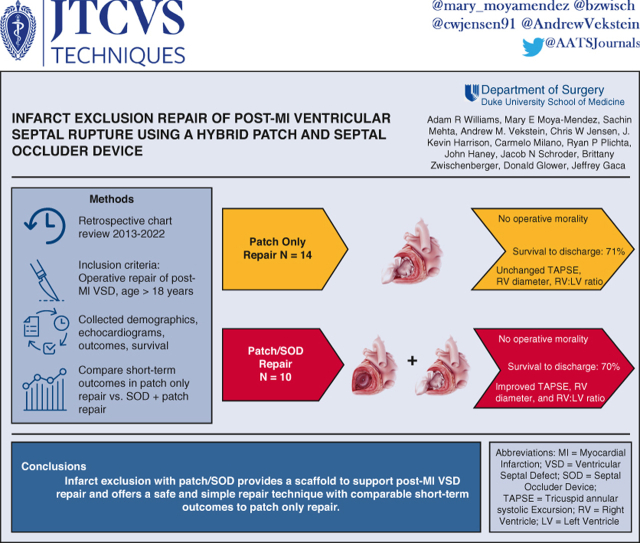
Abstract
Objective
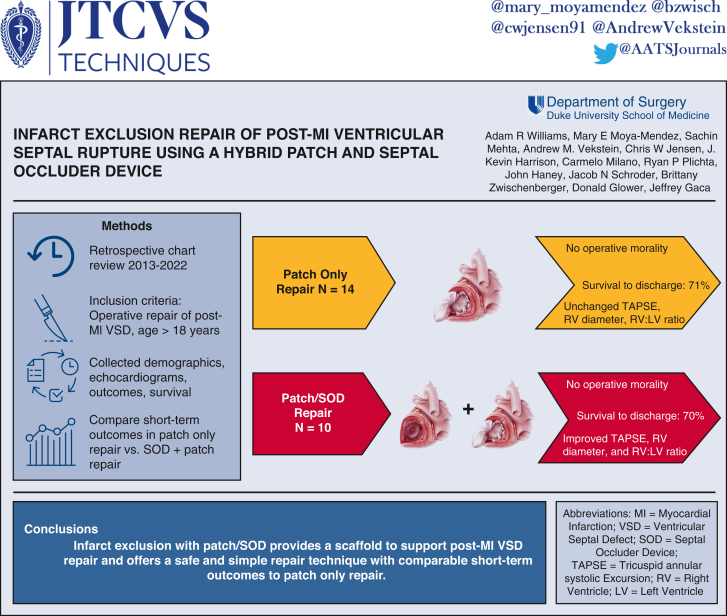
We developed a hybrid technique for repairing post-myocardial infarction (MI) ventricular septal defect (VSD) that combines infarct exclusion with patch and a nitinol-mesh septal occluder device (SOD) to provide a scaffold to support the damaged septal wall. Here, we compare outcomes of patients with post-MI VSD repaired using patch only or hybrid patch/SOD.
Methods
Patients undergoing post-MI VSD repair at our institution from 2013 to 2022 who received patch alone or patch/SOD repair were analyzed. Primary outcome was survival to hospital discharge. Clinical outcomes and echocardiograms were also analyzed.
Results
Over a 9-year period, 24 patients had post-MI VSD repair at our institution with either hybrid patch/SOD (n = 10) or patch only repair (n = 14). VSD size was 18 ± 5.8 mm for patch/SOD and 17 ± 4.6 mm for patch only. In the patch/SOD repair cohort, average size of SOD implant was 23.6 ± 5.6 mm. Mild left ventricular dysfunction was present prerepair and was unchanged postrepair in both groups; however, moderate-to-severe right ventricular (RV) dysfunction was common in both groups before repair. RV function worsened or persisted as severe in 10% of hybrid versus 54% of patch-only patients postrepair. Tricuspid annular systolic excursion and RV:left ventricle diameter ratio, quantitative metrics of RV function, improved after patch/SOD repair. No intraoperative mortality occurred in either group. Postoperative renal, hepatic, and respiratory failure requiring tracheostomy was common in both groups. Survival to hospital discharge in both cohorts was 70%.
Conclusions
Post-MI VSD repair with patch/SOD has comparable short-term outcomes with patch alone. Addition of a SOD to patch repair provides a scaffold that may enhance the repair of post-MI VSD with patch exclusion.
Key Words: ventricular septal defect, VSD, myocardial infarction, MI, post-MI VSD, coronary artery disease, CAD
Graphical abstract
Hybrid patch/SOD repair technique of a post-MI VSD involving the posterior basal septum.
Central Message.
Hybrid repair of postmyocardial infarction ventricle septal rupture using a patch plus septal occluder device adds a scaffold to enhance the defect closure and support infarct exclusion.
Perspective.
Ventricular septal rupture is a devastating mechanical complication of MI, with >90% mortality without surgical intervention. We have developed a hybrid repair technique with open deployment of a catheter-based septal occluder device combined with infarct exclusion with patch for post-MI VSD. Comparison with patch-only repair showed similar short-term outcomes including a 71% survival-to-discharge.
See Discussion on page 237.
