Abstract
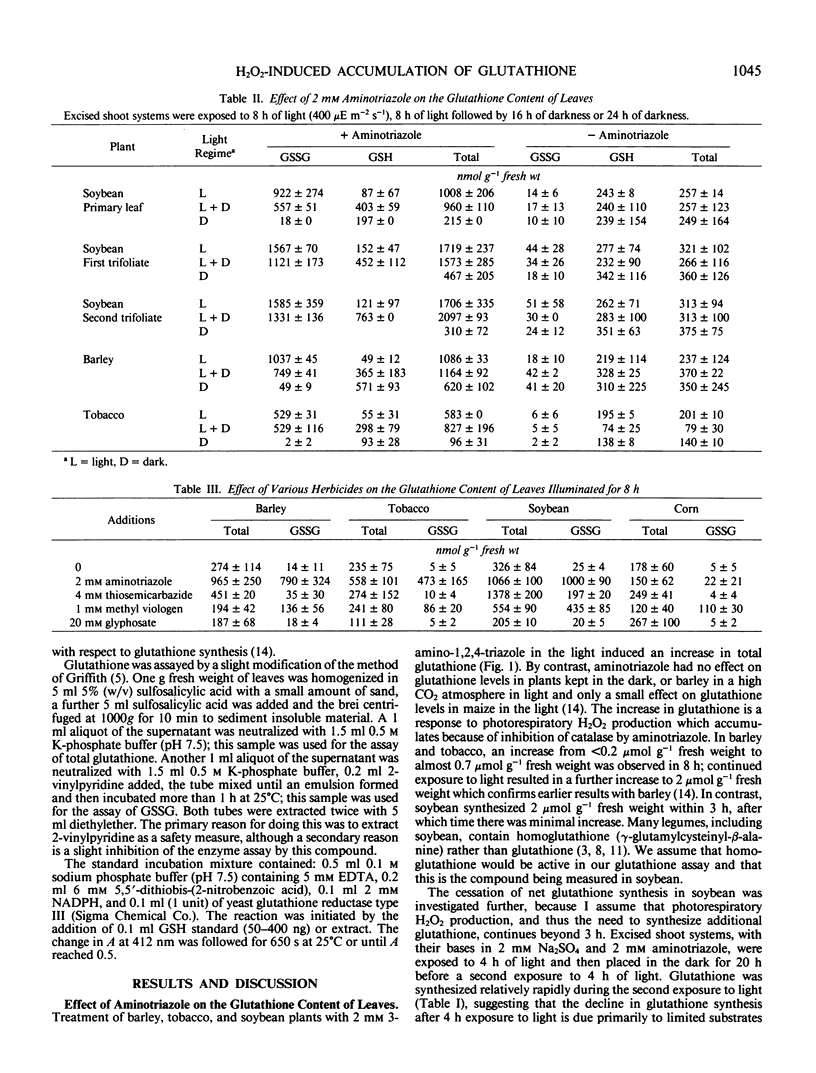
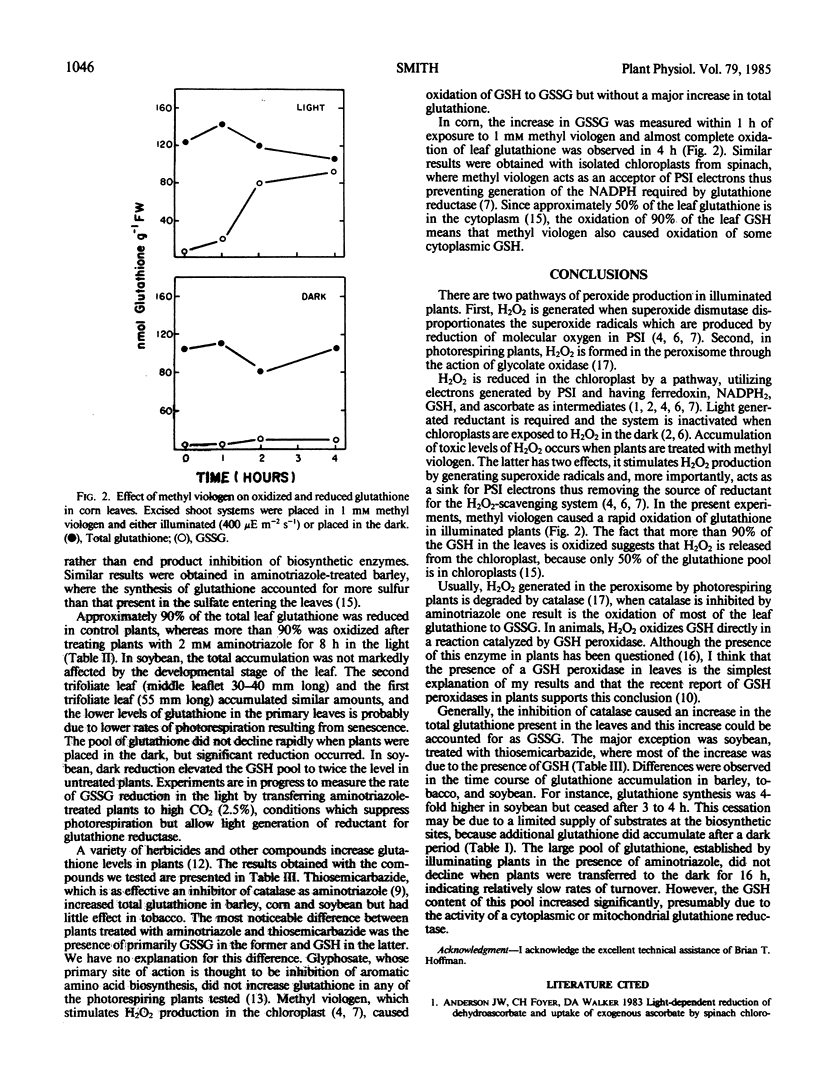
The effect of various herbicides on glutathione levels in barley (Hordeum vulgare L.), tobacco (Nicotiana tabacum L.), soybean (Glycine max [L.] Merr.), and corn (Zea mays L.) was examined. Illumination of excised barley, tobacco, and soybean plants for 8 hours in solution containing 2 millimolar aminotriazole (a catalase inhibitor) resulted in an increase in leaf glutathione from 250 to 400 nanomoles per gram fresh weight to 600 to 1800 nanomoles per gram fresh weight, depending on the species tested. All of this increase could be accounted for as oxidized glutathione. Between 25 and 50% of this oxidized glutathione was reduced when plants were darkened for 16 hours, but there was no significant decline in total glutathione. Another catalase inhibitor, thiosemicarbazide, was as effective as aminotriazole in elevating glutathione in soybean but was less effective in barley and tobacco. Glyphosate, an inhibitor of aromatic amino acid biosynthesis, had no significant effect on glutathione levels in any of the plants examined. Whereas methyl viologen (paraquat), which is a sink for photosystem I electrons, caused oxidation of leaf glutathione in all of the plants but did not increase the total amount of glutathione present.
