Abstract
Rationale:
Ayahuasca has been proposed as a potential treatment of alcohol (ethanol) use disorder (AUD). The serotonin 5-HT2A receptor agonist N,N-dimethyltryptamine (DMT) is the main psychoactive component of ayahuasca, suggesting that its therapeutic effects may be mediated by 5-HT2A receptors.
Objectives:
The aim of the present study was to investigate the effects of ayahuasca on the expression of ethanol self-administration using a two-bottle choice procedure, and the role of 5-HT2A receptors in those effects.
Methods:
Male mice had intermittent access to ethanol (10% v/v) in a two-bottle choice procedure for 30 days. Animals were then submitted to 3 treatment phases, each followed by ethanol re-exposure tests. During the treatment phase, every 3 days animals received i.p. injections of either vehicle or the 5-HT2A receptor antagonist M100907 (M100, 1 mg/kg) followed by an i.g. (gavage) administration of vehicle or ayahuasca (100 mg/kg) and were exposed to the self-administration apparatus with no ethanol availability. During re-exposure tests, animals were submitted to the same conditions as during acquisition, with no treatments prior to those sessions.
Results:
Treatment with ayahuasca blocked the expression of ethanol self-administration, decreasing ethanol intake and preference during re-exposure tests. Pretreatment with M100 blocked the effects of ayahuasca on ethanol drinking without significantly attenuating ethanol self-administration.
Conclusions:
Treatment with ayahuasca during alcohol abstinence blocked the expression of alcohol self-administration in mice, and 5-HT2A receptor activation is critical for those effects to emerge. Our findings support a potential for ayahuasca and other 5-HT2A receptor agonists as adjunctive pharmacotherapies for the treatment of AUD.
Keywords: ayahuasca, ethanol, serotonin, 5-HT2A, self-administration, mice
Introduction
Alcohol (ethanol) use disorder (AUD) is a chronic, relapsing disease that currently represents a global public health burden (WHO 2018). In the U.S. alone, nearly one-third of adults experience AUD at some point during their lifetime (Grant et al. 2015). Current pharmacotherapies, such as naltrexone and acamprosate, and behavioral treatments may assist patients in reducing ethanol use or facilitating ethanol abstinence (Witkiewitz et al. 2019). However, relapse rates remain high, and treatment efficacy in supporting abstinence are a particular challenge (Maisel et al. 2013), emphasizing the needs for more research focused on identifying the factors most critical in the etiology and treatment of this disease and further avenues for treatment.
Several studies have shown that the hallucinogenic beverage ayahuasca blocks the abuse-related behavioral effects of ethanol (for review, see Hamill et al. 2019). We have previously demonstrated that ayahuasca blocks the development and expression of behavioral sensitization to ethanol (Oliveira-Lima et al. 2015) and the reinstatement of ethanol-induced conditioned place preference in mice (Cata-Preta et al. 2018). Corroborating evidence showing that ayahuasca-assisted therapy decreases drug use and craving in drug-dependent individuals (Argento et al. 2019; Thomas et al. 2013; Loizaga-Velder and Verres 2014), we have also found a lower prevalence of AUD and ethanol-related problems in religious ayahuasca users relative to control groups (Barbosa et al. 2016, 2018).
Ayahuasca is a brew frequently prepared from the decoction of the plants Banisteriopsis caapi, which contains β-carbolines, and Psychotria viridis, which contains N,N-dimethyltryptamine (DMT) (Oliveira-Lima et al. 2015; Cata-Preta et al. 2018). Because of its high concentration in the brew, DMT has been proposed as the main psychoactive component of ayahuasca, with β-carboline alkaloids such as harmine and harmaline facilitating its effects (McKenna 2004). DMT is a serotonin 5HT2A/2C receptor agonist with higher affinity for 5-HT2A receptors, which suggests that its therapeutic effects may be mediated by DMT-induced activation of 5-HT2A receptors (Glennon et al. 2000). However, the extent to which 5-HT2A receptor activation is sufficient to explain the therapeutic effects of ayahuasca on the abuse-related behavioral effects of ethanol remains unknown.
The aim of the present study was to investigate the effects of treatment with ayahuasca during ethanol abstinence on the expression of ethanol self-administration using a two-bottle choice procedure in male mice. We also investigated the role of 5-HT2A receptors in the therapeutic effects of ayahuasca by administering the selective 5-HT2A receptor antagonist M100907 prior to ayahuasca treatments. Our hypothesis was that ayahuasca would prevent the expression of ethanol self-administration in a two-bottle choice procedure, and that 5-HT2A receptor blockade would inhibit the therapeutic effects of ayahuasca in this model.
Material and Methods
Animals
Three-month-old Swiss male mice from our own colony were used. Animals weighing 35–40 g were group-housed (10 per cage) for most of the study, except during self-administration sessions, during which animals were single-housed for 15h every other day (see description below). When in groups, animals were housed in polypropylene cages (32 × 42 × 18 cm) under controlled temperature (22–23 °C) and light (12h light, 12h dark; lights on at 06h40) conditions. Food and water were available ad libitum throughout the experiments. Animals were maintained according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th Edition, revised 2011) and in accordance with the Brazilian Law for Procedures for Animal Scientific Use (#11794/2008). The Institutional Animal Care and Use Committee of UESC approved the experimental procedures (protocol #020/20).
Drugs and compounds
A batch of ayahuasca was obtained from a member of the Santo Daime Church. The batch was lyophilized and quantified to obtain concentrations of its main constituents, as previously described: DMT: 0.4 mg/100 mg; tetrahydroharmine; 3.07 mg/100 mg; harmine: 3.85 mg/100 mg; harmaline: 0.17 mg/100 mg (Oliveira-Lima et al. 2015). The 5-HT2A receptor antagonist R(+)-MDL100,907 HCl (M100) was synthesized at the Chemical Biology Research Branch, National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism at the National Institutes of Health (Bethesda, MD, USA). Ayahuasca (100 mg/kg, 10 mg/ml) and M100 (1 mg/kg, 0.1 mg/ml) were both diluted in saline (0.9%), with saline (0.9%) as a vehicle solution (Veh). Ayahuasca and its vehicle were administered intragastrically (gavage, i.g.), and M100 and its vehicle were administered intraperitoneally (i.p.). Ethanol 95% (Eth, Merck®) made available for drinking was diluted in water at a 10% volume solution. The dose of ayahuasca was chosen based on its ability to block other abuse-related behavioral effects of ethanol, including the development and expression of ethanol-induced behavioral sensitization and conditioned place preference (Oliveira-Lima et al. 2015; Cata-Preta et al. 2018). In those studies, a higher dose of ayahuasca was included and showed a similar magnitude of effect compared to the dose of 100 mg/kg. Therefore, we chose the minimally effective dose of ayahuasca for the present study. The dose of M100 was chosen based on its ability to block the expression of cocaine self-administration in mice (Nic Dhonnchadha et al. 2009; Cunningham et al. 2013).