Left ventricular (LV) rupture is a devasting mechanical complication from acute myocardial infarction (MI), associated with greater than 90% mortality without surgical repair. Most ventricular ruptures occur in the LV free wall, with less than 20% originating in the interventricular septum.1 Post-MI ventricular septal defect (VSD) leads to left–right shunting of blood, right ventricular (RV) volume and pressure overload, and RV dysfunction. Closure of the septal wall defect is the only therapy that reverses this process and prevents end-organ failure and death.
The original operative technique for post-MI VSD consisted of infarctectomy and reconstruction of the septum with a patch.2 In the early 1990s, Tirone David introduced a repair technique that excluded the infarcted myocardium of the septum with a pericardial patch sutured to the endocardium of the left ventricle without resecting any myocardial tissue.3 The infarct exclusion with patch technique increased survival in post-MI VSD by avoiding infarctectomy and additional damage to the right ventricle.4 The infarct exclusion with patch technique has remained the gold standard for repairing post-MI VSD for over 3 decades.
Over the past several decades, percutaneous closure using catheter-based septal occluder devices (SODs) have emerged, which attempt to close the defect and provide a scaffold to the damaged wall.5, 6, 7, 8, 9 SODs are collapsible, self-expanding nitinol mesh frames commercially available in multiple sizes that expand into a framework that consists of a waist and disks on the RV and LV septal wall. Outcomes with percutaneous closure have largely been disappointing due to difficulty in precise placement within VSD and the potential interaction with the tricuspid and mitral valves. Furthermore, percutaneous closure of VSDs carries high procedural complications, mortality, and persistent shunts.6,7 However, direct open placement of a SOD into a VSD is simple, straightforward, and allows for changes to easily be made to the device and prevent interference with the sub-valvular apparatus.
We first described a hybrid repair technique of direct deployment of a SOD alone via right atriotomy for post-MI VSD10 and recently combined traditional infarct exclusion with patch and SOD deployment via left ventriculotomy.11 Here, we report our early outcomes in patients post-MI VSD using our hybrid patch exclusion and SOD technique compared with infarct exclusion with patch alone.
Methods
Study Approval and Research Ethics
This study was approved by the institutional review board of Duke University Medical Center (institutional review board #Pro00107284) on December 21, 2020. As a retrospective study, the need for individual patient consent was waived.
Patient Selection and Data Collection
We retrospectively reviewed our institution's electronic medical record for all patients seen between January 2013 and May 2022 with a diagnosis of a post-MI VSD as recorded by the appropriate International Classification of Diseases, Tenth Revision code I23.2, ventricular septal defect as current complication following acute myocardial infarction. A diagnosis of post-MI VSD was confirmed by direct chart review. Patients were then screened for surgical intervention and included in the analysis if they received operative repair of the VSD.
Extracted data included baseline demographics, comorbidities, inotrope use, mechanical circulatory support, and preoperative percutaneous coronary interventions. Operative reports were reviewed to determine repair technique, location of ventriculotomy, size of SOD if used, cardiopulmonary bypass time, crossclamp time, any associated cardiac procedures. The primary outcome was survival to hospital discharge. Additional outcomes were assessed for operative mortality, 30-day survival, cause of death, intensive care unit length of stay, ventilation time, renal failure requiring dialysis, and re-exploration for bleeding.
Echocardiogram assessment of ventricular structure and function were performed. Intraoperative transesophageal echocardiograms of prerepair and postrepair were reviewed and analyzed. Variables extracted included size and location of VSD, LV ejection fraction, RV function, valvular regurgitation, and residual shunt after VSD repair. RV function was further quantified using tricuspid annular plane systolic excursion (TAPSE) and RV:LV diameter ratio. TAPSE was measured in a 4-chamber view, placing the M mode line at the lateral tricuspid annulus and measuring the displacement of the annulus toward the apex during systole. RV and LV diameters were measured in end-diastole. The RV:LV diameter ratio was calculated by dividing the RV diameter by the LV diameter in diastole.
Operative Technique
All patients underwent median sternotomy with central aortic cannulation, bicaval venous cannulation, and myocardial protection using cold blood cardioplegia delivered via antegrade and retrograde. Moderate systemic hypothermia was used, 28 to 32 °C. Valve interventions and coronary artery bypass grafting were performed as indicated. The VSD was accessed via ventriculotomy performed parallel to either the posterior descending artery for posterior VSDs or left anterior descending artery for anterior VSDs. Pledgeted retraction stiches were placed in the ventricular wall to facilitate exposure to the VSD.
For the patch-alone repair group, a sheet of bovine pericardium was fashioned to the allow for sufficient oversizing of the VSD. Interrupted mattress stitches were placed circumferentially around the defect and then passed through the patch and secured. In some cases, a second layer of running PROLENE stitch was placed around the circumference of the patch. The patch was brought out the ventriculotomy and incorporated into the closure. Two sheets of Teflon felt were used to close the ventriculotomy with 2 layers of running suture incorporating the patch in the closure. Glue was also applied to the ventriculotomy closure in some cases to provide additional hemostasis.
