Abstract
Necrotizing enterocolitis (NEC) is the leading cause of morbidity and mortality in preterm infants. NEC is multifactorial and the result of a complex interaction of feeding, dysbiosis, and exaggerated inflammatory response. Feeding practices in the neonatal intensive care units (NICUs) can vary among institutions and have significant impact on the vulnerable gastointestinal tract of preterm infants. . These practices encompass factors such as the type of feeding and fortification, duration of feeding, and rate of advancement, among others. The purpose of this article is to review the data on some of the most common feeding practices in the NICU and their impact on the development of NEC in preterm infants. Data on the human milk bioactive component glycosaminoglycans, specifically hyaluronan, will also be discussed in the context of postnatal intestinal development and NEC prevention.
Keywords: Necrotizing enterocolitis, Preterm infants, NICU feeding practices, Human milk bioactive component, Human milk glycosaminoglycans, Hyaluronan, Inflammatory bowel disease
Introduction
Necrotizing enterocolitis (NEC) is the most common gastrointestinal emergency in preterm infants. Despite decades of research, mortality and morbidity from NEC have not changed1 Approximately, 30% of infants who develop NEC require surgical intervention, and 30-40% do not survive2. NEC is also associated with significant long-term morbidities such as short-gut syndrome, growth failure, cerebral palsy, and learning disabilities2-6. The total annual costs to care for infants with NEC in the United States alone are estimated to be between $500 million and $1 billion. As such, NEC is one of the most devastating diseases in preterm infants with an urgent need for preventive or treatment strategies.
Although the pathogenesis of NEC is unclear, studies suggest that NEC is multifactorial and a result of the complex interaction of feeding, abnormal bacterial colonization, and exaggerated inflammatory response of the preterm intestinal epithelium7,8. Enteral feeding is widely recognized as a significant risk factor with nearly 99% of infants who develop NEC already on feeds. Although variations in feeding practices exist among institutions, it’s widely recognized that exclusive human milk (HM) feeding is protective against NEC9-14. Both human and animal studies have demonstrated that bovine-based formula increases intestinal permeability, upregulates oxidative stress, and is directly toxic to intestinal epithelial cells15,16. Preterm infants who receive HM in the first two weeks after birth experience a six to ten-fold decrease in the incidence of NEC compared to formula-fed infants17,18. Similarly, formula feeding in preterm infants born less than 1500 grams is associated with an increased risk of NEC development compared to donor milk feeds19,20. The protective effects of HM are believed to be mediated through its bioactive components such as lactoferrin, immunoglobulin (IgA), and HM oligosaccharides. Our laboratory focuses on studying glycosaminoglycans (GAGs), a class of polysaccharides prevalent in HM, as potential bioactive factors in HM21-24. Though our understanding of the functions of this class of molecules is still evolving, their elevated concentrations in early HM and protective capabilities against pathogens indicate their importance in the prevention of intestinal pathologies25-27. This article aims to provide a brief overview of the available literature on the various feeding practices in the NICU and their impact on the development of NEC in preterm infants. Data on the human milk GAGs, specifically hyaluronan, will also be discussed in the context of postnatal intestinal development and NEC.
Feeding practices in preterm infants and NEC
Various feeding practices such as the timing of initiation of feeds, rate of advancement of feeds, and type of fortification can influence the immature intestinal tract and affect the risk of NEC development28. Included in this section are some of the most common practices and their influence on the incidence of NEC.
Standardized feeding protocols
Multiple studies have emphasized the significance of standardized feeding protocols (SFP) in the NICU in reducing the incidence of NEC29,30. The most recent meta-analysis was published in 2017 and included a total of ~18,000 preterm infants from 15 studies31. The authors showed an almost 80% reduction in the incidence of NEC with the use of SFP in preterm infants born less than 37 weeks32. To account for potential variations in practices in the NICU over time that can influence the incidence of NEC, the authors divided the studies into two epochs: 1978 to 2004 and 2004 to 2016. Notably, even when comaring the two epochs, a statistically significant reduction in NEC incidence was observed, high-lighting the importance of a standardized approach for feeding in the NICU. Although, the mechanism by which SFP prevents NEC remains unclear, the benefits associated with early initiation of feeds, trophic feeds in preterm infants, and the consistency in NICU clinical practice are likely contributing factors.
Initiation and progression of enteral feeds
Delayed initiation of enteral feeds is often practiced in unstable infants due to concerns of feeding intolerance or increased risk of NEC. However, a recent meta-analysis published in 2022 showed that delaying initiation of feeds did not provide any benefits in terms of prevention of NEC or mortality, but rather, was associated with a slight increase in invasive infection33-35. In addition, Nangia et al conducnted a randomized study in which very- low birth weight infants were assigned to start feeding at 80mL/kg/day on the first day of life, as compared to the conventional trophic feeds of 20mL/kg/day36. They showed that a higher volume of firstday feeds was safe and did not lead to an increased risk of NEC or mortality when compared to conventional trophic feeds. Infants receiving early higher volumes reached goal feeds quicker, had fewer complications, and shorter hospital stay36. In summary, delaying the initiation of feeds does not decrease the risk of NEC and may even be associated with an increased risk of infection. A higher volume of feeds initiated early after birth seems to be safe, but larger studies are needed to confirm those results.
