Abstract
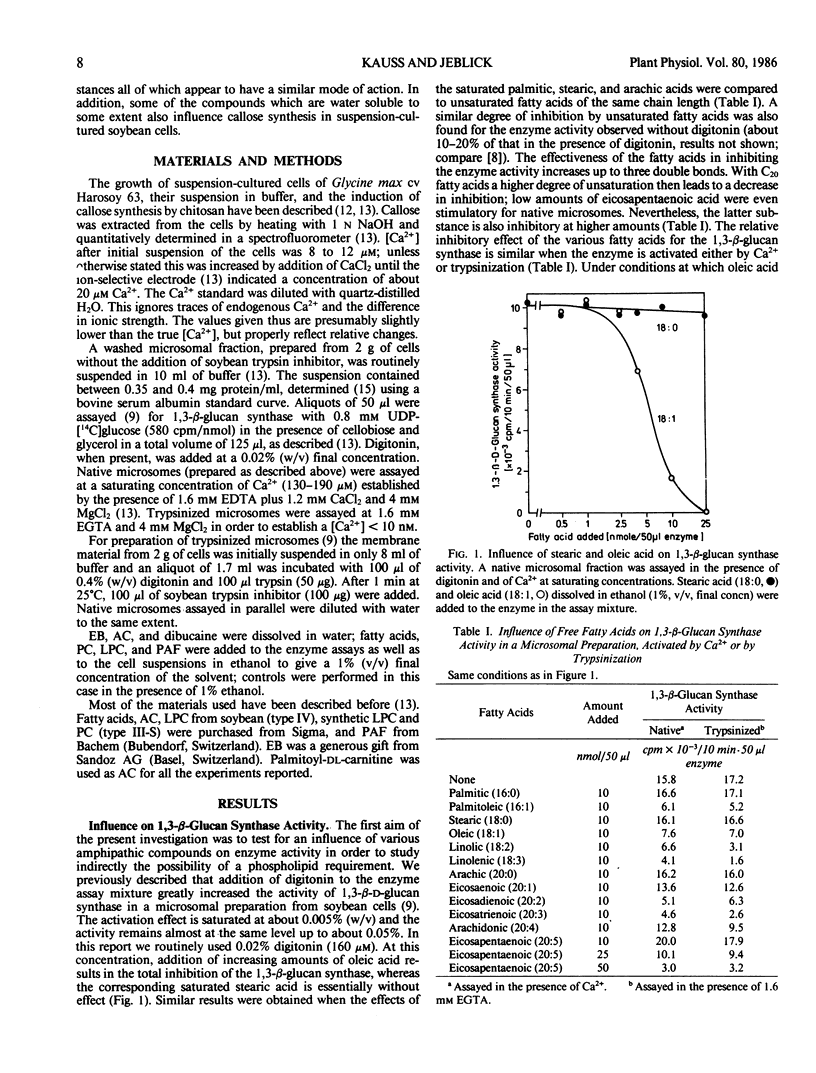
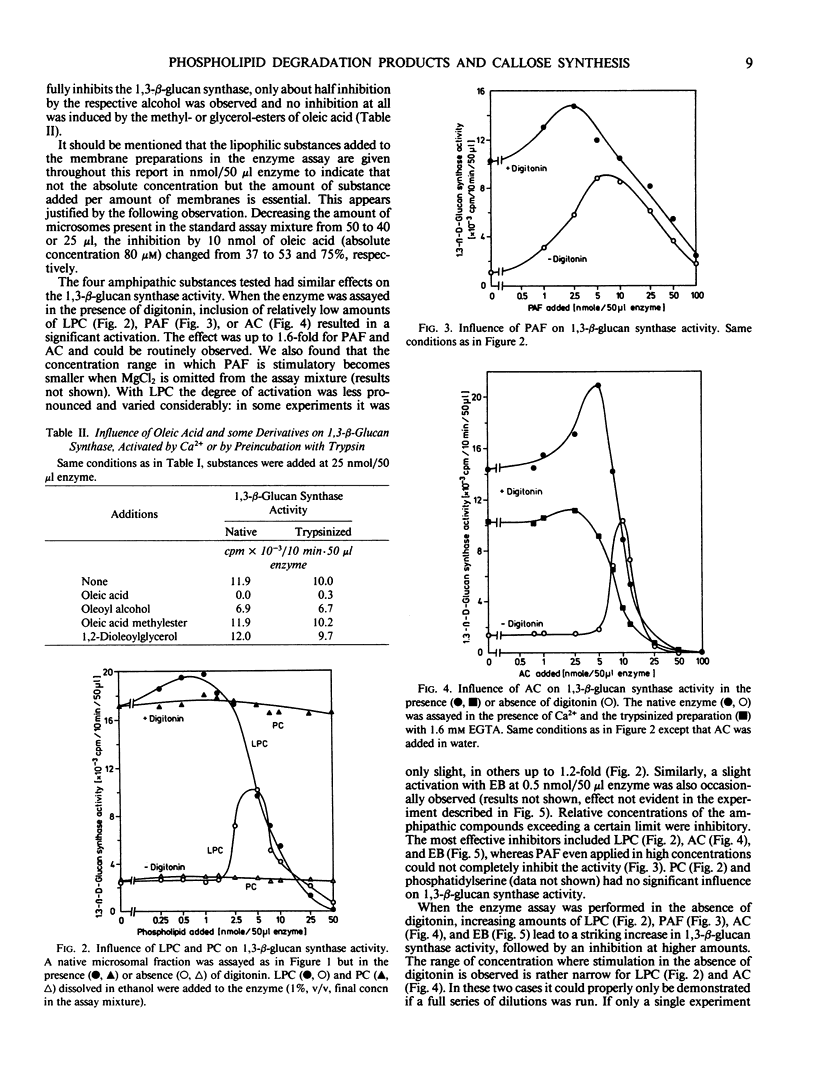
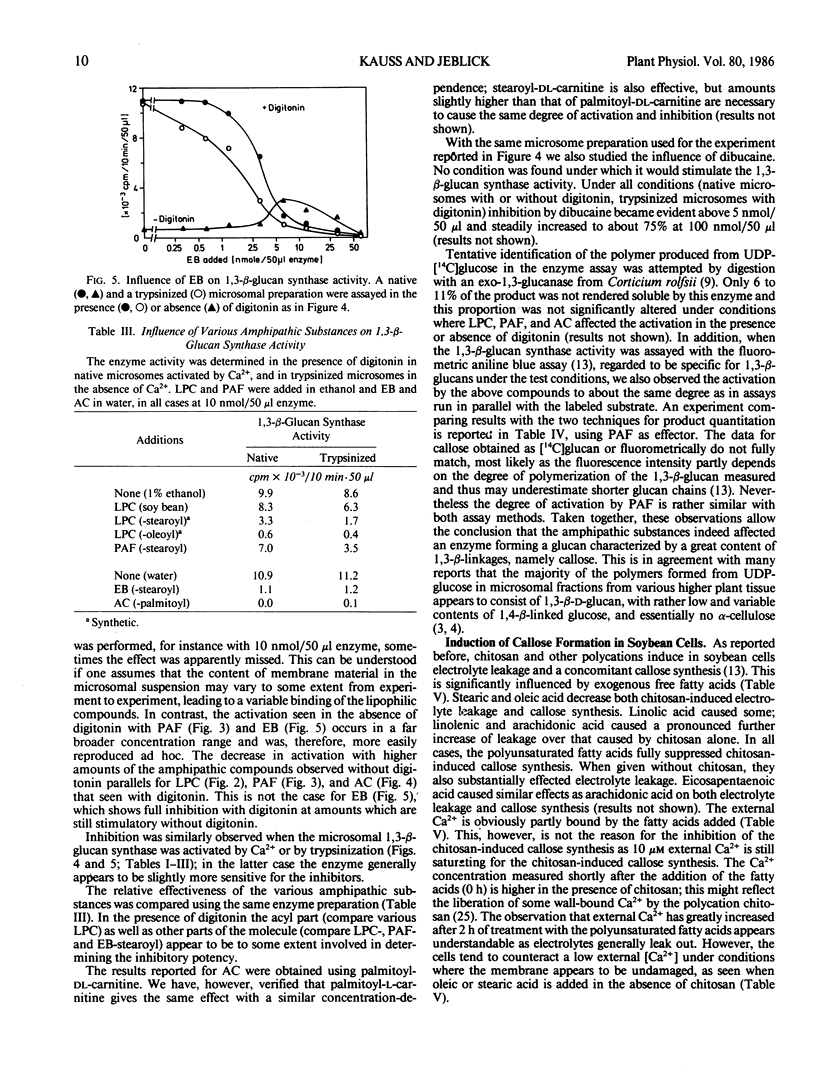
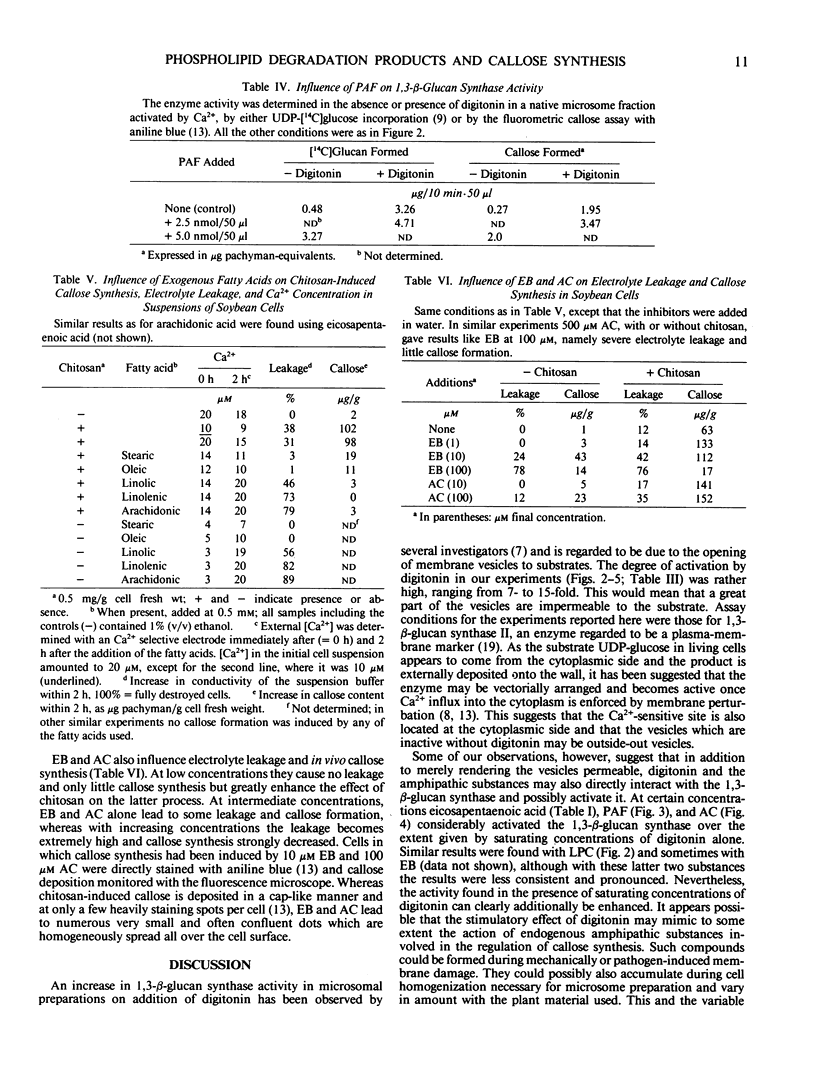
The activity of 1,3-β-d-glucan synthase assayed in the presence of digitonin in a microsomal preparation from suspension-cultured cells of Glycine max can be fully inhibited by unsaturated fatty acids, trienoic acids being most effective. Lysophosphatidylcholine, platelet-activating factor, acylcarnitine, and Echinocandin B can also fully inhibit the enzyme. Inhibition is observed both when the enzyme is activated by Ca2+ or by trypsinization. At low amounts some of the substances can also cause stimulation. These effects all may result from a displacement of certain endogenous phospholipids necessary for optimal activity of the 1,3-β-d-glucan synthase.