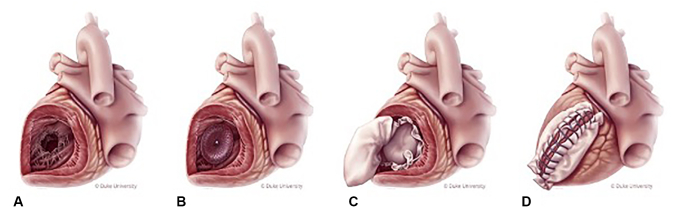
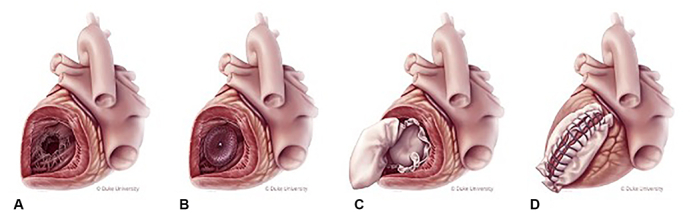
For the hybrid patch and SOD repair group, an Amplatzer Septal Occluder (Abbott Laboratories) was loaded onto the catheter delivery system, inserted into the VSD, and deployed under direct vision (Figure 1). In recent cases, we have no longer used the catheter delivery system and simply collapse the nitinol device with our hand and insert it into the defect manually and reposition as necessary. A sheet of bovine pericardium was fashioned to allow for sufficient oversizing of the device. Interrupted mattress stitches were placed circumferentially around the defect, and include the SOD, and then passed through the patch. Once all the stiches were placed, the knots are then secured. The patch was brought out the ventriculotomy and closed as described previously. Additional details of the hybrid patch and SOD repair have been previously described and include a video of the technique.11
Figure 1.
Hybrid patch and SOD repair of a post-MI VSD involving the posterior basal septum. A, Inferior-wall VSD exposed via ventriculotomy; B, SOD deployed in VSD; C, infarct exclusion with bovine pericardial patch; and D, closure of ventriculotomy with Teflon felt.
Statistical Analysis
Clinical and operative outcomes after post-MI VSD repair are reported. We stratified patients into 2 cohorts: (1) hybrid infarct exclusion using patch and SOD, and (2) infarct exclusion with patch alone for comparison. Descriptive statistics were used to report the baseline, operative, and postoperative characteristics for patients and are expressed as mean ± standard deviation for continuous data and as numerical count with percentage in parentheses for categorical data. All statistical comparisons were done between the patch with SOD repair and the patch alone repair. A Mann–Whitney U test was used to compare nonparametric data separated by patch with SOD versus patch alone. A Fisher exact test was used to compare categorical data separated by patch with SOD versus patch alone. Statistical analysis was performed using GraphPad Software.
Results
Over a 9-year period, 24 patients had post-MI VSD repair at our institution. Baseline characteristics are shown in Table 1, and there was no difference between groups other than hypertension being more frequent in the hybrid patch and SOD group. The average age was 67 ± 6.9 years, and most were men (67%). An infarct exclusion with patch only repair was performed in 14 patients, and 10 patients underwent hybrid patch and SOD repair. Same admission percutaneous coronary intervention was performed in 38% of patients prior to repair of VSD. Cardiogenic shock at presentation was common in the setting of post-MI VSD with preoperative intra-aortic balloon pump (IABP) support used in 92% of patients.
Table 1.
Baseline characteristics
| Variable∗ | Total cohort (n = 24) | Patch + SOD (n = 10) | Patch only (n = 14) | P value† |
|---|---|---|---|---|
| Demographics | ||||
| Age at surgery, y | 67 ± 6.9 | 69.4 ± 7.3 | 64.3 ± 5.5 | .07 |
| Male sex | 16 (67) | 6 (60) | 10 (71) | .67 |
| Comorbidities | ||||
| Hypertension | 13 (54) | 9 (90) | 4 (40) | .004 |
| Diabetes | 9 (38) | 4 (40) | 5 (36) | >.99 |
| CAD | 11 (46) | 5 (50) | 6 (43) | >.99 |
| NYHA class IV | 7 (29) | 3 (30) | 4 (29) | >.99 |
| Previous CABG | 2 (8) | 2 (20) | 0 (0) | .16 |
| Previous PCI | ||||
| Remote | 4 (17) | 0 (0) | 4 (29) | .11 |
| Same admission | 9 (38) | 4 (40) | 5 (36) | >.99 |
| Acute renal failure | 3 (13) | 2 (20) | 1 (7) | .55 |
| Cardiogenic shock‡ | 20 (84) | 8 (80) | 12 (86) | >.99 |
| Preoperative IABP | 22 (92) | 9 (90) | 13 (93) | >.99 |
SOD, Septal occluder device; CAD, coronary artery disease; NYHA, New York Heart Association; CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention; IABP, intra-aortic balloon pump.
Continuous variables are presented as mean ± standard deviation and categorical data as number (%).
Fisher exact test was used for categorical variables. Unpaired t test was used for parametric continuous variables.
Cardiogenic shock was defined as CI <2.0 or SBP <90 mm Hg.
Operative characteristics are detailed in Table 2, and there was no significant difference in operative times, associated procedures, or use of mechanical support. Average CPB time was 205.5 ± 67.3 minutes, and crossclamp time was 112.6 ± 39.8 minutes for the overall cohort. Most VSDs were exposed via left ventriculotomy (88%). Coronary artery bypass grafting was the most common associated procedure, performed in 58% of patients, and tricuspid valve repair or replacement was the most common associated valve procedure. All patients had bovine pericardium used to repair the VSD. The average size SOD implanted in the hybrid patch and SOD group was 23.6 ± 5.6 mm. Postoperative extracorporeal circulation was used in 2 patients in the hybrid patch and SOD cohort and 3 patients in the patch-only repair cohort. There was no intraoperative mortality in either group.