Furthermore, studies have shown that delaying early progressive enteral feeds, although associated with a slight benefit in terms of feeding tolerance, does not have an impact on in-hospital mortality and NEC37,38. The most recent meta-analysis which encompassed a total of 4033 preterm infants confirmed that a slow advancement in feeding (15-24ml/kg/day) compared to a faster increase in feeding (30-40mL/kg/day) did not provide any advantages in terms of NEC prevention, in-hospital mortality, or feeding tolerance33. On the contrary, a slower rate of increasing feeds was associated with a slight increase in invasive infection in preterm infants38. Taken together, the current level of evidence supports the earlier initiation of feeds and a faster progression approach.
Continuous versus intermittent bolus feeding
Enteral feedings in preterm infants can be given intermittently every two to three hours, via a nasogastric or orogastric tube, or continuously over 24-hour period. While theoretical benefits exist for each method, the data regarding clinical outcomes, specifically NEC, remains unclear33,39-41. A meta-analysis published in 2020 found that infants born < 37 weeks and < 2500 grams who were bolus-fed reached goal feeds sooner compared to continuous feeds, with no differences in the incidence of NEC, rate of growth, or length of hospital stay. Moreover, no differences were found between two- and three-hourly feeding intervals in terms of feeding complications, in particular NEC, in preterm infants42.
Type and timing of fortification of HM
Controversy exists regarding the risks and benefits of early versus late fortification of HM for preterm infants. Three relatively recent systematic reviews showed that early fortification, defined as fortification before reaching a volume of 100ml/kg/day and within 7 days of life, versus later fortification, defined as fortification after reaching a volume of 100mg/kg/day and after 7 days of age, did not significantly impact the incidence of NEC, time to achieve full feeds, mortality rates, and length of hospital stay43-45. However, it is important to note that the certainty of evidence from the included studies was considered low due to the small sample size and the lack of blinding in the included studies. Consequently, larger and well-designed studies are required to provide more conclusive evidence regarding the potential benefits of early fortification.
Moreover, there is limited available evidence comparing the use of HM-derived fortification versus bovine milk-derived fortifiers46. O’Connor et al conducted a study in preterm infants and found no differences in NEC incidence, feeding intolerance, or mortality when HM or bovine milk-derived fortifiers were used47. However, in a subgroup analysis of a study comparing outcomes in infants receiving their mother’s milk fortified with either HM-derived fortification or powdered bovine milk-derived fortification, it was observed that the bovine-milk derived group had a higher risk of NEC and the combined outcome of NEC, surgery, or death i48,49. It is worth noting that no studies have yet compared the use of the liquid form of bovine milk fortification with HM-derived fortification on the incidence of NEC in preterm infants.
Human milk glycosaminoglycans
HM feeding decreases the risk of NEC development in preterm infants but does not completely prevent the disease. This likely is due to the multifactorial nature of the disease and the heterogeneity of bioactive components in HM. Our laboratory studies glycosaminoglycans (GAGs), a class of polysaccharides compro-mised of repeating disaccharides50,51, as potential bioactive factors that protect against NEC21-24. GAGs are classified based on the composition of monosaccharide composition, the glycosidic linkage, and the sulfation levels into four categories: hyaluronic acid (HA); chondroitin sulfate (CS), and dermatan sulfate; heparan sulfate (HS) and heparin; and keratan sulfate52. GAGs are synthesized in the mammary gland through the sequential action of specific glycosyltransferases, linked to a protein “core” and excreted as proteoglycans. It is important to note that milk GAGs reach the small intestine intact as there are no specific enzymes capable of degrading them in the GI tract. Therefore, these molecules are thought to play important roles in intestinal development and protection against enteric pathogens.
Multiple factors influence the composition of GAGs in milk. Quantitative and qualitative differences in GAG composition exist in human versus bovine milk. HM contains 7 times higher concentration of GAG than bovine milk53, mainly in the form of under-sulfated CS (~55% of GAGs), followed by HS (~40%)54. The influence of preterm birth and the stage of lactation on the composition of GAGs has also been demonstrated. Preterm HM contains about three times more GAGs than term HM milk, with the highest values found in colostrum. In colostrum, the GAG concentration is around 9.3 and 3.8 g/l in preterm and term HM, respectively, with a progressive decrease to 4.3 and 0.4 g/l, by the end of the first month of lactation. The degree of sulfation also varies during the lactation period, with sulfation levels of HS and CS increasing in the early months of lactation before subsequently declining55. This variability based on gestational age and the changing sulfation pattern during the breastfeeding period has not yet been evaluated, but likely have significant implications for protection against enteric infections or NEC56. However, further reseach is needed to fully understand the impact of these factors on the protective properties of GAGs in milk.
Studies also indicate that maternal health can directly influence GAG composition in HM. For example, alterations in the structure and sulfation levels of CS have been observed in the milk of a breast affected by invasive carcinoma compared to the unaffected breast of the same mother57. Moreover, Cerdó et al.58 showed that the gut microbiome of infants born to obese mothers has a greater capacity for GAG degradation compared to infants born to mothers of normal weight. The specific implications of such findings on intestinal health or clinical outcomes are currently unknown and require further investigation.