Two-bottle choice ethanol self-administration and experimental design
Mice were given the opportunity to drink 10% ethanol using an intermittent access, two-bottle choice procedure, as described in earlier studies (Li et al. 2010). The protocol was divided into 3 phases: acquisition, treatment and re-exposure (see Figure 1). For all phases, when animals were not in the individual drinking cages, animals remained group-housed in their home cages. Food was available ad libitum during all sessions.
Figure 1.
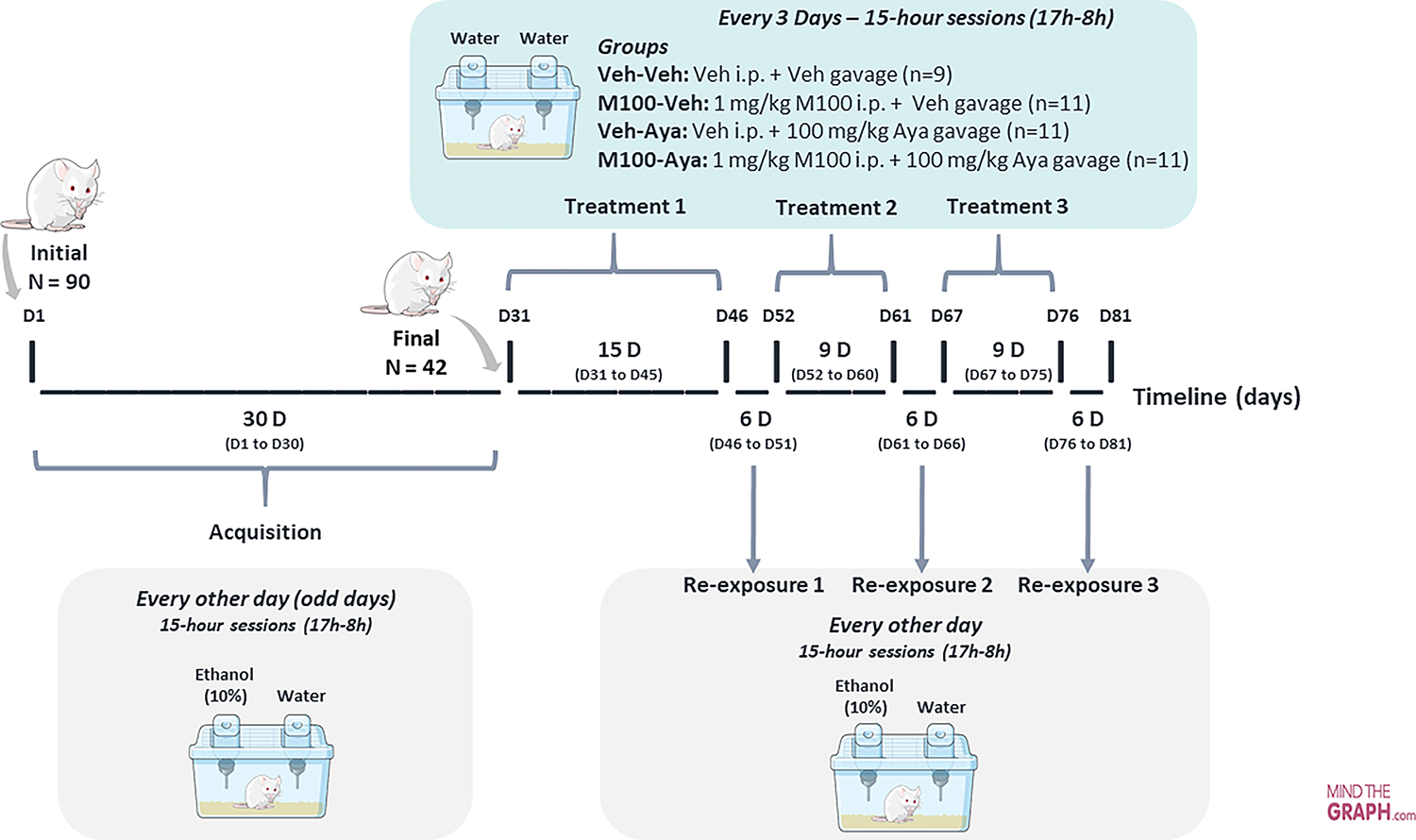
Protocol design across experimental days. Ninety animals were submitted to the ethanol two-bottle choice procedure acquisition phase, during which they were exposed to individual cages with access to two bottles, one of water and one of 10% ethanol solution, for 15h, every other day. At the end of the acquisition phase, the 42 animals that showed the highest ethanol drinking/preference were randomly assigned to 4 groups: Veh-Veh, M100-Veh, Veh-Aya or M100-Aya. Animals were then submitted to the treatment phase, with each treatment phase being followed by 3 re-exposure tests.
Acquisition:
Every other day (odd days) for 15 days, 90 animals were housed individually in polypropylene boxes (30 × 19 × 13 cm) for 15 hours (17h00–08h00) and given access to two bottles, one containing 10 mL of water and the other containing 10 mL of 10% ethanol solution. Therefore, the majority of the 15-hour ethanol access took place during the animals’ dark (active) phase. At the end of 15 days, the average ethanol intake between all animals was 1.57 g/kg, and animals that did not reach 1.5 g/kg of ethanol intake were then excluded from the study. The remaining (42) animals with ethanol intake above 1.5 g/kg were maintained in the study and continued to be submitted to the acquisition protocol for another 15 days (total of 15 acquisition sessions over the course of 30 days, with sessions happening every other day).