Table 2.
Operative and echocardiogram findings
| Variable∗ | Total cohort (n = 24) | Patch + SOD (n = 10) | Patch only (n = 14) | P value† |
|---|---|---|---|---|
| Operative characteristics | ||||
| Location of VSD | ||||
| Anterior | 5 (21) | 2 (20) | 3 (21) | >.99 |
| Posterior | 19 (79) | 8 (80) | 11 (79) | >.99 |
| CPB, min | 205.5 ± 67.3 | 196.2 ± 55.8 | 220.8 ± 73.8 | .40 |
| Crossclamp, min | 112.6 ± 39.8 | 105.8 ± 29.5 | 128.7 ± 44 | .18 |
| Concomitant procedure | ||||
| CABG | 14 (58) | 7 (70) | 7 (50) | .67 |
| TVR/r | 6 (25) | 3 (30) | 3 (14) | >.99 |
| AVR | 1 (4) | 1 (10) | 0 (0) | .42 |
| MVR/r | 2 (8) | 1 (10) | 1 (7) | >.99 |
| Size of SOD, mm | – | 23.6 ± 5.6 | – | – |
| Echo characteristics | ||||
| Size of VSD, mm | 17 ± 5.4 | 18 ± 5.9 | 16.3 ± 5.2 | .46 |
| LVEF, % | ||||
| Preop | 45.5 ± 11.1 | 44.5 ± 9.6 | 46.2 ± 12.3 | .49 |
| Postop | 44.2 ± 10.2 | 46 ± 5.7 | 42.3 ± 12.5 | .77 |
| RV dysfunction | 14 (58) | 5 (50) | 9 (64) | .68 |
| Pre-mod-severe | 14 (58) | 5 (50) | 9 (64) | .68 |
| Post same/worse | 8 (34) | 1 (10) | 7 (50) | .08 |
| RVEDD, cm | ||||
| Preop | 4 ± 0.59 | 4 ± 0.68 | 4.1 ± 0.54 | .79 |
| Postop | 3.8 ± 0.74 | 3.4 ± 0.48 | 4.1 ± 0.78 | .03 |
| LVEDD, cm | ||||
| Preop | 4.8 ± 0.84 | 4.8 ± 1 | 4.9 ± 0.75 | .89 |
| Postop | 4.7 ± 0.87 | 4.7 ± 1.1 | 4.7 ± 0.75 | .74 |
| RV:LV ratio | ||||
| Preop | 0.85 ± 0.14 | 0.85 ± 0.16 | 0.86 ± 0.13 | .97 |
| Postop | 0.84 ± 0.21 | 0.76 ± 0.19 | 0.89 ± 0.21 | .08 |
SOD, Septal occluder device; VSD, ventricular septal defect; CPB, cardiopulmonary bypass; CABG, coronary artery bypass graft; TVR, tricuspid valve replacement; TVr, tricuspid valve repair; AVR, aortic valve replacement; MVR, mitral valve replacement; MVr, mitral valve repair; LVEF, left ventricular ejection fraction; preop, preoperative; postop, postoperative; RV, right ventricle; RVEDD, right ventricle end-diastolic diameter; LVEDD, left ventricle end-diastolic diameter; LV, left ventricle.
Continuous variables are presented as mean ± standard deviation and categorical data as number (%).
Fisher exact test was used for categorical variables. Unpaired t test was used for parametric continuous variables. Mann–Whitney U test was used for nonparametric continuous variables.
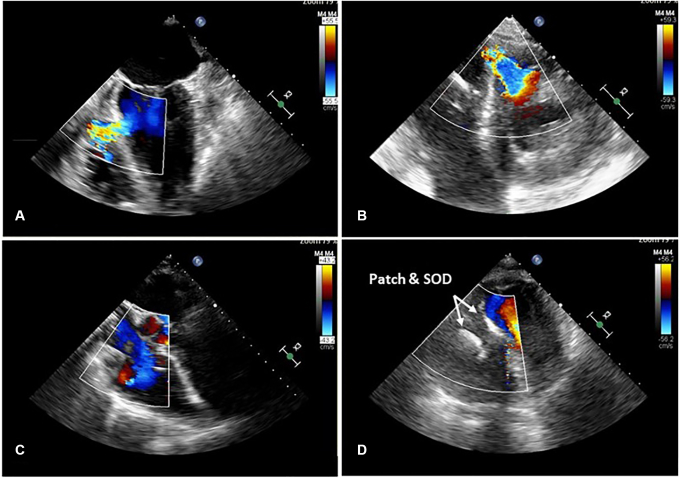
Average VSD size measured by echocardiography was 17 ± 5.4 mm and was comparable between groups (Table 2). The VSD location was posterior in 79% of patients and 21% located anterior. Pre- and postrepair echocardiogram images showed complete closure of the VSD with no residual shunt postrepair greater than trivial (Figure 2). Preoperative LV ejection fraction (EF) was 45.5 ± 11.1%, with no difference between groups (hybrid repair: 44.5 ± 9.6% vs patch-only repair: 46.2 ± 12.3%). Postoperative LVEF remained unchanged at 44.2 ± 10.2% in the entire cohort (hybrid repair: 46 ± 5.7% vs patch-only repair: 42.3 ± 12.5%). Preoperative RV dysfunction was common, with 58% of patients presenting with moderate-to-severe dysfunction. Postoperative RV function worsened or persisted in 34% of the entire cohort, but only 10% worsening in hybrid patch and SOD repair group compared with 50% in patch-only repair patients (P = .08). RV function as assessed by TAPSE RV diameter, and RV:LV diameter ratio was preserved in the hybrid patch and SOD repair cohort versus worsening in the patch only repair cohort (Table 2 and Figure 3).