Finally, since donor milk is the recommended substitute when a mother’s milk is unavailable, the effect of holder pasteurization on HM GAG was investigated. Notably, though this holder pasteurization can affect HM anti-infective properties, the overal GAG concentrations, and relative proportions are largely unaffected by this method or by processing and storage59,60. This suggests that the beneficial effects of GAGs in donor milk may still be preserved even after the pasteurization process.
The role of HM GAGs in models of intestinal inflammation, NEC, and the microbiome
While the specific functions of HM GAGs are still being investigated, their high concentration in HM and protective role against pathogens suggest a potential protective role in the prevention of NEC and enteric infections50,61. Studies have demonstrated the ant-infection and anti-inflammatory properties of GAGs, as well as their physiological roles in complex interactions such as cell growth and differentiation, cell–cell and cell–matrix interactions62,63. Importantly, due to a lack of digestive enzymes capable of breaking down GAGs64, HM GAGs remain undigested throughout the majority of the gastrointestinal tract50,64-66. As a consequence, HM GAGs act as soluble receptors binding to pathogens and preventing invasion of the intestinal mucosa67 as demonstrated against HIV, RSV, and CMV67,68.
One of the most prominent GAGs in HM is CS, compromising ~ 55% of the total GAG content69. Extensive research on CS demonstrates important antibacterial, antiviral, anti-inflammatory, and antioxidant properties of this particular molecule70-72. Convincing evidence from clinical studies shows that oral supplementation with CS increases the predominance of Bacteroides with a reduction of Staphylococcus, Enterococcus, and Clostridium in the gut, which may play an important role in maintaining intestinal health73. Furthermore, in an obesity-induced intestinal inflammation model, oral administration of CS resulted in an elevation of beneficial bacteria such as Lactobacillus and short-chain fatty acid (SCFA)-producing bacteria, including Lactobacillus, Bifidobacterium, and Lachnospiraceae NK4A136 group. It also led to a reduction in the lipopolysaccharide (LPS) producer (Escherichia coli). These changes were associated with the alleviation of inflammation, as evidenced by a decreased pro-inflammatory cytokines in both the circulation and intestine. Furthermore, in a prospective observational study in patients with inflammatory bowel disease (IBD) in remission, CS treatment for osteoarthritis was associated with a lower incidence of IBD relapse compared to expected rates74. In a randomized double-blinded, placebo-controlled trial in dogs with IBD, oral CS supplementation along with prebiotics led to a significant reduction in clinical illness, improvement in intestinal histology, and decreased levels of serum markers of inflammation and oxidative injury75. Moreover, CS has been shown to reduce bacterial invasion and translocation in vitro69. Burge et al. demonstarted that CS resulted in a significant decrease in bacterial translocation and invasion across the cell monolayer, in a concentrationdependent manner, with no effect on cell viability. Lastly, in vivo, the combined administration of CS prenatally and postnatally has been shown to be protective in a murine model of NEC. This protection is likely achieved through the reduction of intestinal dysbiosis and an increase in the relative abundance of lactobacillus76.
Effects of HA on intestinal development, inflammation, and the microbiome
Our previous work highlights the protective roles of exogenous HA, free or bound to proteins, in models of sepsis and intestinal bacterial invasion and translocation69,77,78. HA is a non-sulfated GAG composed of repeating D-glucuronic acid and N-acetyl-D-glucosamine units79. HA is synthesized by three HA synthases (HAS1, HAS2, and HAS3) in the plasma membrane and secreted into the extracellular space. In the small intestine and colon, HA is found in the extracellular space adjacent to crypt epithelial cells and peri cryptal macrophages80. Compelling evidence suggests a critical role of endogenous HA in intestinal development and inflammation81. Stenson et al. showed that HA binding to TLR4 on macrophages regulates intestinal and colonic growth by promoting crypt fission and LGR5+ stem cell proliferation80. In a subsequent study, the same group showed that blocking HA binding to TLR4 and CD44 receptors in the first weeks of life in mouse pups resulted in a 30% decrease in the length of the small intestine and colon compared to controls, demonstrating the critical role of endogenous HA in intestinal development80,82.
The effects of exogenous HA treatment on the intestine have been investigated in multiple studies in vivo. While earlier studies suggested that HMW HA is anti-inflammatory, whereas LMW is proinflammatory, more recent literature supports the notion that the effects of HA are context dependent, influenced by the environment, mode of administration, and specific pathology83. For instance, Riehl et al. showed that long-term systemic administration of HA750 over 5 weeks induced epithelial proliferation and promoted small intestinal and colonic lengthening in mice in a TLR4-dependent manner82. They further showed that intraperitoneal HA750 treatment, given 8 hours before irradiation, provided radioprotection and increased crypt survival in mice. We and others explored oral administration of lower molecular weight HA, specifically HA35 on intestinal development and inflammation. Hill et al. demonstartedthat oral administration of HA35, not larger or smaller sizes, had similar effects to HM-derived HA on the intestinal epithelium both in vitro and in vivo25. They showed that HA isolated from HM or HA 35 increased the expression of beta defensin-2, a key antimicrobial peptidem in vitro and in vivo via CD44 and TLR-4. Furthermore, they observed that HA35 inhibited Salmonella enterica infection in vitro27. Similar results were reported on the protective effects of HA35 on Citrobacter infection and in ethanol-induced intestinal injury models.