Treatment and re-exposure:
Animals were submitted to 3 treatment periods, each followed by 3 ethanol re-exposure sessions. During the 1st treatment period, animals were treated every 3 days with ayahuasca and/or M100 for a period of 15 days (total of 5 treatment sessions), forming the following groups (n=9–11 per group): Vehicle-Vehicle (Veh-Veh); 1 mg/kg M100-Vehicle (M100-Veh); Vehicle-100 mg/kg Ayahuasca (Veh-Aya) and 1 mg/kg M100–100 mg/kg-Ayahuasca (M100-Aya). M100 or its vehicle were administered i.p. and, immediately afterwards, ayahuasca or its vehicle were administered i.g. (gavage). Thirty minutes after the ayahuasca/vehicle administration, each animal was individually placed in the self-administration cage with access to 2 bottles of water. Forty-eight hours after the last treatment session, animals were submitted to the 1st ethanol re-exposure session, being individually placed in the self-administration cage with access to one bottle of water and one bottle of 10% ethanol solution, similarly to the acquisition phase. Re-exposure sessions happened every other day for 6 days (total of 3 re-exposure sessions) before the next treatment phase. The 2 subsequent treatment phases followed the same protocol described for the 1st treatment phase, except they were shorter: 9 days each, with treatments every 3 days (3 treatments total per treatment phase). Each treatment phase was also followed by a 6-day re-exposure phase, with re-exposure tests every other day (3 re-exposures each).
Consumption from each bottle (water or ethanol) was measured at the end of each session (acquisition, treatment or re-exposure). Bottles were refilled with fresh water or fresh solutions before each session. Bottle sides were switched at every session. Each bottle of water or ethanol was weighed on a precision scale before and after the session to determine the consumption of water/ethanol. Animals were also weighed daily. To ensure data were not affected by liquid loss due to bottle leaks or evaporation, two bottles were left in an empty cage for one week during each session, during which time liquid loss was measured and found to be less than 0.1 mL/day. After obtaining the final weight of each ethanol bottle, ethanol consumption in g/kg was calculated for each session using the following formula:
To calculate each animal’s preference for the ethanol bottle compared to the water bottle, the following formula was used:
Statistical analysis
All variables were checked for normality (Shapiro–Wilk test) and homogeneity of variances (Levene’s test). Data for the acquisition phase were grouped by 6-day periods (3 sessions/period), and data for the re-exposure test were also grouped as the 3 re-exposure sessions being conducted after each treatment period. Statistical analyses were performed using analysis of variance (ANOVA), with or without repeated measures (RM), and sphericity was assumed for all our tests. For multiple comparison analyses, time (D1-D6, D7-D12, D13-D18, D19-D24, D25-D30, R1, R2, R3) and solution (ethanol vs water) were categorized as within-subject factors, while M100 (Veh vs M100) and ayahuasca (Veh vs ayahuasca) treatments were defined as between-subject factors. Multiple comparisons were performed using Tukey’s post hoc tests. ANOVAs with more than 3 factors (Figure 2) were performed using IBM SPSS Statistics (v. 27). All other analyses and graphic representations were performed using GraphPad Prism (v. 9.1.2). A probability of p<0.05 was considered a statistically significant difference.
Figure 2.
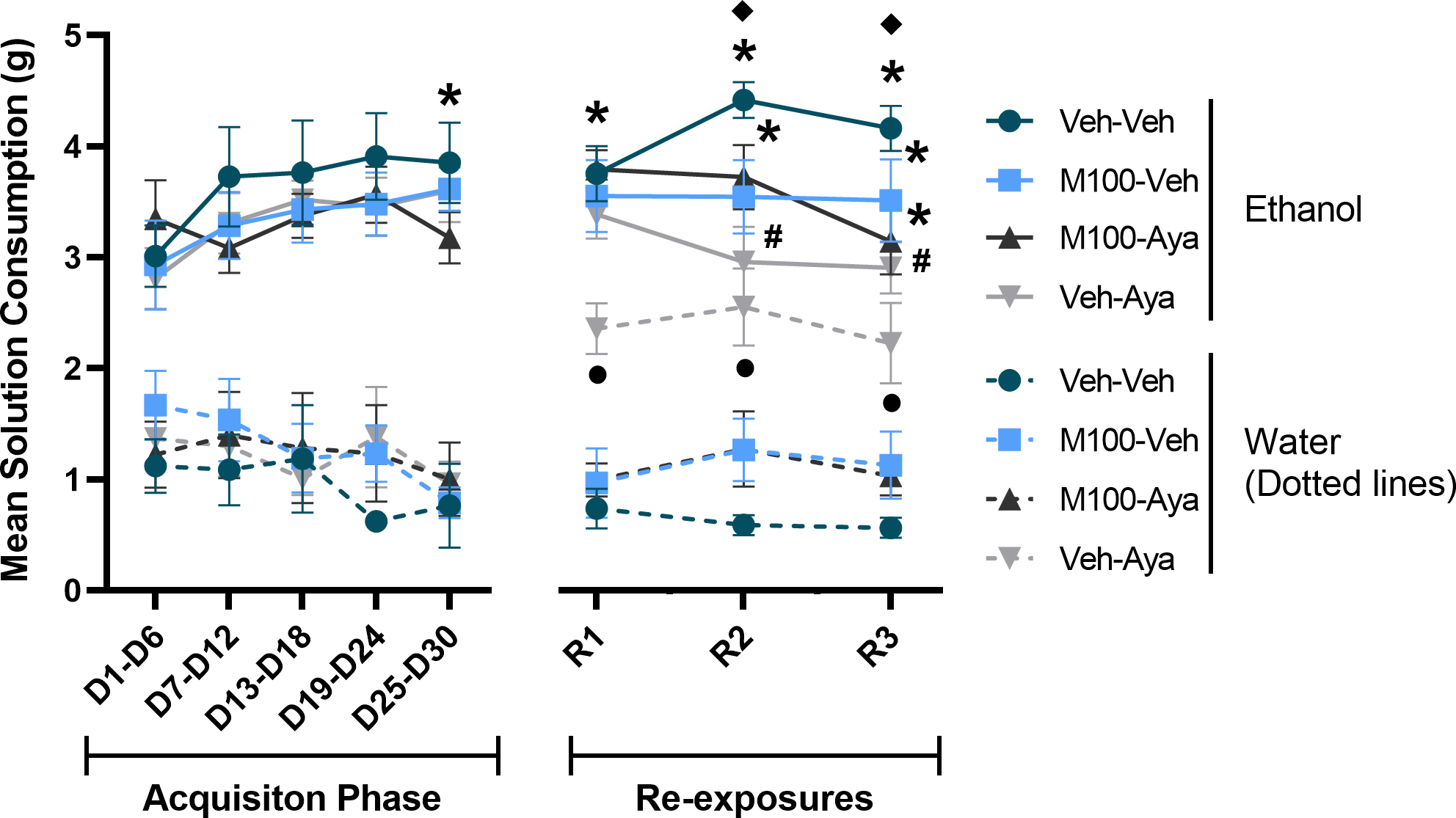
Mean consumption of ethanol and water solutions during the acquisition of two-bottle choice ethanol self-administration and during ethanol re-exposures following a treatment period with vehicle (Veh), the 5-HT2A antagonist M100907 (M100, 1 mg/kg, i.p.) and/or ayahuasca (100 mg/kg, i.g.). Each time-point is an average of 3 sessions within the same phase (i.e., 3 acquisition sessions or 3 re-exposure sessions). Data are shown as mean±SEM. *p<0.05 compared to water within the same group; υp<0.05 compared to the same group/condition during D1-D6; λp<0.05 compared to Water for Veh-Veh; #p<0.05 compared to Ethanol for Veh-Veh.