Figure 2.
Echocardiogram images of hybrid patch and SOD repair of post-MI VSD. Prerepair (A) long-axis and (B) short-axis views of basal, inferior wall VSD with Doppler showing large left-to-right shunt. C, Long-axis image showing no residual shunt after hybrid repair and D, short-axis view showing SOD. SOD, Septal occluder device.
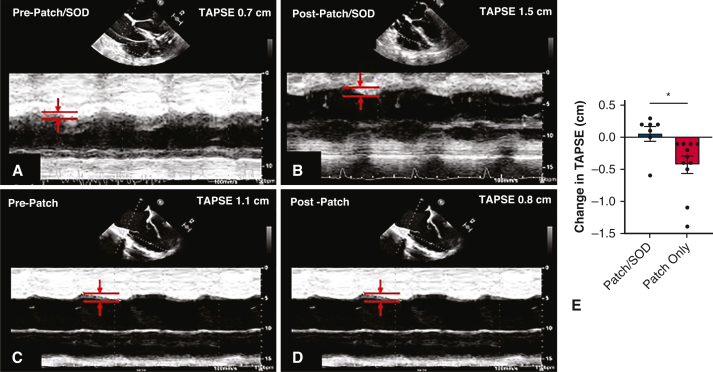
Figure 3.
Echocardiogram M-mode images of tricuspid annular plane systolic excursion (TAPSE), a metric of RV function that measures the distance of base-to-apex shortening, before and after post-MI VSD repair. Improved TAPSE after patch/SOD repair (A and B) compared with worsening TAPSE after patch only repair (C and D). E, TAPSE after patch/SOD repair was improved compared with worsening TAPSE in patch-only repair, ∗P = .005. SOD, Septal occluder device.
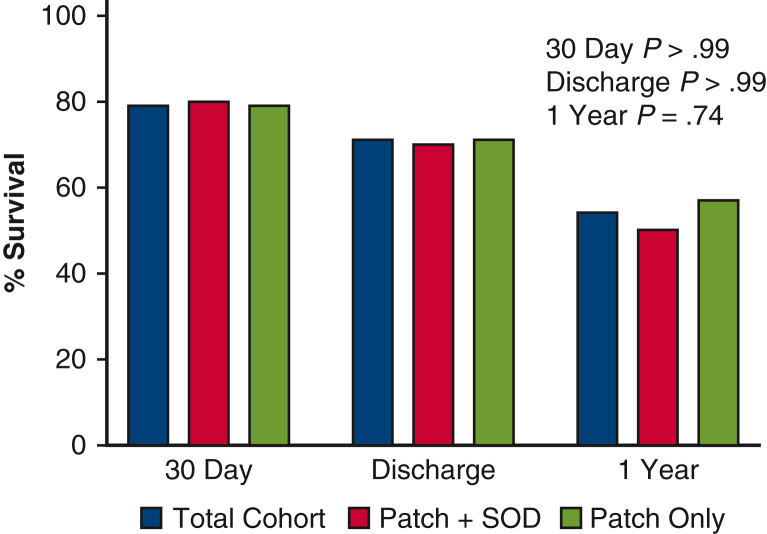
Clinical outcomes are shown Table 3 and survival outcomes in Figure 4. There was no intraoperative mortality in either group. Survival to discharge after post-MI VSD repair was 71% (n = 17), with no difference between repair strategies (hybrid repair: 70% vs patch-only repair: 71%). One-year survival dropped to 54% in the overall cohort with no difference between groups. The most common causes of death were multiorgan system failure (57%, n = 4) and heart failure (43%, n = 3). Prolonged intensive care unit course, liver failure, renal failure, and respiratory failure rates were high and emphasize the severe illness associated with post-MI VSD.
Table 3.
Clinical outcomes
| Variable∗ | Total cohort (n = 24) | Patch + SOD (n = 10) | Patch only (n = 14) | P value† |
|---|---|---|---|---|
| Clinical outcomes | ||||
| ICU stay, d | 18.3 ± 15.1 | 21.1 ± 18.4 | 16.4 ± 12.4 | .46 |
| Ventilator, h | 200 ± 246.6 | 153.6 ± 157.7 | 233.1 ± 295.8 | .45 |
| Tracheostomy | 6 (25) | 3 (30) | 3 (21) | .67 |
| Low CO syndrome | 7 (29) | 3 (30) | 4 (29) | >.99 |
| Atrial fibrillation | 14 (58) | 6 (60) | 8 (57) | >.99 |
| Pacemaker | 5 (21) | 0 (0) | 5 (36) | .045 |
| Dialysis | 9 (38) | 6 (60) | 3 (21) | .092 |
| Stroke | 3 (13) | 0 (0) | 3 (21) | .24 |
| Pneumonia | 4 (17) | 1 (10) | 3 (31) | .61 |
| Liver failure | 5 (21) | 2 (20) | 3 (31) | >.99 |
| Re-exploration for bleeding | 5 (21) | 2 (20) | 3 (31) | >.99 |
| Intraoperative mortality | 0 (0) | 0 (0) | 0 (0) | - |
| 30-d mortality | 5 (21) | 2 (20) | 3 (21) | >.99 |
| Survival to discharge | 17 (71) | 7 (70) | 10 (71) | >.99 |
| 1-y survival | 13 (54) | 5 (50) | 8 (57) | .74 |
SOD, Septal occluder device; ICU, intensive care unit; CO, cardiac output.