Our lab recently investigated the effect of oral HA35 on small intestinal (SI) maturation, epithelial proliferation, and differentiation. We showed that oral treatment of HA35 in mouse pups for 7 days was associated with increased ileal villus length and crypt depth, and enhanced intestinal epithelial cell proliferation79. Oral HA35 treatment also induced an increase in the goblet and Paneth cells in the small intestine of mouse pups. The effects on the goblet and Paneth cell numbers are particularly important, as both cells play crucial roles in the protection against enteric infection and NEC. Goblet cells produce the mucus layer of the intestine which acts as a physical barrier preventing the interaction between pathogenic bacteria and the epithelium79,84,85. Paneth cells are pivotal for intestinal homeostasis through their role in maintaining stem cell health and releasing antimicrobial proteins (e.g., defensins) that influence microbiome composition79,86-89 and protect against bacterial infection90.
Effect of HA on intestinal microbiome
Microbial colonization of the infant gastrointestinal tract is generally agreed to begin at birth, but the evolution of the gut microbiome continues until relative stabilization, several years into childhood91,92. Microbial seeding of the infant microbiome is affected by many environmental factors, including mode of delivery, medication use, and infant diet. HM contains bioactive factors, such as HM oligosaccharides (HMOs), secretory IgA (sIgA), lactoferrin, and GAGs, potentially responsible, in part, for the more typically favorable microbiome established in breastfed infants. Many of these HM factors, such as HA, are not significantly degraded along the length of the intestine93 and are thus postulated to function as a prebiotic50. Supraphysiological levels of HA35 for 7 d in breastfeeding mice induced increases in Clostridiales Ruminococcaceae, Lactobacillales Lactobacillaceae, and Clostridiales Lachnospiraceae, and decreases in Bacillales, Staphylococcaceae, Bacteroidales Rikenellaceae, and others21. Notably, Clostridiales Ruminococcaceae and Lachnospiraceae are known producers of beneficial short-chain fatty acids94, while Lactobacillales Lactobacillaceae represent potential prophylactic probiotics against NEC95. In the colon, nanomolecular HA bound to bilirubin results in increases in the protective Clostridium XIVα and Akkermansia muciniphila, both types of bacteria are implicated in gut homeostasis96. Finally, HA may physically inhibit adhesion and invasion of pathogenic bacteria in the gut, a property widely exploited in bioengineered orthopedic scaffolding to prevent infection following surgery97.
HA in necrotizing enterocolitis
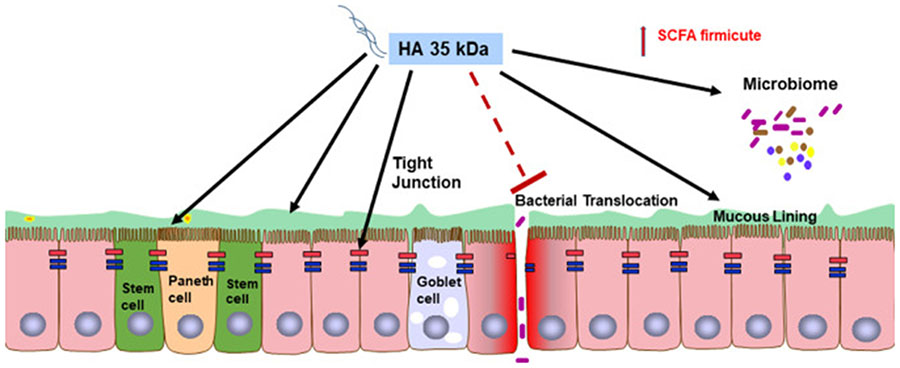
One of the most significant benefits provided by HA is the ability to reduce intestinal inflammation98, particularly in neonatal NEC99. In premature infants, NEC occurs, in part, due to bacterial activation of TLR4, resulting in hyperinflammation, intestinal epithelial injury, bacterial epithelial translocation, and systemic dissemination100. In a murine NEC model that incorporate Paneth cell disruption Klebsiella pneumoniae dysbiosis101, and intestinal development and maturity roughly equivalent to that of human 22-24 week preterm infants102, pre-treatment of HA35, at doses of 15 mg/kg body weight or 30 mg/kg body weight, once daily for three days, increased survival of P14-16 murine pups in a dose-dependent manner99. Improved survival via HA35 appeared to be mediated through reductions in intestinal histological injury, bacterial translocation, and systemic release of the proinflammatory cytokines IFN-γ (interferon-γ), TNF-α (tumor necrosis factor-α), Gro-α, IL (interleukin)-12ρ70, and IL-6. In addition, HA35 reduced intestinal permeability and upregulated expression of the tight junction proteins, claudin-2, −3, −4, occludin, and zonula occludens-1 (ZO-1). Fig. 1
Fig. 1.
Oral HA35kDa (HA35) promotes postnatal intestinal development, increasing intestinal stem cell proliferation, and differentiation into Paneth cells and goblet cells. Oral HA35 in mouse pups leads to increased predominance of Short chain fatty acid (SCFA) producing bacteria, suggesting a potential role as a prebiotics. Oral HA35 also protects against NEC development through increasing TJ protein expression reduction in bacterial transloation and subsequent intestinal inflammation and injury.
