Abstract
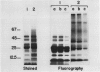
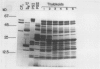
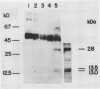
The accessibility of various Photosystem II (PSII)-associated polypeptides to the protease pronase and the chemical modifier trinitrobenzene-sulfonic acid (TNBS) has been investigated. Three polypeptides with apparent molecular weight of 32, 21, and 16 kilodaltons, known to be associated with O2 evolution, are all resistant to pronase digestion and TNBS labeling in intact thylakoids. All the polypeptides in the isolated PSII preparation were labeled with TNBS while a different pattern of labeling was observed when the PSII complex was isolated from TNBS-modified thylakoids. Attempts to prepare PSII particles from pronase-treated thylakoids using the Triton X-100 solubilization method were unsuccessful. Pronase-treated thylakoids were probed with antisera against the chlorophyll proteins of PSII using immunoblotting techniques. This allowed for a positive identification of proteolytic fragments from the respective proteins. The results are discussed in relation to the transmembrane organization of PSII in spinach thylakoids.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B., Anderson J. M., Ryrie I. J. Transbilayer organization of the chlorophyll-proteins of spinach thylakoids. Eur J Biochem. 1982 Apr 1;123(2):465–472. doi: 10.1111/j.1432-1033.1982.tb19790.x. [DOI] [PubMed] [Google Scholar]
- Holschuh K., Bottomley W., Whitfeld P. R. Structure of the spinach chloroplast genes for the D2 and 44 kd reaction-centre proteins of photosystem II and for tRNASer (UGA). Nucleic Acids Res. 1984 Dec 11;12(23):8819–8834. doi: 10.1093/nar/12.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E., Hauska G. A cytochrome f/b6 complex of five polypeptides with plastoquinol-plastocyanin-oxidoreductase activity from spinach chloroplasts. Eur J Biochem. 1981 Jul;117(3):591–595. doi: 10.1111/j.1432-1033.1981.tb06379.x. [DOI] [PubMed] [Google Scholar]
- Lam E., Malkin R. Reconstruction of the chloroplast noncyclic electron transport pathway from water to NADP with three integral protein complexes. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5494–5498. doi: 10.1073/pnas.79.18.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E., Oritz W., Mayfield S., Malkin R. Isolation and Characterization of a Light-Harvesting Chlorophyll a/b Protein Complex Associated with Photosystem I. Plant Physiol. 1984 Mar;74(3):650–655. doi: 10.1104/pp.74.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J., Herrmann R. G. Nucleotide sequence of the gene for the P680 chlorophyll alpha apoprotein of the photosystem II reaction center from spinach. Nucleic Acids Res. 1984 Mar 26;12(6):2837–2850. doi: 10.1093/nar/12.6.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullet J. E., Burke J. J., Arntzen C. J. Chlorophyll proteins of photosystem I. Plant Physiol. 1980 May;65(5):814–822. doi: 10.1104/pp.65.5.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz W., Lam E., Chollar S., Munt D., Malkin R. Topography of the protein complexes of the chloroplast thylakoid membrane : studies of photosystem I using a chemical probe and proteolytic digestion. Plant Physiol. 1985 Feb;77(2):389–397. doi: 10.1104/pp.77.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strotmann H., Hesse H., Edelmann K. Quantitative determination of coupling factor CF1 of chloroplasts. Biochim Biophys Acta. 1973 Aug 31;314(2):202–210. doi: 10.1016/0005-2728(73)90135-7. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Bohnert H. J., Whitfeld P. R., Bottomley W. Nucleotide sequence of the gene for the M(r) 32,000 thylakoid membrane protein from Spinacia oleracea and Nicotiana debneyi predicts a totally conserved primary translation product of M(r) 38,950. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7699–7703. doi: 10.1073/pnas.79.24.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]