Continuous variables are presented as mean ± standard deviation and categorical data as number (%).
Fisher exact test was used for categorical variables. Unpaired t test was used for parametric continuous variables.
Figure 4.
Percentage survival to 30 days, discharge and 1 year after post-MI VSD repair. SOD, Septal occluder device.
Discussion
Here, we present our hybrid post-MI VSD repair technique using a SOD and infarct exclusion patch and compare outcomes with patch-only repair. The main findings of this study are (1) RV failure and use of intra-aortic balloon pump support is common in post-MI VS; (2) post-MI VSD repair carries significant in-hospital mortality and remains high during the first year even after hospital discharge; (3) postoperative RV structure and function may be supported by using a SOD along with infarct exclusion with patch; (4) repair of post-MI VSD using a hybrid repair with patch and SOD is safe and has comparable short-term outcomes to patch-only repair (Figure 5). Our hybrid repair technique combines an easy to deploy SOD under direct vision with patch, which reinforces the post-MI VSD repair with a scaffold to support the septal wall.
Figure 5.
The methodology and major findings of our study, including comparable survival outcomes between the patch-only and patch/SOD repair groups, and improved postoperative right ventricular function in the patch/SOD repair group. MI, Myocardial infarction; VSD, ventricular septal defect; TAPSE, tricuspid annular plane systolic excursion; RV, right ventricle; LV, left ventricle; SOD, septal occluder device.
Hybrid Post-MI VSD Repair Technique
The hybrid patch and SOD repair technique combines the gold standard surgical intervention of infarct exclusion with patch with an open deployment of a catheter-based SOD. Commercially available SODs are readily available in a wide range of sizes and have been primarily used to percutaneously close atrial septal defects. We have adopted this technology to be deployed on the arrested heart under direct vision. For our hybrid post-MI repair technique, we have used the Amplatzer Septal Occluder (Abbott, Inc) device, which was approved by the Food and Drug Administration more than 20 years ago to percutaneously close atrial septal defects and is available in sizes ranging from 4 to 38 mm. Since the Amplatzer Septal Occluder device is delivered in our hybrid technique under direct vision rather than the percutaneously, this is technically an off-label use of the device. We use intraoperative transesophageal echocardiograms to measure the VSD size as well as direct intraoperative measurements to size the device. In our initial cases, we were oversizing the SOD by ∼20%; however, more recently we have found that one-to-one sizing of the VSD and SOD is adequate to provide enough closure and a framework that allows for sutures to be passed through normal adjacent myocardium, as well as the device to further anchor the patch in the LV.
Our technique for hybrid post-MI repair has evolved: initially, the VSD was approached via right atriotomy and only the device was implanted without any patch.10 We now perform a left ventriculotomy 1-cm parallel to the infarcted vessel to deploy the SOD and then sew a patch to the adjacent myocardium, and also include the SOD frame in the patch closure.11 This allows for precise deployment in the LV to ensure the device does not interfere with the mitral subvalvular apparatus. We also sew a patch over the SOD to ensure there is no residual shunt, as SODs are totally occlusive at the time of implantation. The Amplatzer occluder device is designed with a sheet of Dacron fabric inside a nitinol stent frame that is semipermeable at implantation and can take several days to weeks to completely occlude the defect and reverse the left to right shunt.5,6 Therefore, the patch provides for immediate cessation of left-to-right shunting, providing a “belt and suspenders” repair. We have also found that the catheter delivery system for the SOD is not necessary during open deployment. The nitinol frame is easily compressed by the surgeon's fingers into a small tubular structure that can be inserted manually into the VSD opening, and the nitinol frame self-expands into the bi-disk structure. If the initial placement is not correct, it can easily be recompressed and repositioned into the correct location within the VSD.
The key operative steps for hybrid patch and SOD repair include central aortic and bicaval cannulation, cardioplegic arrest, left ventriculotomy 1-cm parallel to the infarcted vessel, retraction sutures to expose the VSD, deployment of the SOD under direct vision, oversized patch sewn to the adjacent normal myocardium incorporating the wire SOD frame, and closure of ventriculotomy with felt incorporating the tongue of the patch (Figure 1). Indeed, the hybrid patch and SOD repair is a simple and easily reproducible technique that provides a reinforced patch repair of post-MI VSD.