Similarly, in a murine NEC model incorporating formula feeding and Paneth cell knockdown, pups pretreated with HA35 for 7 d before NEC induction experienced significantly reduced intestinal histological injury and improved survival. RNAseq analysis showed that HA35-protected pups were characterized by increased ileal expression of TRIM58 (tripartite motif containing 58), ST6GALNAC1 (ST6 N-acetylgalactosaminide alpha-2,6-sialytransferase 1), and RETNLB (resistin-like beta), genes associated with innate immunity and goblet cell barrier function. Results from both models suggest an important role of HA35 in protection against NEC by enhancing barrier function, through increasing TJ protein expression, number of mucous-producing goblet cells, and antimicrobial-producing Paneth cells.
Conclusion
Feeding practices remains a significant factor influencing the risk of NEC development in preterm infants. Wide variation in NICU feeding practices exists in preterm infants, potentially contributing to the difference in NEC incidences among different institutions. While HM feeding reduces the risk of NEC in preterm infants, it does not completely eliminate the disease. This likely is due to the multifactorial aspect of the disease and the heterogeneity of bioactive components in HM. Future studies are needed to determine the specific roles of feeding practices and HM bioactive factors, specifically GAGs, on morbidity and mortality in preterm infants. By gaining a deeper understanding of these factors, we can strive to optimize feeding strategies and enhance the protective benefits of HM in reducing the incidence of NEC and improving outcomes for preterm infants.
Dedication
Charlie was born prematurely at 26 weeks. She developed NEC at 5 weeks of age and lost approximately 70% of her small intestine. Due to the ongoing support and care of her knowledgeable NICU team, Intestinal Rehabilitation team, and Early Intervention services, Charlie is a creative, silly 3.5-year-old who loves dance, gymnastics, soccer, and school. She is a central line graduate and receives nutrition via G-tube.

Abbreviations:
- NEC
Necrotizing enterocolitis
- HM
Human milk
- NICU
Neonatal intensive care
- GAGs
Glycosaminoglycans
- HA
Hyaluronan
- SFP
Standard feeding protocols
- CS
Chondroitin Sulfate
- TLR4
Toll-like receptor 4
References
- 1.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368(9543):1271–1283. [DOI] [PubMed] [Google Scholar]
- 2.Patel RM, et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med. 2015;372(4):331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hintz SR, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005;115(3):696–703. [DOI] [PubMed] [Google Scholar]
- 4.Hickey M, Georgieff M, Ramel S. Neurodevelopmental outcomes following necrotizing enterocolitis. Semin Fetal Neonatal Med. 2018;23(6):426–432. [DOI] [PubMed] [Google Scholar]
- 5.Hull MA, et al. Mortality and management of surgical necrotizing enterocolitis in very low birth weight neonates: a prospective cohort study. J Am Coll Surg. 2014;218(6):1148–1155. [DOI] [PubMed] [Google Scholar]
- 6.Rowe MI, et al. Necrotizing enterocolitis in the extremely low birth weight infant. J Pediatr Surg. 1994;29(8):987–990 discussion 990-1. [DOI] [PubMed] [Google Scholar]
- 7.Duffy LC. Interactions mediating bacterial translocation in the immature intestine. J Nutr. 2000;130(2S Suppl):432s–436s. [DOI] [PubMed] [Google Scholar]
- 8.Hackam D, Caplan M. Necrotizing enterocolitis: Pathophysiology from a historical context. Semin Pediatr Surg. 2018;27(1):11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He Y, Lawlor NT, Newburg DS. Human Milk Components Modulate Toll-Like Receptor-Mediated Inflammation. Adv Nutr. 2016;7(1):102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker WA, Iyengar RS. Breast milk, microbiota, and intestinal immune homeostasis. Pediatr Res. 2015;77(1-2):220–228. [DOI] [PubMed] [Google Scholar]
- 11.Westerbeek EA, et al. The effect of enteral supplementation of a prebiotic mixture of non-human milk galacto-, fructo- and acidic oligosaccharides on intestinal permeability in preterm infants. Br J Nutr. 2011;105(2):268–274. [DOI] [PubMed] [Google Scholar]
- 12.van den Berg A, et al. The effect of glutamine-enriched enteral nutrition on intestinal permeability in very-low-birth-weight infants: a randomized controlled trial. JPEN J Parenter Enteral Nutr. 2006;30(5):408–414. [DOI] [PubMed] [Google Scholar]
- 13.Foster JP, Seth R, Cole MJ. Oral immunoglobulin for preventing necrotizing enterocolitis in preterm and low birth weight neonates. Cochrane Database Syst Rev. 2016;4:Cd001816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maffei D, Schanler RJ. Human milk is the feeding strategy to prevent necrotizing enterocolitis!. Semin Perinatol. 2017;41(1):36–40. [DOI] [PubMed] [Google Scholar]
- 15.Burge K, et al. Lipid Composition, Digestion, and Absorption Differences among Neonatal Feeding Strategies: Potential Implications for Intestinal Inflammation in Preterm Infants. Nutrients. 2021;13(2) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel AL, Kim JH. Human milk and necrotizing enterocolitis. Semin Pediatr Surg. 2018;27(1):34–38. [DOI] [PubMed] [Google Scholar]
- 17.Schanler RJ, et al. Randomized trial of donor human milk versus preterm formula as substitutes for mothers’ own milk in the feeding of extremely premature infants. Pediatrics. 2005;116(2):400–406. [DOI] [PubMed] [Google Scholar]