Full text
PDF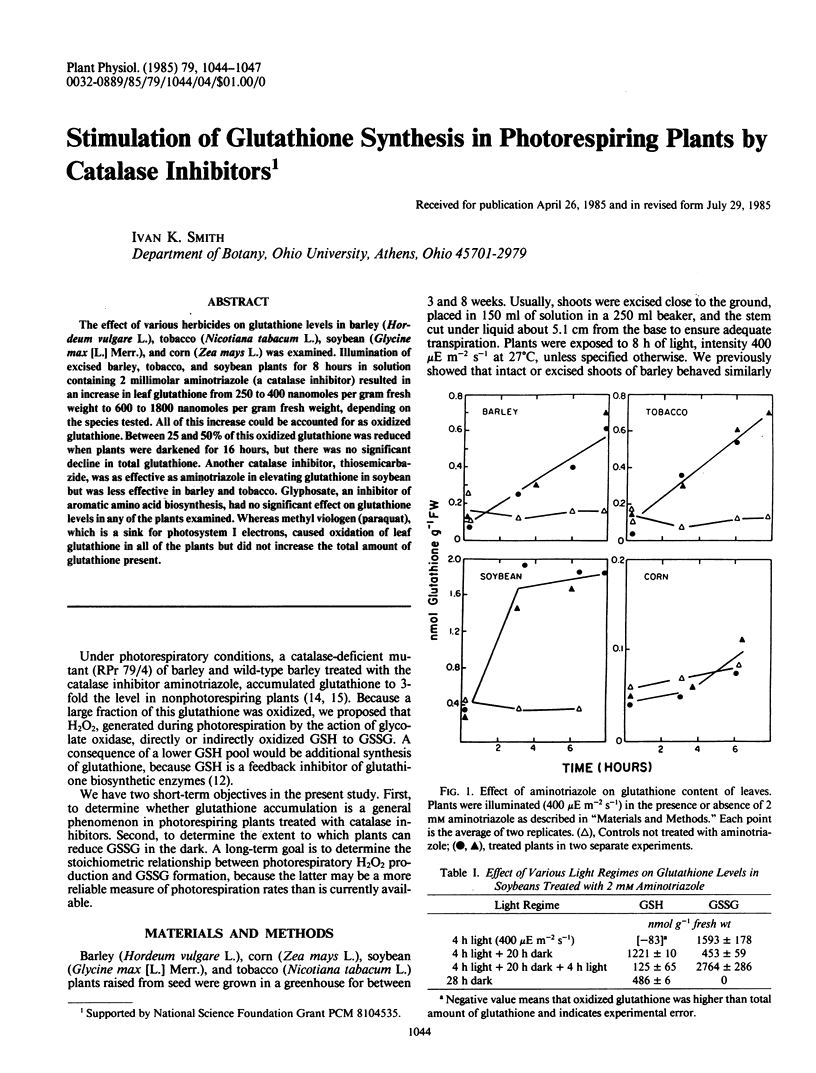

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CARNEGIE P. R. ISOLATION OF A HOMOLOGUE OF GLUTATHIONE AND OTHER ACIDIC PEPTIDES FROM SEEDLINGS OF PHASEOLUS AUREUS. Biochem J. 1963 Dec;89:459–471. doi: 10.1042/bj0890459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Law M. Y., Charles S. A., Halliwell B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of Paraquat. Biochem J. 1983 Mar 15;210(3):899–903. doi: 10.1042/bj2100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIASH E., NOVOGRODSKY A., SCHEJTER A. Irreversible reaction of 3-amino-1:2:4-triazole and related inhibitors with the protein of catalase. Biochem J. 1960 Feb;74:339–348. doi: 10.1042/bj0740339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbaugh J. M., Fall R. Characterization of a Selenium-Independent Glutathione Peroxidase From Euglena gracilis. Plant Physiol. 1985 Feb;77(2):437–442. doi: 10.1104/pp.77.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRICE C. A. A new thiol in legumes. Nature. 1957 Jul 20;180(4577):148–149. doi: 10.1038/180148a0. [DOI] [PubMed] [Google Scholar]
- Rubin J. L., Gaines C. G., Jensen R. A. Glyphosate Inhibition of 5-Enolpyruvylshikimate 3-Phosphate Synthase from Suspension-Cultured Cells of Nicotiana silvestris. Plant Physiol. 1984 Jul;75(3):839–845. doi: 10.1104/pp.75.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J., Shrift A. Phylogenetic distribution of glutathione peroxidase. Comp Biochem Physiol B. 1979;63(1):39–44. doi: 10.1016/0305-0491(79)90231-1. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E. Metabolic pathways in peroxisomes and glyoxysomes. Annu Rev Biochem. 1981;50:133–157. doi: 10.1146/annurev.bi.50.070181.001025. [DOI] [PubMed] [Google Scholar]