Surgery for post-MI VSD is technically challenging due to friable and necrotic myocardium that poorly holds suture.12 Increased stress on the sutures holding a patch to friable myocardium may lead to breakdown of the patch with residual or recurrent shunting. Furthermore, ongoing infarct expansion can lead to breakdown of suture lines. The hybrid patch and SOD repair provides a scaffold with the ability to expand and conform to any early changes after repair. The wire mesh framework also provides a structure in addition to the necrotic edges to secure the sutures of the patch, providing additional attachment of the patch. SODs remain porous for some time until fibrotic scarring and neoendothelization occurs, making the patch an integral aspect of the hybrid repair to provide immediate reversal of the left-right shunting.
Structural and Functional Changes After Post-MI VSD Repair
In our study, there was a high proportion of posterior VSDs, which are considered to be more challenging repairs compared with anterior defects and carry greater operative risk.12 In both the hybrid patch and SOD and patch-alone groups, baseline LV function was mild depressed with EF ∼45% and postoperatively remained unchanged at 42%. Not surprisingly, most patients presented with at least moderate-to-severe RV dysfunction. RV dysfunction is common after a post-MI VSD due to the left-to-right shunting that leads to volume and pressure overload of the RV. Interestingly, postoperative RV dysfunction worsened or remained severe in 50% of the patch-only repair cohort versus only 10% in the patch and SOD repair cohort.
TAPSE has been shown to be a highly reproducible and easy-to-calculate echocardiography derived metric of RV function.13,14 Using M-mode echocardiography, systolic excursion is measured from the lateral tricuspid valve annulus towards the apex in a 4-chamber view to calculate TAPSE. Normal TAPSE in healthy volunteers is approximately 26 mm and RV dysfunction is present when TAPSE is less than 17 mm.13,14 Our overall VSD cohort had a prerepair average TAPSE value of 11 mm, indicating the presence of significant RV dysfunction. TAPSE remained stable or slightly improved in the hybrid SOD/patch repair cohort, whereas it worsened in the patch only repair.
Structural changes to the heart were assessed using LV and RV diameters derived from echocardiography. The RV chamber is typically smaller than the LV chamber, usually about two-thirds the size of the LV chamber.13 The relationship between the RV and LV can be assessed using the RV:LV diameter ratio. The RV:LV ratio remained unchanged in the patch-only repair cohort but showed improvement in the patch and SOD repair cohort. Taken together with improved TAPSE, postoperative RV function may be supported with the use of a patch and SOD repair technique for post-MI VSD. Adding a SOD to infarct exclusion with patch repair may provide a scaffold to the septal wall and restore structural integrity. Furthermore, the additional step of adding a SOD to patch repair does not prolong cross clamp time or lead to other risks, such as the need for permanent pacemaker implantation.
Outcomes After Repair of Post-MI VSD
A recent review of the Society of Thoracic Surgeons (STS) database of post-MI VSD repair used a primary outcome of operative mortality, defined by death in the hospital or within 30 days, and showed a mortality rate of 43%.12 There was a temporal trend toward improving outcomes likely secondary to the advancement of mechanical circulatory support. The data presented in our cohort showed a significant number of postoperative comorbidities in these patients, with a high prevalence of organ failure and need for dialysis and tracheostomy. Post-MI VSD is a catastrophic event that occurs after a massive MI and, despite surgical correction, these patients still suffer from major comorbidities even if they survive beyond 30 days. We observed an overall survival to discharge in our total cohort of ∼70%; however, these patients remained at significant risk of mortality during the first year after discharge with survival at one year of only 54%. Indeed, post-MI VSD remains a highly lethal event and one of the most humbling operations for cardiac surgeons.
These patients often present in very critical condition with large left-to-right shunts that cause significant RV dysfunction, cardiogenic shock, and end-organ dysfunction, as demonstrated with our data. We think immediate placement of an IABP provides critical hemodynamic support in post-MI VSD repair patients. Our cohort had more than 90% of IABPs placed preoperatively, much greater than the 60% in the STS report. IABPs unload the LV and therefore increase systemic flow and reduce shunting across the VSD. Additionally, IABPs increase coronary perfusion, which is a key determinant of RV function and provides additional support to the failing RV. IABPs are easy to insert and can even be placed in remote cardiac catheterization laboratories. IABP placement is a critical first step in the management post-MI VSD patients to stabilize and support hemodynamics until definitive operative repair can be performed.
The STS database ranks repair of post-MI VSD as the greatest reported mortality of all adult cardiac operations, which highlights the challenges of repairing these critically ill patients.12 Dr David pioneered an operation that has made surgical treatment of post-MI VSD simple and reproducible, transforming the surgical treatment of this devastating mechanical complication of MI. After being introduced nearly 30 years ago, Dr David's infarct exclusion with patch repair technique remains the gold standard surgical repair of post-MI VSD. We have added another simple step with direct deployment of a SOD that may reinforce the infarct exclusion with patch repair. The SOD may restore structural integrity to the septal wall and provide a reinforced “belt and suspenders” repair of post-MI VSD.
Limitations
The major limitation of the study is the small sample size, which is due to the rare nature of surviving a post-MI VSD long enough to make it to operative repair. Our institution is a large, tertiary referral center and we typically see only 2 to 3 post-MI VSDs per year. Moreover, we recently developed this technique over the past several years; therefore, follow-up time is limited and we only have short-term outcomes to 1 year in our cohort. In addition, many of our cases are referred from rural areas, which also limits our follow-up. Interestingly, our series had a high prevalence of inferior wall post-MI VSDs, which is considered a more challenging repair and is associated with worse outcomes.