Results
Figure 2 shows the total amount (g) of ethanol solution or water solution consumed during each phase of the study. A mixed-measures ANOVA of all time-points for the study with time (all time-points) and solution as within-subject factors and M100 and ayahuasca treatments as between-subject factors showed a significant effect of time [F(7,266) = 3.537; p<0.01] and of solution [F(1,38) = 149.962; p<0.0001]. The same analysis also showed as a significant interaction between time vs ayahuasca treatment [F(7,266) = 2.01; p<0.05], time vs solution [F(7,266) = 2.513; p<0.05], time vs solution vs ayahuasca treatment [F(7,266) = 2.26; p<0.01] and time vs solution vs ayahuasca treatment vs M100 treatment [F(7,266)= 2.32, p<0.01]. Tukey’s post hoc test showed that during the last week of the acquisition phase (D25-D30) all 4 groups showed significantly higher intake of ethanol solution compared to water (p<0.0001 for all groups). During the first re-exposure test, while ethanol solution consumption remained stable for all 4 groups, the control group (Veh-Veh), the group treated with M100 (M100-Veh) and the group treated with M100 and ayahuasca (M100-Aya) showed a significant higher ethanol solution consumption compared to water (p<0.0001 for all 3 groups). However, animals treated with ayahuasca (Veh-Aya) showed a significant increase in water solution consumption compared to the control group (Veh-Veh) (p=0.0052), with the Veh-Aya group showing no significant difference between ethanol and water solution consumption during the first re-exposure test (R1, p>0.05). During the second and third re-exposure tests, the control group (Veh-Veh), the group treated with M100 (M100-Veh) and the group treated with M100 and ayahuasca (M100-Aya) continued to show a significantly higher ethanol solution consumption compared to water (p<0.0001 for all 3 groups on both tests). Importantly, the group treated with ayahuasca (Veh-Aya) showed a significantly lower ethanol solution consumption compared to the control group (Veh-Veh) (p<0.05 for both tests). Animals treated with ayahuasca (Veh-Aya) again showed a significant increase in water consumption compared to the control group (Veh-Veh) (p<0.01 for both tests), with this group not showing a significant difference between ethanol and water solution consumption during the second and third re-exposure tests (p>0.7 for both tests). When ethanol solution consumption was compared over time, the control group (Veh-Veh) was the only group to show a significant increase in ethanol solution consumption during the second and third re-exposure compared to itself during the first week of the acquisition phase (D1-D6) (p<0.05 for both tests).
A mixed-measures ANOVA of the total ethanol intake in g/kg across the experimental phases (Figure 3) with time (all time-points) as within-subject factor and M100 and ayahuasca treatments as between subject factors showed only a significant effect of time [F(7,266) = 3.376; p<0.01]. Tukey’s post hoc analysis showed that the control group (Veh-Veh) also showed an increased ethanol intake during the second re-exposure test compared to itself during the first week of the acquisition phase (D1-D6) (p<0.05). Because all groups received similar treatment during the ethanol acquisition phase, we performed an additional analysis restricting the mixed-measures ANOVA to the re-exposure tests (R1, R2 and R3), and we used a more stringent alpha level criterion (1%) to avoid committing a Type I error due to increased number of statistical tests. This test showed a significant interaction between time vs ayahuasca treatment [F(2,76) = 6.023; p<0.01]. The Tukey’s post hoc test showed that animals treated with ayahuasca (Veh-Aya) consumed a significantly lower dose of ethanol compared to the control group (Veh-Veh) during the second and third re-exposure tests (p<0.01 for both tests).