- 18.Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2014(4):Cd002971. [DOI] [PubMed] [Google Scholar]
- 19.Quigley M, Embleton ND, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2018;6(6):Cd002971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu AS, Harrison CM. Formula feeding results in better growth and weight gain compared to donor breast milk in preterm and low birthweight infants, with a greater risk in necrotising enterocolitis. Arch Dis Child Educ Pract Ed. 2020;105(6):381–382. [DOI] [PubMed] [Google Scholar]
- 21.Chaaban H, et al. Acceleration of Small Intestine Development and Remodeling of the Microbiome Following Hyaluronan 35 kDa Treatment in Neonatal Mice. Nutrients. 2021;13(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burge K, et al. The Role of Glycosaminoglycans in Protection from Neonatal Necrotizing Enterocolitis: A Narrative Review. Nutrients. 2020;12(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gunasekaran A, et al. Hyaluronan 35 kDa enhances epithelial barrier function and protects against the development of murine necrotizing enterocolitis. Pediatr Res. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunasekaran A, et al. Hyaluronan 35 kDa enhances epithelial barrier function and protects against the development of murine necrotizing enterocolitis. Pediatr Res. 2020;87(7):1177–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill DR, et al. Specific-sized hyaluronan fragments promote expression of human beta-defensin 2 in intestinal epithelium. J Biol Chem. 2012;287(36):30610–30624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim Y, de la Motte CA. The Role of Hyaluronan Treatment in Intestinal Innate Host Defense. Front Immunol. 2020;11:569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill DR, et al. Human milk hyaluronan enhances innate defense of the intestinal epithelium. J Biol Chem. 2013;288(40):29090–29104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meek JY, Noble L. Policy Statement: Breastfeeding and the Use of Human Milk. Pediatrics. 2022;150(1). [DOI] [PubMed] [Google Scholar]
- 29.Patole SK, de Klerk N. Impact of standardised feeding regimens on incidence of neonatal necrotising enterocolitis: a systematic review and meta–analysis of observational studies. Arch Dis Child Fetal Neonatal Ed. 2005;90(2):F147–F151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moschino L, et al. Optimizing Nutritional Strategies to Prevent Necrotizing Enterocolitis and Growth Failure after Bowel Resection. Nutrients. 2021;13(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jasani B, Patole S. Standardized feeding regimen for reducing necrotizing enterocolitis in preterm infants: an updated systematic review. J Perinatol. 2017;37(7):827–833. [DOI] [PubMed] [Google Scholar]
- 32.Jasani B, Patole S. Standardized feeding regimen for reducing necrotizing enterocolitis in preterm infants: an updated systematic review. J Perinatol. 2017;37(7):827–833. [DOI] [PubMed] [Google Scholar]
- 33.Ou J, et al. Nutrition in Necrotizing Enterocolitis and Following Intestinal Resection. Nutrients. 2020;12(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan J, Bombell S, McGuire W. Early trophic feeding versus enteral fasting for very preterm or very low birth weight infants. Cochrane Database of Systematic Reviews. 2013(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young L, Oddie SJ, McGuire W. Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database of Systematic Reviews. 2022(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nangia S, et al. Early Total Enteral Feeding versus Conventional Enteral Feeding in Stable Very-Low-Birth-Weight Infants: A Randomised Controlled Trial. Neonatology. 2019;115(3):256–262. [DOI] [PubMed] [Google Scholar]
- 37.Young L, Oddie SJ, McGuire W. Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev. 2022;1(1):Cd001970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oddie SJ, Young L, McGuire W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev. 2021;8(8):Cd001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Zhu W, Luo BR. Continuous feeding versus intermittent bolus feeding for premature infants with low birth weight: a meta-analysis of randomized controlled trials. Eur J Clin Nutr. 2020;74(5):775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olieman JF, et al. Enteral nutrition in children with short-bowel syndrome: current evidence and recommendations for the clinician. J Am Diet Assoc. 2010;110(3):420–426. [DOI] [PubMed] [Google Scholar]
- 41.Davis TA, Fiorotto ML, Suryawan A. Bolus vs. continuous feeding to optimize anabolism in neonates. Curr Opin Clin Nutr Metab Care. 2015;18(1):102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ibrahim NR, et al. Short versus long feeding interval for bolus feedings in very preterm infants. Cochrane Database Syst Rev. 2021;8(8):Cd012322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alyahya W, et al. Early versus Delayed Fortification of Human Milk in Preterm Infants: A Systematic Review. Neonatology. 2020;117(1):24–32. [DOI] [PubMed] [Google Scholar]
- 44.Thanigainathan S, Abiramalatha T. Early fortification of human milk versus late fortification to promote growth in preterm infants. Cochrane Database Syst Rev. 2020;7(7):Cd013392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Basu S, et al. Early versus late fortification of breast milk in preterm infants: a systematic review and meta-analysis. Eur J Pediatr. 2020;179(7):1057–1068. [DOI] [PubMed] [Google Scholar]