In the absence of digitonin the enzyme activity is greatly stimulated by lysophosphatidylcholine, platelet-activating factor, acylcarnitine, and Echinocandin B within a certain concentration range, presumably by rendering the microsomal vesicles permeable to the substrate and Ca2+. Dibucaine does not cause such an effect.
Acylcarnitine and Echinocandin B at low concentrations can induce callose synthesis in vivo; this effect is enhanced by chitosan. At higher concentrations the two substances and polyunsaturated fatty acids cause severe electrolyte leakage. The effects are discussed in regard to the induction of callose synthesis by enforced Ca2+ influx, and its modulation by membrane lipids.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beatrice M. C., Palmer J. W., Pfeiffer D. R. The relationship between mitochondrial membrane permeability, membrane potential, and the retention of Ca2+ by mitochondria. J Biol Chem. 1980 Sep 25;255(18):8663–8671. [PubMed] [Google Scholar]
- Delmer D. P. Biosynthesis of cellulose. Adv Carbohydr Chem Biochem. 1983;41:105–153. doi: 10.1016/s0065-2318(08)60057-8. [DOI] [PubMed] [Google Scholar]
- Köhle H., Jeblick W., Poten F., Blaschek W., Kauss H. Chitosan-elicited callose synthesis in soybean cells as a ca-dependent process. Plant Physiol. 1985 Mar;77(3):544–551. doi: 10.1104/pp.77.3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Low P. S., Lloyd D. H., Stein T. M., Rogers J. A., 3rd Calcium displacement by local anesthetics. Dependence on pH and anesthetic charge. J Biol Chem. 1979 May 25;254(10):4119–4125. [PubMed] [Google Scholar]
- Mauco G., Chap H., Douste-Blazy L. Platelet activating factor (PAF-acether) promotes an early degradation of phosphatidylinositol-4,5-biphosphate in rabbit platelets. FEBS Lett. 1983 Mar 21;153(2):361–365. doi: 10.1016/0014-5793(83)80643-7. [DOI] [PubMed] [Google Scholar]
- Preisig C. L., Kuć J. A. Arachidonic acid-related elicitors of the hypersensitive response in potato and enhancement of their activities by glucans from Phytophthora infestans (Mont.) deBary. Arch Biochem Biophys. 1985 Jan;236(1):379–389. doi: 10.1016/0003-9861(85)90638-1. [DOI] [PubMed] [Google Scholar]
- Sandermann H., Jr Regulation of membrane enzymes by lipids. Biochim Biophys Acta. 1978 Sep 29;515(3):209–237. doi: 10.1016/0304-4157(78)90015-1. [DOI] [PubMed] [Google Scholar]
- Sawistowska-Schröder E. T., Kerridge D., Perry H. Echinocandin inhibition of 1,3-beta-D-glucan synthase from Candida albicans. FEBS Lett. 1984 Jul 23;173(1):134–138. doi: 10.1016/0014-5793(84)81032-7. [DOI] [PubMed] [Google Scholar]
- Weltzien H. U. Cytolytic and membrane-perturbing properties of lysophosphatidylcholine. Biochim Biophys Acta. 1979 Aug 20;559(2-3):259–287. doi: 10.1016/0304-4157(79)90004-2. [DOI] [PubMed] [Google Scholar]
- Wise B. C., Glass D. B., Chou C. H., Raynor R. L., Katoh N., Schatzman R. C., Turner R. S., Kibler R. F., Kuo J. F. Phospholipid-sensitive Ca2+-dependent protein kinase from heart. II. Substrate specificity and inhibition by various agents. J Biol Chem. 1982 Jul 25;257(14):8489–8495. [PubMed] [Google Scholar]
- Young D. H., Kauss H. Release of Calcium from Suspension-Cultured Glycine max Cells by Chitosan, Other Polycations, and Polyamines in Relation to Effects on Membrane Permeability. Plant Physiol. 1983 Nov;73(3):698–702. doi: 10.1104/pp.73.3.698. [DOI] [PMC free article] [PubMed] [Google Scholar]