Figure 3.
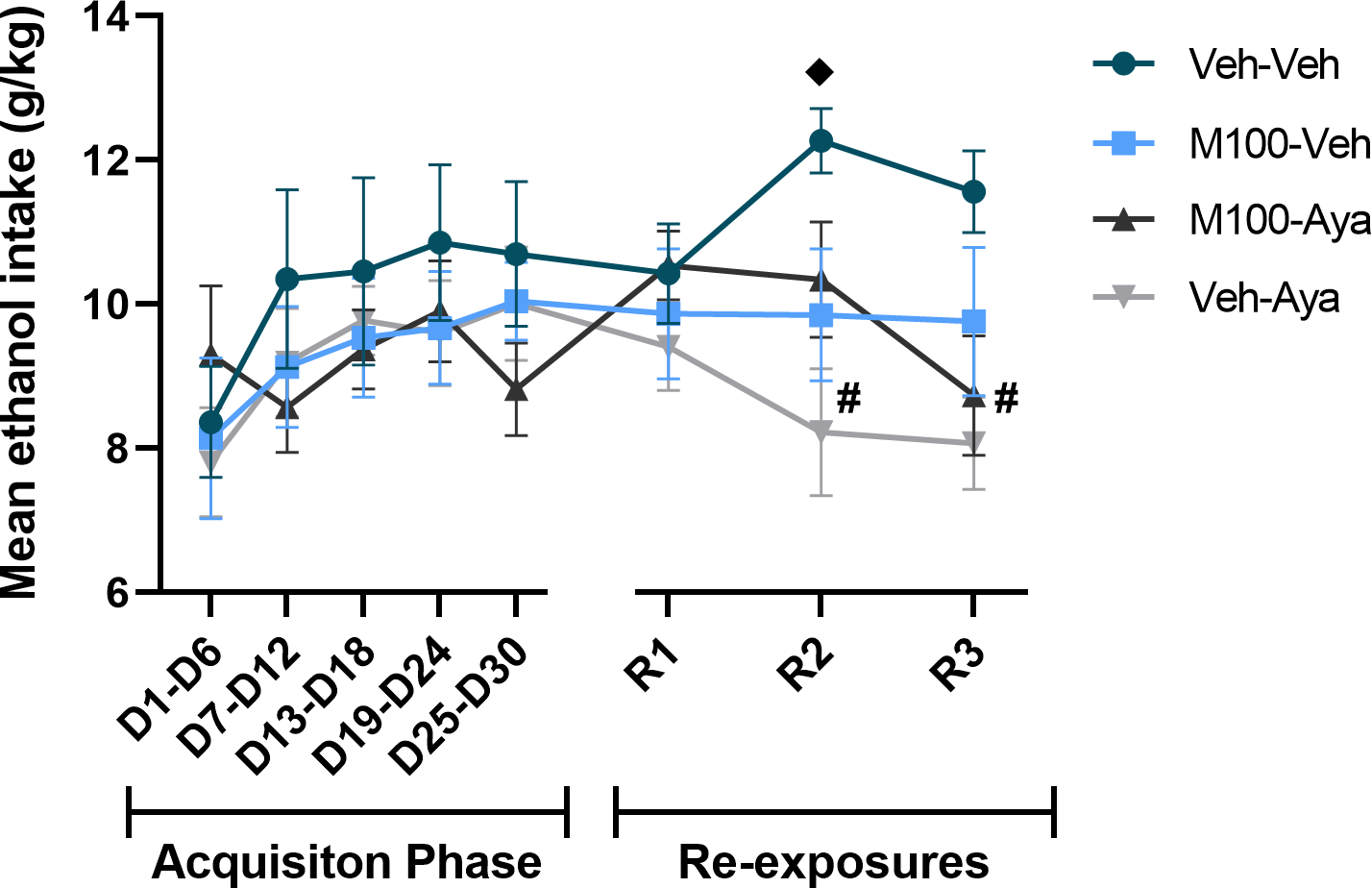
Ethanol dose (g/kg) consumed during the acquisition of two-bottle choice ethanol self-administration and during ethanol re-exposures following a treatment period with vehicle (Veh), the 5-HT2A antagonist M100907 (M100, 1 mg/kg, i.p.) and/or ayahuasca (100 mg/kg, i.g.). Each time-point is an average of 3 sessions within the same phase (i.e., 3 acquisition sessions or 3 re-exposure sessions). Data are shown as mean±SEM. υp<0.05 compared to the same group during D1-D6; #p<0.05 compared to Veh-Veh.
A mixed-measures ANOVA of the preference (%) for the ethanol bottle across the experimental phases (Figure 4) with time (all time-points) as within-subject factor and M100 and ayahuasca treatments as between subject factors showed a significant effect of time [F(7,266) = 3.678; p<0.001] and significant interactions between time vs ayahuasca treatment [F(7,266) = 3.619; p<0.01], ayahuasca vs M100 treatments [F(1,38) = 5.452; p<0.05] and time vs ayahuasca vs M100 treatments [F(7,266) = 2.751; p<0.01]. All groups showed a preference (greater than 50%) for the ethanol bottle during the acquisition phase, with no difference between groups during acquisition (p>0.05 for all time-points). During all re-exposure tests, the ayahuasca-treated group showed a significantly lower ethanol preference compared to itself during the last acquisition period (D25-D30, p<0.01 for al tests). Again, an additional analysis was performed restricting the mixed-measures ANOVA to the re-exposure tests (R1, R2 and R3), using a more stringent alpha level criterion (1%) to avoid committing a Type I error due to increased number of statistical tests. This test showed a significant effect of ayahuasca treatment [F(1,38) = 12.28; p<0.01] and a significant interaction between ayahuasca vs M100 treatments [F(1,38) = 13.55; p<0.001]. Animals treated with ayahuasca (Veh-Aya) showed a significantly lower preference compared to the control group (Veh-Veh) during all re-exposure tests (p<0.01 for all tests). The group treated with ayahuasca also showed a significantly lower ethanol preference during the first and second re-exposure tests compared to the two other experimental groups (M100-Veh and M100-Aya, p<0.05 for all tests/conditions).
Figure 4.
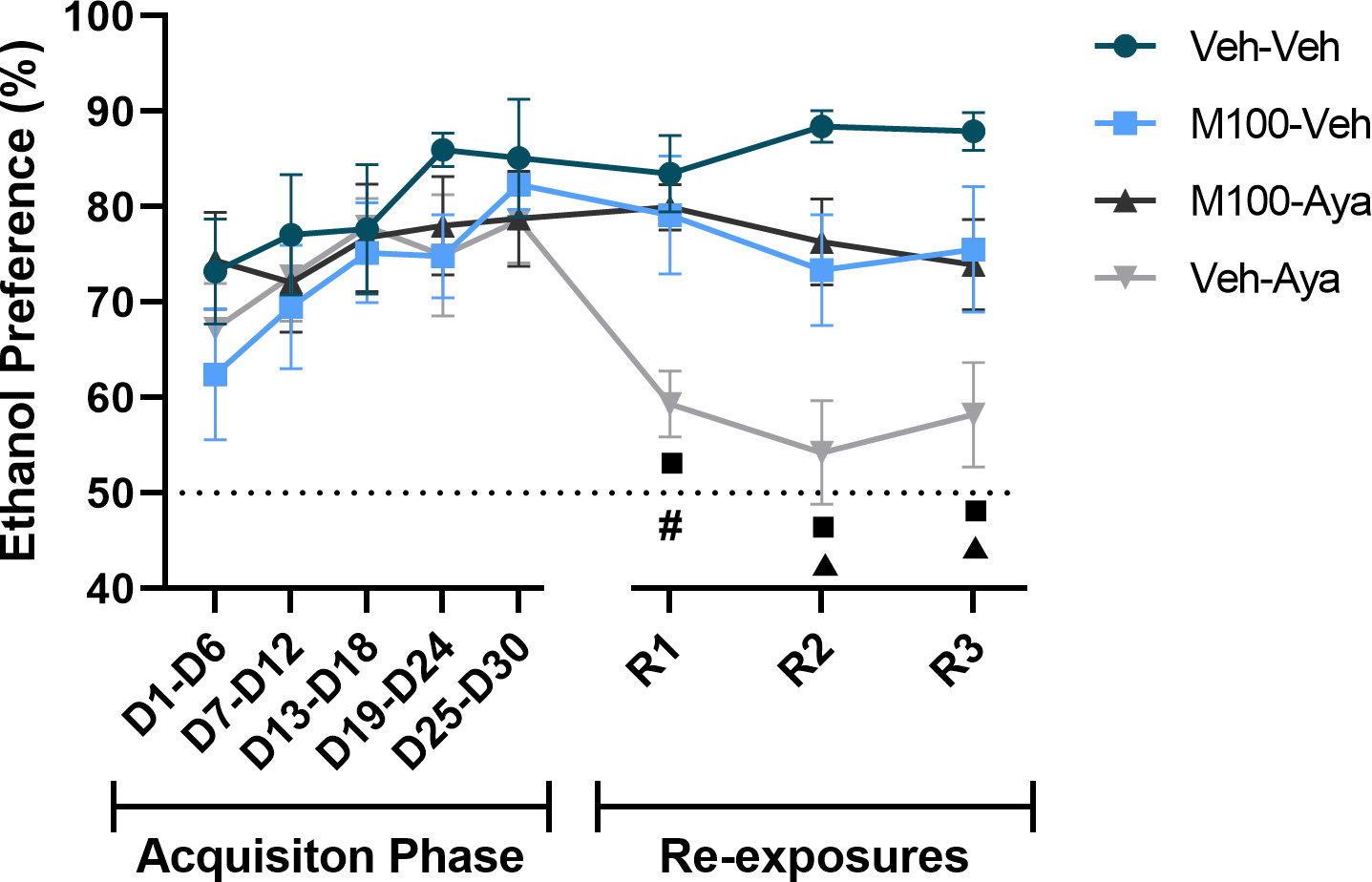
Preference (%) for the ethanol solution during the acquisition of two-bottle choice ethanol self-administration and during ethanol re-exposures following a treatment period with vehicle (Veh), the 5-HT2A antagonist M100907 (M100, 1 mg/kg, i.p.) and/or ayahuasca (100 mg/kg, i.g.). Each time-point is an average of 3 sessions within the same phase (i.e., 3 acquisition sessions or 3 re-exposure sessions). Data are shown as mean±SEM. #p<0.05 compared to Veh-Veh; πp<0.05 compared to all other groups (Veh-Veh, M100-Veh and Veh-Aya); νp<0.05 compared to the same group during D25-D30.
Only a significant main effect of time was observed for total water consumption over the treatment days (when animals had access to two bottles of water) (Figure 5, mixed-measures ANOVA [F(10,350) = 4.39; p<0.0001]) and for weight across the experimental protocol (Figure 6, mixed-measures ANOVA [F(10,350) = 39.53; p<0.0001]. No significant post hoc differences were observed for either measure.
Figure 5.
Total water consumption during the treatment phase, when animals only had access to two bottles of water. During treatment days (D), animals received treatment with vehicle (Veh), the 5-HT2A antagonist M100907 (M100, 1 mg/kg, i.p.) and/or ayahuasca (100 mg/kg, i.g.). Data are shown as mean±SEM.
Figure 6.