- 46.Hair AB, Scottoline B, Good M. Dilemmas in human milk fortification. J Perinatol. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Connor DL, et al. Nutrient enrichment of human milk with human and bovine milk-based fortifiers for infants born weighing <1250 g: a randomized clinical trial. Am J Clin Nutr. 2018;108(1):108–116. [DOI] [PubMed] [Google Scholar]
- 48.Sullivan S, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr. 2010;156(4):e1. [DOI] [PubMed] [Google Scholar]
- 49.Lucas A, Boscardin J, Abrams SA. Preterm Infants Fed Cow’s Milk-Derived Fortifier Had Adverse Outcomes Despite a Base Diet of Only Mother’s Own Milk. Breastfeed Med. 2020;15(5):297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burge K, et al. The Role of Glycosaminoglycans in Protection from Neonatal Necrotizing Enterocolitis: A Narrative Review. Nutrients. 2020;12(2):546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richard-Blum S. Glycosaminoglycans: major biological players. Glycoconj. J. 2017;34:275–276. [DOI] [PubMed] [Google Scholar]
- 52.Coppa GV, et al. Human milk glycosaminoglycans: the state of the art and future perspectives. Ital J Pediatr. 2013;39(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coppa GV, et al. Composition and structure elucidation of human milk glycosaminoglycans. Glycobiology. 2011;21(3):295–303. [DOI] [PubMed] [Google Scholar]
- 54.Coppa GV, et al. Glycosaminoglycan content in term and preterm milk during the first month of lactation. Neonatology. 2012;101(1):74–76. [DOI] [PubMed] [Google Scholar]
- 55.Wang C, et al. Glycosaminoglycanomic profiling of human milk in different stages of lactation by liquid chromatography-tandem mass spectrometry. Food Chem. 2018;258:231–236. [DOI] [PubMed] [Google Scholar]
- 56.Soares da Costa D, Reis RL, Pashkuleva I. Sulfation of Glycosaminoglycans and Its Implications in Human Health and Disorders. Annu Rev Biomed Eng. 2017;19:1–26. [DOI] [PubMed] [Google Scholar]
- 57.Mannello F, et al. Chondroitin sulfate structure is modified in human milk produced by breast affected by invasive carcinoma. Breast. 2011;20(6):586–587. [DOI] [PubMed] [Google Scholar]
- 58.Cerdo T, et al. Maternal obesity is associated with gut microbial metabolic potential in offspring during infancy. J Physiol Biochem. 2018;74(1):159–169. [DOI] [PubMed] [Google Scholar]
- 59.Wiederschain GY, Newburg DS. Glycoconjugate stability in human milk: glycosidase activities and sugar release. J Nutr Biochem. 2001;12(10):559–564. [DOI] [PubMed] [Google Scholar]
- 60.Francese R, et al. Human milk glycosaminoglycans inhibit cytomegalovirus and respiratory syncytial virus infectivity by impairing cell binding. Pediatr Res. 2022. [DOI] [PubMed] [Google Scholar]
- 61.Knowles TA, et al. It’s all in the milk: chondroitin sulfate as potential preventative therapy for necrotizing enterocolitis. Pediatr Res. 2021;89(6):1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jackson RL, Busch SJ, Cardin AD. Glycosaminoglycans: molecular properties, protein interactions, and role in physiological processes. Physiol Rev . 1991;71(2):481–539. [DOI] [PubMed] [Google Scholar]
- 63.Raman R, Sasisekharan V, Sasisekharan R. Structural insights into biological roles of protein-glycosaminoglycan interactions. Chem Biol. 2005;12(3):267–277. [DOI] [PubMed] [Google Scholar]
- 64.Barthe L, et al. In vitro intestinal degradation and absorption of chondroitin sulfate, a glycosaminoglycan drug. Arzneimittelforschung. 2004;54(5):286–292. [DOI] [PubMed] [Google Scholar]
- 65.Maccari F, et al. Metabolic fate of milk glycosaminoglycans in breastfed and formula fed newborns. Glycoconj J. 2016;33(2):181–188. [DOI] [PubMed] [Google Scholar]
- 66.Zuniga M, Monedero V, Yebra MJ. Utilization of Host-Derived Glycans by Intestinal Lactobacillus and Bifidobacterium Species. Front Microbiol. 2018;9:1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coppa GV, et al. Human milk glycosaminoglycans inhibit in vitro the adhesion of Escherichia coli and Salmonella fyris to human intestinal cells. Pediatr Res. 2016;79(4):603–607. [DOI] [PubMed] [Google Scholar]
- 68.Coppa GV, et al. Composition and structure elucidation of human milk glycosaminoglycans. Glycobiology. 2010;21(3):295–303. [DOI] [PubMed] [Google Scholar]
- 69.Burge KY, et al. The Protective Influence of Chondroitin Sulfate, a Component of Human Milk, on Intestinal Bacterial Invasion and Translocation. J Hum Lact. 2019;35(3):538–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shmagel A, et al. The Effects of Glucosamine and Chondroitin Sulfate on Gut Microbial Composition: A Systematic Review of Evidence from Animal and Human Studies. Nutrients. 2019;11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shang Q, et al. Structural modulation of gut microbiota by chondroitin sulfate and its oligosaccharide. Int J Biol Macromol. 2016;89:489–498. [DOI] [PubMed] [Google Scholar]
- 72.Knowles TA, et al. It’s all in the milk: chondroitin sulfate as potential preventative therapy for necrotizing enterocolitis. Pediatr Res. 2021;89(6):1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shmagel A, et al. The Effects of Glucosamine and Chondroitin Sulfate on Gut Microbial Composition: A Systematic Review of Evidence from Animal and Human Studies. Nutrients. 2019;11(2):294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Linares PM, et al. Effect of Chondroitin Sulphate on Pro-Inflammatory Mediators and Disease Activity in Patients with Inflammatory Bowel Disease. Digestion. 2015;92(4):203–210. [DOI] [PubMed] [Google Scholar]
