–513. [DOI] [PubMed] [Google Scholar]
- 35.Cadarelli R, Rober KL, Carderelli K. Identifying community perspectives for a lung cancer screening awareness campaign in Appalachia Kentucky: the Terminate Lung Cancer (TLC) study. J Canc Educ. 2017;32:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smieliauskas F, Macmahon H, Salgia R. Geographic variation in radiologist capacity and widespread implementation of lung cancer CT screening. J Med Screen. 2014;21:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ersek JL, Eberth JM, McDonnell KK. Knowledge of, attitudes toward, and the use of low-dose computed tomography for lung cancer screening among family physicians. Cancer. 2016;122:2324–2331. [DOI] [PubMed] [Google Scholar]
- 38.United States Census Bureau. New Census Data Show Differences between Urban and Rural Populations; 2016. Release Number CB16–210 www.census.gov/nersrrom.pressreleases/2016/cb16-210. Accessed on April 23, 2020.
- 39.ERS. Rural Population and Migration Briefing Room. www.ers.usda.gov/briefing/population/. Accessed on April 23, 2020.
- 40.Barrett NJ, Ingraham KL, Bethea K, et al. Project PLACE: enhancing community and academic partnerships to describe and address health disparities. Adv Canc Res. 2020;146:167–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hilty DM, Gentry MT, MCKean AJ, Cowan KE, Lim RF, Lu FG. Telehealth for rural diverse populations: telebehavioral and cultural competencies, clinical outcomes and administrative approaches. mHealth. 2020. Apr 5;6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tailor TD, Tong BC, Gao J, Choudhury KR, Rubin GD. A geospatial analysis of factors affecting access to CT facilities: implications for lung cancer screening. J Am Coll Radiol. 2019;16(12):1663–1668. [DOI] [PubMed] [Google Scholar]
- 43.Van den Bergh KAM, Essink-Bot ML, van Klavaren RJ, de Koning HJ. Informed participation in a randomized controlled trial of computed tomography screening for lung cancer. Eur Respir J. 2009;34:711–720. [DOI] [PubMed] [Google Scholar]
- 44.Ali N, Lifford KJ, Carter B, et al. Barriers to uptake among high-risk individuals declining participation in lung cancer screening: a mixed method analysis of the UK Lung Cancer Screening (UKLS) trial. BMJ Open. 2015:5e008254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel D, Akporobaro A, Chinyanganya N, et al. Attitudes to participation in a lung cancer screening trial: a qualitative study. Thorax. 2012;67:418–425. [DOI] [PubMed] [Google Scholar]
- 46.Cutler DM, Lleras-Muney A. Education and Health: Evaluating Theories and Evidence. The Health Effects of Non-health Policies. National Bureau of Economic Research; 2006. [Google Scholar]
- 47.Beeken RJ, Simon AE, von Wagner C, Whitaker KL, Wardle J. Cancer fatalism: deterring early presentation and increasing social inequalities? Cancer Epidemiol Biomark Prev. 2011;20(10):2127–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niederdeppe J, Levy AG. Fatalistic beliefs about cancer prevention and three prevention behaviors. Cancer epidemiology and Prevention Biomarkers. 2007;16: 998–1003. [DOI] [PubMed] [Google Scholar]
- 49.Jensen LF, Pedersen AF, Andersen B, Fenger-Grøn M, Vedsted P. Distance to screening site and non-participation in screening for breast cancer: a population-based study. J Public Health. 2014. Jun;36(2):292–299. [DOI] [PubMed] [Google Scholar]
- 50.Rubin G, Berensen A, Crawford SM, et al. The expanding role of primary care in cancer control. Lancet Oncol. 2015;16:1231–1272. [DOI] [PubMed] [Google Scholar]
- 51.McIlfatrick S, Kenney S, McKenna H, McCarley N, McElwee G. Investigating role of the general practitioner in cancer prevention: a mixed methods study. BMC Fam Pract. 2013;14:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eberth JM, Crouch EL, Josey MJ, et al. Rural-urban differences in access to thoracic surgery in the United States, 2010 to 2014. Ann Thorac Surg. 2019;108(4):1087–1093. [DOI] [PubMed] [Google Scholar]