Subjects’ weights across the experimental protocol. Each time-point is an average of 3–5 sessions within the same phase (i.e., 3 acquisition sessions). Weights were evaluated during acquisition of bottle choice ethanol self-administration and during ethanol re-exposures (R) following a treatment (T) period with vehicle (Veh), the 5-HT2A antagonist M100907 (M100, 1 mg/kg, i.p.) and/or ayahuasca (100 mg/kg, i.g.). Data are shown as mean±SEM.
Discussion
Accumulating evidence suggests that the hallucinogenic beverage ayahuasca may have therapeutic effects for AUD (for review, see Hamill et al. 2019). Studies report a lower prevalence of AUD and ethanol-related problems in religious ayahuasca users relative to control groups (Barbosa et al. 2016, 2018). A recent study also has shown that use of ayahuasca in naturalistic settings is associated with lower self-reported current consumption of ethanol, even after adjusting for religious or social group effects (Perkins et al. 2021). In this regard, pre-clinical studies have been fundamental in determining whether the pharmacological properties of ayahuasca modulate the abuse-related effects of ethanol, or if the ritualistic context in which ayahuasca is predominantly consumed plays a major role in its therapeutic effects. Our present findings show that treatment with ayahuasca during abstinence of ethanol self-administration using a two-bottle choice paradigm in mice decreased ethanol intake and ethanol choice (preference) during a subsequent ethanol re-exposure.
Importantly, these effects were observed at a dose of ayahuasca that had been previously shown to block the expression of ethanol-induced behavioral sensitization and conditioned place preference in mice (Oliveira-Lima et al. 2015; Cata-Preta et al. 2018). The dose used in the present study (and up to 500 mg/kg) does not affect locomotor activity per se compared to vehicle in the same strain of mice (Oliveira-Lima et al. 2018), suggesting that those effects were not a result of motor impairment. However, it is important to note that ayahuasca also induced rewarding effects per se in the conditioned place preference model in mice at the dose used in the present study (Cata-Preta et al. 2018; Reis et al. 2020), which may have influenced our results. Specifically, we saw a significant increase in water intake during the re-exposure tests in the group treated with ayahuasca (Veh-Aya) compared to the control group (Veh-Veh). During the second and third re-exposure tests (R2 and R3), an increase in water intake is accompanied by a significant decrease in ethanol solution consumption (and in total ethanol intake in g/kg), suggesting that a shift in overall consumption from ethanol to water was observed. However, the fact that water intake was increased in the ayahuasca-treated group (Veh-Aya) during the first re-exposure test (R1), when ethanol intake was unchanged compared to other groups, suggests that animals could have developed a preference for water during the treatment days due, possibly due to the rewarding effects of ayahuasca having been paired with water. Corroborating this assumption, no differences were observed between groups in water consumption during the treatment phases (when animals only had access to two bottles of water), suggesting that, in the absence of alcohol, ayahuasca does not affect total water intake. Therefore, future studies should investigate the effects of non-rewarding doses of ayahuasca using a similar paradigm. Of note, in our previous study, a higher, non-rewarding dose of ayahuasca also exerted therapeutic effects on ethanol-induced conditioned place preference (Cata-Preta et al. 2018), indicating that the expression of its rewarding effects is not necessary for its therapeutic effects to emerge.