- 75.Segarra S, et al. Oral chondroitin sulfate and prebiotics for the treatment of canine Inflammatory Bowel Disease: a randomized, controlled clinical trial. BMC Veterinary Research. 2016;12(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hosfield B, et al. Perinatal Administration of Chondroitin Sulfate Mitigates the Severity of Necrotizing Enterocolitis by Reducing Intestinal Dysbiosis. J Am Coll Surg. 2020;231(4). [Google Scholar]
- 77.Chaaban H, et al. Inter-alpha inhibitor protein level in neonates predicts necrotizing enterocolitis. J Pediatr. 2010;157(5):757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chaaban H, et al. Inter-α inhibitor protein and its associated glycosaminoglycans protect against histone-induced injury. Blood. 2015;125(14):2286–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chaaban H, et al. Acceleration of Small Intestine Development and Remodeling of the Microbiome Following Hyaluronan 35 kDa Treatment in Neonatal Mice. Nutrients. 2021;13(6):2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stenson WF, Ciorba MA. Nonmicrobial Activation of TLRs Controls Intestinal Growth, Wound Repair, and Radioprotection. Front Immunol. 2021;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim Y, de la Motte CA. The Role of Hyaluronan Treatment in Intestinal Innate Host Defense. Front Immunol. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Riehl TE, Ee X, Stenson WF. Hyaluronic acid regulates normal intestinal and colonic growth in mice. Am J Physiol Gastrointest Liver Physiol. 2012;303(3):G377–G388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O’Neill LAJ. A Feed-Forward Loop Involving Hyaluronic Acid and Toll-Like Receptor-4 as a Treatment for Colitis? Gastroenterology. 2009;137(6):1889–1891. [DOI] [PubMed] [Google Scholar]
- 84.Kim JJ, Khan WI. Goblet Cells and Mucins: Role in Innate Defense in Enteric Infections. Pathogens. 2013;2:55–70. doi: 10.3390/pathogens2010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pelaseyed T, et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev. 2014;260(1):8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sicard J-F, et al. Interactions of Intestinal Bacteria with Components of the Intestinal Mucus. Frontiers in Cellular and Infection Microbiology. 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salzman NH, Underwood MA, Bevins CL. Paneth cells, defensins, and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa. Semin Immunol. 2007;19(2):70–83. [DOI] [PubMed] [Google Scholar]
- 88.Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9(5):356–368. [DOI] [PubMed] [Google Scholar]
- 89.Sato T, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature . 2011;469(7330):415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. 2012;12(7):503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dzidic M, et al. Gut Microbiota and Mucosal Immunity in the Neonate. Medical Sciences. 2018;6. doi: 10.3390/medsci6030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dalby MJ, Hall LJ. Recent advances in understanding the neonatal microbiome. F1000Res. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kessler SP, et al. Multifunctional Role of 35 Kilodalton Hyaluronan in Promoting Defense of the Intestinal Epithelium. J Histochem Cytochem. 2018;66(4):273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sinha SR, et al. Dysbiosis-Induced Secondary Bile Acid Deficiency Promotes Intestinal Inflammation. Cell Host Microbe. 2020;27(4):e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiao X, et al. Bifidobacterium and Lactobacillus for preventing necrotizing enterocolitis in very-low-birth-weight preterm infants: a systematic review and meta-analysis. World J Pediatr. 2020;16(2):135–142. [DOI] [PubMed] [Google Scholar]
- 96.Lee Y, et al. Hyaluronic acid-bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat Mater. 2020;19(1):118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carlson GA, et al. Bacteriostatic properties of biomatrices against common orthopaedic pathogens. Biochem Biophys Res Commun. 2004;321(2):472–478. [DOI] [PubMed] [Google Scholar]
- 98.Kim Y, et al. Hyaluronan 35 kDa treatment protects mice from Citrobacter rodentium infection and induces epithelial tight junction protein ZO-1 in vivo. Matrix Biol. 2017;62:28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gunasekaran A, et al. Hyaluronan 35 kDa enhances epithelial barrier function and protects against the development of murine necrotizing enterocolitis. Pediatr Res. 2020;87(7):1177–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hackam DJ, Sodhi CP. Bench to bedside — new insights into the pathogenesis of necrotizing enterocolitis. Nat Rev Gastroenterol Hepatol. 2022;19(7):468–479. [DOI] [PubMed] [Google Scholar]
- 101.Zhang C, et al. Paneth cell ablation in the presence of Klebsiella pneumoniae induces necrotizing enterocolitis (NEC)-like injury in the small intestine of immature mice. Dis Model Mech. 2012;5(4):522–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stanford AH, et al. A direct comparison of mouse and human intestinal development using epithelial gene expression patterns. Pediatr Res. 2020;88(1):66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]