Conclusions
Post-MI VSD repair with a patch and SOD is safe and has comparable short-term outcomes to patch-alone repair. Addition of a SOD to infarct exclusion with patch repair provides a scaffold that may enhance the repair of post-MI VSD.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: aats.org/resources/infarct-exclusion-repair-of-post-mi-ventricular-septal-rupture-using-a-hybrid-patch-and-septal-occluder-device-compared-to-patch-alone.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Acknowledgments
The authors thank Megan Llewellyn for the medical illustration of the hybrid patch and septal occluder device repair in Figure 1.
Footnotes
Drs Williams and Moya-Mendez contributed equally to this article.
References
- 1.Reddy S.G., Roberts W.C. Frequency of rupture of the left ventricular free wall or ventricular septum among necropsy cases of fatal acute myocardial infarction since introduction of coronary care units. Am J Cardiol. 1989;63:906–911. doi: 10.1016/0002-9149(89)90137-9. [DOI] [PubMed] [Google Scholar]
- 2.Daggett W.M. Surgical technique for early repair of posterior ventricular septal rupture. J Thorac Cardiovasc Surg. 1982;84:306–312. [PubMed] [Google Scholar]
- 3.Komeda M., Fremes S.E., David T.E. Surgical repair of postinfarction ventricular septal defect. Circulation. 1990;82:IV243–IV247. [PubMed] [Google Scholar]
- 4.David T.E., Dale L., Sun Z. Postinfarction ventricular septal rupture: repair by endocardial patch with infarct exclusion. J Thorac Cardiovasc Surg. 1995;110:1315–1322. doi: 10.1016/S0022-5223(95)70054-4. [DOI] [PubMed] [Google Scholar]
- 5.Assenza G.E., McElhinney D.B., Valente A.M., Pearson D.D., Volpe M., Martucci G., et al. Transcatheter closure of post-myocardial infarction ventricular septal rupture. Circ Cardiovasc Interv. 2013;6:59–67. doi: 10.1161/CIRCINTERVENTIONS.112.972711. [DOI] [PubMed] [Google Scholar]
- 6.Calvert P.A., Cockburn J., Wynne D., Ludman P., Rana B.S., Northridge D., et al. Percutaneous closure of postinfarction ventricular septal defect: in-hospital outcomes and long-term follow-up of UK experience. Circulation. 2014;129:2395–2402. doi: 10.1161/CIRCULATIONAHA.113.005839. [DOI] [PubMed] [Google Scholar]
- 7.Egbe A.C., Poterucha J.T., Rihal C.S., Taggart N.W., Cetta F., Cabalka A.K., et al. Transcatheter closure of postmyocardial infarction, iatrogenic, and postoperative ventricular septal defects: the Mayo Clinic experience. Catheter Cardiovasc Interv. 2015;86:1264–1270. doi: 10.1002/ccd.25989. [DOI] [PubMed] [Google Scholar]
- 8.Thiele H., Kaulfersch C., Daehnert I., Schoenauer M., Eitel I., Borger M., et al. Immediate primary transcatheter closure of postinfarction ventricular septal defects. Eur Heart J. 2008;30:81–88. doi: 10.1093/eurheartj/ehn524. [DOI] [PubMed] [Google Scholar]
- 9.Holzer R., Balzer D., Amin Z., Ruiz C.E., Feinstein J., Bass J., et al. Transcatheter closure of postinfarction ventricular septal defects using the new Amplatzer muscular VSD occluder: results of a U.S. Registry. Catheter Cardiovasc Interv. 2004;61:196–201. doi: 10.1002/ccd.10784. [DOI] [PubMed] [Google Scholar]
- 10.Lee M.S., Kozitza R., Mudrick D., Williams M., Lodge A.J., Harrison J.K., et al. Intraoperative device closure of postinfarction ventricular septal defects. Ann Thorac Surg. 2010;89:e48–e50. doi: 10.1016/j.athoracsur.2010.03.081. [DOI] [PubMed] [Google Scholar]
- 11.Madou I.D., Williams A.R., Gaca J.G. Patch exclusion technique with Amplatzer septal occluder device for the treatment of postinfarction ventricular septal defect. JTCVS Techniques. 2020;3:198–201. doi: 10.1016/j.xjtc.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnaoutakis G.J., Zhao Y., George T.J., Sciortino C.M., McCarthy P.M., Conte J.V. Surgical repair of ventricular septal defect after myocardial infarction: outcomes from the Society of Thoracic Surgeons National Database. Ann Thorac Surg. 2012;94:436–443. doi: 10.1016/j.athoracsur.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bleeker G.B., Steendijk P., Holman E.R., Yu C.M., Breithardt O.A., Kaandorp T.A., et al. Assessing right ventricular function: the role of echocardiography and complementary technologies. Heart. 2006;92(Suppl 1):i19–i26. doi: 10.1136/hrt.2005.082503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Modin D., Møgelvang R., Andersen D.M., Biering-Sørensen T. Right ventricular function evaluated by tricuspid annular plane systolic excursion predicts cardiovascular death in the general population. J Am Heart Assoc. 2019;8:e012197. doi: 10.1161/JAHA.119.012197. [DOI] [PMC free article] [PubMed] [Google Scholar]