Our findings contradict recent results by Nolli et al. (2020) showing that ayahuasca did not block ethanol intake (g/kg) in a two-bottle choice procedure in rats. The highest dose of ayahuasca used by those authors had a relatively similar DMT concentration compared to our batch (0.52 vs 0.4 mg/kg, respectively) (Nolli et al. 2020). Other than the species (rats vs mice), the main difference in the two protocols was the treatment administration. While in the present study ayahuasca was administered during ethanol abstinence (and ethanol intake was assessed at a later, ayahuasca-free re-exposure test), Nolli et al. (2020) administered ayahuasca immediately before ethanol self-administration sessions. Those findings suggest that ayahuasca may attenuate ethanol self-administration when animals are allowed to experience the compound in the absence the drug. In fact, we have previously hypothesized that ayahuasca may modify the incentive salience of drug-associated environments, which would contribute to its therapeutic effects (Valyear et al. 2017; Cata-Preta et al. 2018). In this regard, in the present study, animals were exposed to the ethanol self-administration apparatus during ayahuasca treatment sessions, and previous findings from our group have shown that the treatment environment influences the therapeutic effects of ayahuasca on the abuse-related behavioral effects of ethanol (Cata-Preta et al. 2018).
DMT is a serotonin 5HT2A/2C receptor agonist that has been proposed as the main psychoactive component of ayahuasca (McKenna 2004). Studies have shown that 5-HT2A receptor agonists, such as psilocybin, may be potential adjunctive pharmacotherapies for the treatment of AUD (Bogenschutz 2013; Bogenschutz et al. 2015). Administration of selective 5-HT2A receptor agonists also has been shown to decrease ethanol self-administration in rats (Maurel and Shceiber 1999; Berquist and Fantegrossi 2021). Because of the high affinity of DMT for 5-HT2A receptors, those findings suggest that DMT-induced activation of 5-HT2A receptors, which are highly expressed in dopaminergic neurons in the ventral tegmental area (VTA) and glutamatergic neurons in the prefrontal cortex (Howell and Cunningham 2015), could mediate the therapeutic effects of ayahuasca. Therefore, we hypothesized that blockade of serotonin 5-HT2A receptors would prevent the therapeutic effects of ayahuasca on ethanol self-administration. In fact, our findings show that pretreatment with a 5-HT2A receptor antagonist before ayahuasca administration prevented ayahuasca from decreasing ethanol intake and preference. These findings show a clear role for 5-HT2A receptors in the effects of ayahuasca on the abuse-related behavioral effects of ethanol.
It is important to note, however, that, while not statistically significant, animals in the groups treated with M100 + ayahuasca showed lower ethanol intake and preference compared to the control group. Therefore, while 5-HT2A receptor activation is necessary for ayahuasca to block ethanol self-administration, other mechanisms also may contribute to its therapeutic effects. Based on 5-HT2A/2C receptor localization in the brain, with 5-HT2C receptors being highly expressed in GABAergic interneurons within the VTA (Howell and Cunningham, 2015), we have previously proposed that the effects of ayahuasca may be modulated by a specific 5-HT2A/2C occupancy ratio (Cata-Preta et al. 2018). Therefore, 5-HT2C receptor activation also may contribute to the effects of ayahuasca. Most importantly, the extract of Banisteriopsis caapi alone, a plant component of ayahuasca that only contains β-carbolines (but not DMT), also blocked the expression of ethanol-induced conditioned place preference in mice (Cata-Preta et al. 2018). Therefore, other DMT-mediated mechanisms, as well as the pharmacological activity of its β-carbolines, also may contribute to ayahuasca-induced blockade of ethanol self-administration. Further studies are needed to better understand these mechanisms.
Similarly, the group treated with the 5-HT2A antagonist M100 (M100-Veh) also showed lower levels of ethanol intake and preference compared to the control group, albeit not statistically significant. Those findings further suggest that 5-HT2A-mediated serotonin neurotransmission is important for the expression of the reinforcing effects of ethanol. In fact, evidence indicates that 5-HT transmission within the VTA can regulate local dopamine neuronal activity and the effects of ethanol. Of note, 5-HT2A agonists potentiate ethanol-induced excitation of VTA dopamine neurons (Brodie et al. 1995), while a 5-HT2A receptor antagonist attenuated operant ethanol self-administration and intra-VTA ethanol infusions in rats (Ding et al. 2009). However, in the present study 5-HT2A receptor antagonism alone was not sufficient to significantly decrease ethanol intake in the two-bottle choice paradigm, emphasizing that other mechanisms also are involved in the reinforcing effects of ethanol.
In summary, the present findings show that ayahuasca treatment during ethanol abstinence blocked the expression of ethanol self-administration in male mice, and that 5-HT2A receptor activation is critical for those effects to emerge. Those findings further support a potential for ayahuasca and other 5-HT2A receptor agonists as adjunctive pharmacotherapies for the treatment of AUD. Of note, the dose of ayahuasca used for the present study was chosen based on its ability to block other abuse-related behavioral effects of ethanol, including the development and expression of ethanol-induced behavioral sensitization and conditioned place preference (Oliveira-Lima et al. 2015; Cata-Preta et al. 2018). While in those studies a higher dose of ayahuasca was included and showed a similar magnitude of effect compared to the dose of 100 mg/kg, an important limitation of the present study was the use of a single dose of ayahuasca and M100907. Further studies evaluating whether lower or higher doses of ayahuasca have the same effects are warranted, particularly doses that do not show rewarding effects per se (Cata-Preta et al. 2018).
Acknowledgments:
The authors thank Mr. José Carlos Santos de Oliveira for his technical support. Funding for this study was provided by internal funds from Universidade Estadual de Santa Cruz (UESC, project number 073.6769.2020.0017856-35), and by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The work of the Drug Design and Synthesis Section, MTMDB, NIDA, and NIAAA was supported by the NIH Intramural Research Programs of the National Institute on Drug Abuse (NIDA) and the National Institute of Alcohol Abuse and Alcoholism (NIAAA).
