Abstract
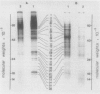
Acer pseudoplatanus cell suspension cultures were used to examine the ability of vacuoles isolated from protoplasts to hydrolyze their endogenous proteins. Total cell proteins were labeled by addition of [3H]leucine to the culture medium. After preparation of the protoplasts, vacuoles were isolated and were shown to be essentially free from other cellular components. Up to 30% of the [3H]leucine-labeled newly synthesized proteins were recovered in the vacuoles. When incubated for 6 hours at 20°C, the vacuoles degraded half of these proteins. The protein breakdown was temperature and pH dependent. Analysis by electrophoresis, in denaturing polyacrylamide gels, revealed that most of the vacuolar proteins were degraded. However, some vacuolar proteins were unaffected during a 6-hour incubation period. The results indicate that vacuoles are able to acquire and degrade intracellular proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Boudet A. M., Canut H., Alibert G. Isolation and Characterization of Vacuoles from Melilotus alba Mesophyll. Plant Physiol. 1981 Dec;68(6):1354–1358. doi: 10.1104/pp.68.6.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor E., Thom M., Maretzki A. Vacuoles from Sugarcane Suspension Cultures : III. PROTONMOTIVE POTENTIAL DIFFERENCE. Plant Physiol. 1982 Jun;69(6):1326–1330. doi: 10.1104/pp.69.6.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkdjian A., Guern J. Vacuolar pH Measurement in Higher Plant Cells : I. EVALUATION OF THE METHYLAMINE METHOD. Plant Physiol. 1981 May;67(5):953–957. doi: 10.1104/pp.67.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMPORT D. T. CELL SUSPENSION CULTURES OF HIGHER PLANTS: ISOLATION AND GROWTH ENERGETICS. Exp Cell Res. 1964 Jan;33:195–206. doi: 10.1016/s0014-4827(64)81026-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin W., Wittenbach V. A. Subcellular localization of proteases in wheat and corn mesophyll protoplasts. Plant Physiol. 1981 May;67(5):969–972. doi: 10.1104/pp.67.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Schmidt G. W., Mishkind M. L. Rapid degradation of unassembled ribulose 1,5-bisphosphate carboxylase small subunits in chloroplasts. Proc Natl Acad Sci U S A. 1983 May;80(9):2632–2636. doi: 10.1073/pnas.80.9.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Wilden W., Herman E. M., Chrispeels M. J. Protein bodies of mung bean cotyledons as autophagic organelles. Proc Natl Acad Sci U S A. 1980 Jan;77(1):428–432. doi: 10.1073/pnas.77.1.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G. J., Mulready P., Cutt J. Vacuole/Extravacuole distribution of soluble protease in hippeastrum petal and triticum leaf protoplasts. Plant Physiol. 1981 Nov;68(5):1081–1089. doi: 10.1104/pp.68.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters S. P., Noble E. R., Dalling M. J. Intracellular Localization of Peptide Hydrolases in Wheat (Triticum aestivum L.) Leaves. Plant Physiol. 1982 Mar;69(3):575–579. doi: 10.1104/pp.69.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A., Lin W., Hebert R. R. Vacuolar localization of proteases and degradation of chloroplasts in mesophyll protoplasts from senescing primary wheat leaves. Plant Physiol. 1982 Jan;69(1):98–102. doi: 10.1104/pp.69.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]