Footnotes
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Argento E, Capler R, Thomas G, Lucas P, Tupper KW (2019) Exploring ayahuasca-assisted therapy for addiction: A qualitative analysis of preliminary findings among an Indigenous community in Canada. Drug Alcohol Rev 38(7):781–789. [DOI] [PubMed] [Google Scholar]
- Barbosa PC, Strassman RJ, da Silveira DX, Areco K, Hoy R, Pommy J, Thoma R, Bogenschutz M (2016) Psychological and neuropsychological assessment of regular hoasca users. Compr Psychiatry 71:95–105. [DOI] [PubMed] [Google Scholar]
- Barbosa PCR, Tófoli LF, Bogenschutz MP, Hoy R, Berro LF, Marinho EAV, Areco KN, Winkelman MJ (2018) Assessment of Alcohol and Tobacco Use Disorders Among Religious Users of Ayahuasca. Front Psychiatry 9:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berquist MD, Fantegrossi WE (2021) Effects of 5-HT2A receptor agonist 2,5-dimethoxy-4-iodoamphetamine on alcohol consumption in Long-Evans rats. Behav Pharmacol 32(5):382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa PC, Strassman RJ (2015) Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol 29(3):289–99. [DOI] [PubMed] [Google Scholar]
- Bogenschutz MP (2013) Studying the effects of classic hallucinogens in the treatment of alcoholism: Rationale, methodology, and current research with psilocybin. Curr Drug Abuse Rev 6(1):17–29. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Trifunovic RD, Shefner SA (1995) Serotonin potentiates ethanol-induced excitation of ventral tegmental area neurons in brain slices from three different rat strains. J Pharmacol Exp Ther 273:1139–1146. [PubMed] [Google Scholar]
- Cata-Preta EG, Serra YA, Moreira-Junior EDC, Reis HS, Kisaki ND, Libarino-Santos M, Silva RRR, Barros-Santos T, Santos LC, Barbosa PCR, Costa JL, Oliveira-Lima AJ, Berro LF, Marinho EAV (2018) Ayahuasca and Its DMT- and beta-carbolines - Containing Ingredients Block the Expression of Ethanol-Induced Conditioned Place Preference in Mice: Role of the Treatment Environment. Front Pharmacol 9:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Anastasio NC, Fox RG, Stutz SJ, Bubar MJ, Swinford SE, Watson CS, Gilbertson SR, Rice KC, Rosenzweig-Lipson S, Moeller FG (2013) Synergism between a serotonin 5-HT2A receptor (5-HT2AR) antagonist and 5-HT2CR agonist suggests new pharmacotherapeutics for cocaine addiction. ACS Chem Neurosci 4(1):110–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Toalston JE, Oster SM, McBride WJ, Rodd ZA (2009) Involvement of local serotonin-2A but not serotonin-1B receptors in the reinforcing effects of ethanol within the posterior ventral tegmental area of female Wistar rats. Psychopharmacology (Berl) 204(3):381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, Dukat M, Grella B, Hong S, Costantino L, Teitler M, Smith C, Egan C, Davis K, Mattson MV (2000) Binding of beta-carbolines and related agents at serotonin (5-HT(2) and 5-HT(1A)), dopamine (D(2)) and benzodiazepine receptors. Drug Alcohol Depend 60(2):121–32. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS (2015) Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 72(8):757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill J, Hallak J, Dursun SM, Baker G (2019) Ayahuasca: Psychological and Physiologic Effects, Pharmacology and Potential Uses in Addiction and Mental Illness. Curr Neuropharmacol 17(2):108–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Cunningham KA (2015) Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol Rev 67(1):176–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khisti RT, Wolstenholme J, Shelton KL, Miles MF (2006) Characterization of the ethanol-deprivation effect in substrains of C57BL/6 mice. Alcohol 40(2):119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Cheng Y, Bian W, Liu X, Zhang C, Ye JH (2010) Region-specific induction of FosB/deltaFosB by voluntary alcohol intake: effects of naltrexone. Alcohol Clin Exp Res 34(10):1742–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loizaga-Velder A, Verres R (2014) Therapeutic effects of ritual ayahuasca use in the treatment of substance dependence--qualitative results. J Psychoactive Drugs 46(1):63–72. [DOI] [PubMed] [Google Scholar]
- Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW (2013) Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction 108(2):275–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel S, De Vry J, Schreiber R (1999) 5-HT receptor ligands differentially affect operant oral self-administration of ethanol in the rat. Eur J Pharmacol 370(3):217–23. [DOI] [PubMed] [Google Scholar]
- McKenna DJ (2004) Clinical investigations of the therapeutic potential of ayahuasca: rationale and regulatory challenges. Pharmacol Ther 102(2):111–29. [DOI] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Fox RG, Stutz SJ, Rice KC, Cunningham KA (2009) Blockade of the serotonin 5-HT2A receptor suppresses cue-evoked reinstatement of cocaine-seeking behavior in a rat self-administration model. Behav Neurosci 123(2):382–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolli LM, de Oliveira DGR, Alves SS, von Zuben MV, Pic-Taylor A, Mortari MR, Caldas ED (2020) Effects of the hallucinogenic beverage ayahuasca on voluntary ethanol intake by rats and on cFos expression in brain areas relevant to drug addiction. Alcohol 84:67–75. [DOI] [PubMed] [Google Scholar]
- Oliveira-Lima AJ, Santos R, Hollais AW, Gerardi-Junior CA, Baldaia MA, Wuo-Silva R, Yokoyama TS, Costa JL, Malpezzi-Marinho EL, Ribeiro-Barbosa PC, Berro LF, Frussa-Filho R, Marinho EA (2015) Effects of ayahuasca on the development of ethanol-induced behavioral sensitization and on a post-sensitization treatment in mice. Physiol Behav 142:28–36. [DOI] [PubMed] [Google Scholar]
- Perkins D, Opaleye ES, Simonova H, Bouso JC, Tófoli LF, GalvÃo-Coelho NL, Schubert V, Sarris J (2021) Associations between ayahuasca consumption in naturalistic settings and current alcohol and drug use: Results of a large international cross-sectional survey. Drug Alcohol Rev [in press]. [DOI] [PubMed] [Google Scholar]
- Reis HS, Rodrigues IRS, Anjos-Santos A, Libarino-Santos M, Serra YA, Cata-Preta EG, Oliveira-Campos D, Kisaki ND, Barros-Santos T, Yokoyama TS, Cruz FC, Oliveira-Lima AJ, Barbosa PCR, Berro LF, Marinho EAV (2020) Ayahuasca blocks the reinstatement of methylphenidate-induced conditioned place preference in mice: behavioral and brain Fos expression evaluations. Psychopharmacology (Berl) 237(11):3269–3281. [DOI] [PubMed] [Google Scholar]
- Thomas G, Lucas P, Capler NR, Tupper KW, Martin G (2013) Ayahuasca-assisted therapy for addiction: results from a preliminary observational study in Canada. Curr Drug Abuse Rev 6(1):30–42. [DOI] [PubMed] [Google Scholar]
- Valyear MD, Villaruel FR, Chaudhri N (2017) Alcohol-seeking and relapse: A focus on incentive salience and contextual conditioning. Behav Processes 141(Pt 1):26–32. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Litten RZ, Leggio L (2019) Advances in the science and treatment of alcohol use disorder. Sci Adv 5(9):eaax4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2018) Global status report on alcohol and health 2018. World Health Organization. https://apps.who.int/iris/handle/10665/274603. License: CC BY-NC-SA 3.0 IGO [Google Scholar]


