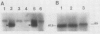Abstract
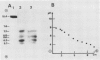
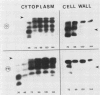
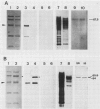
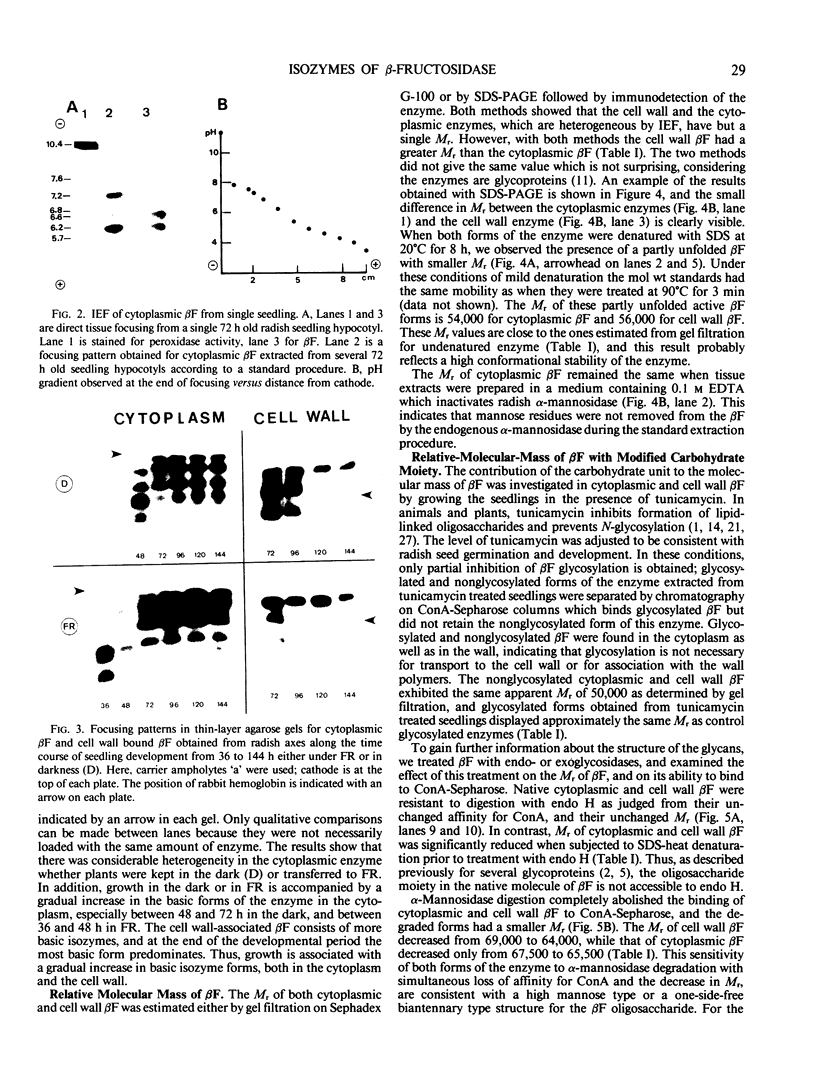
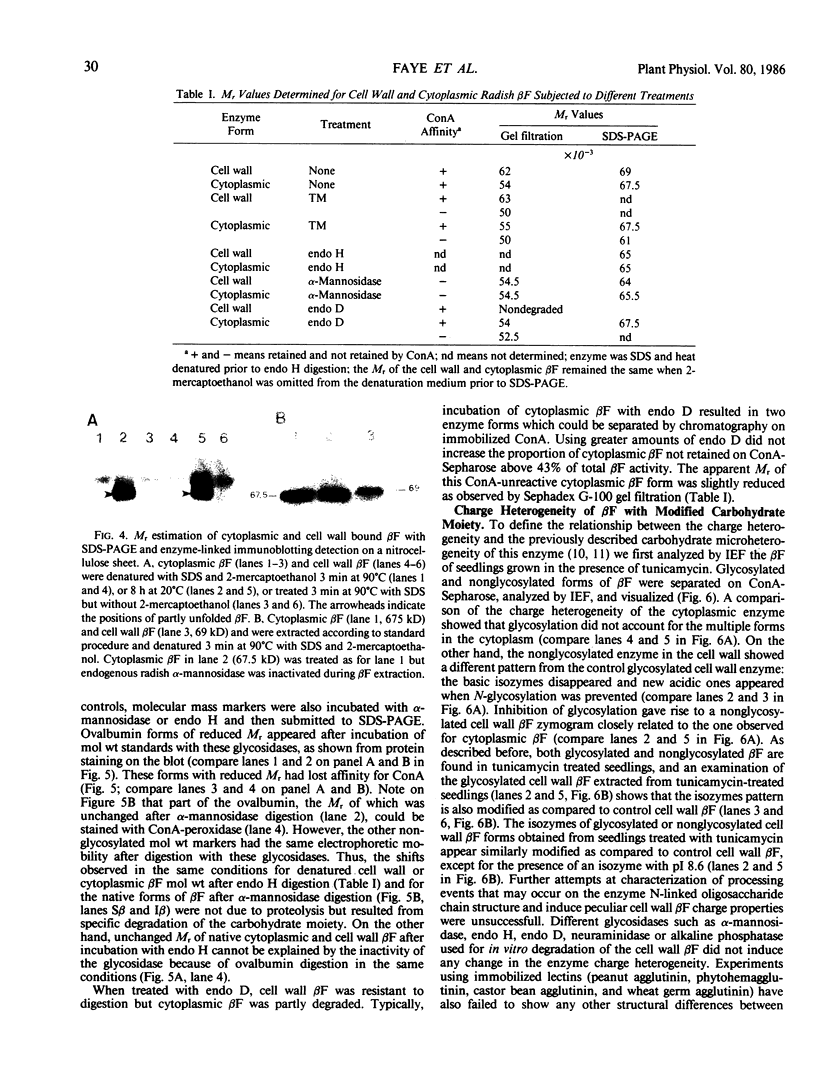
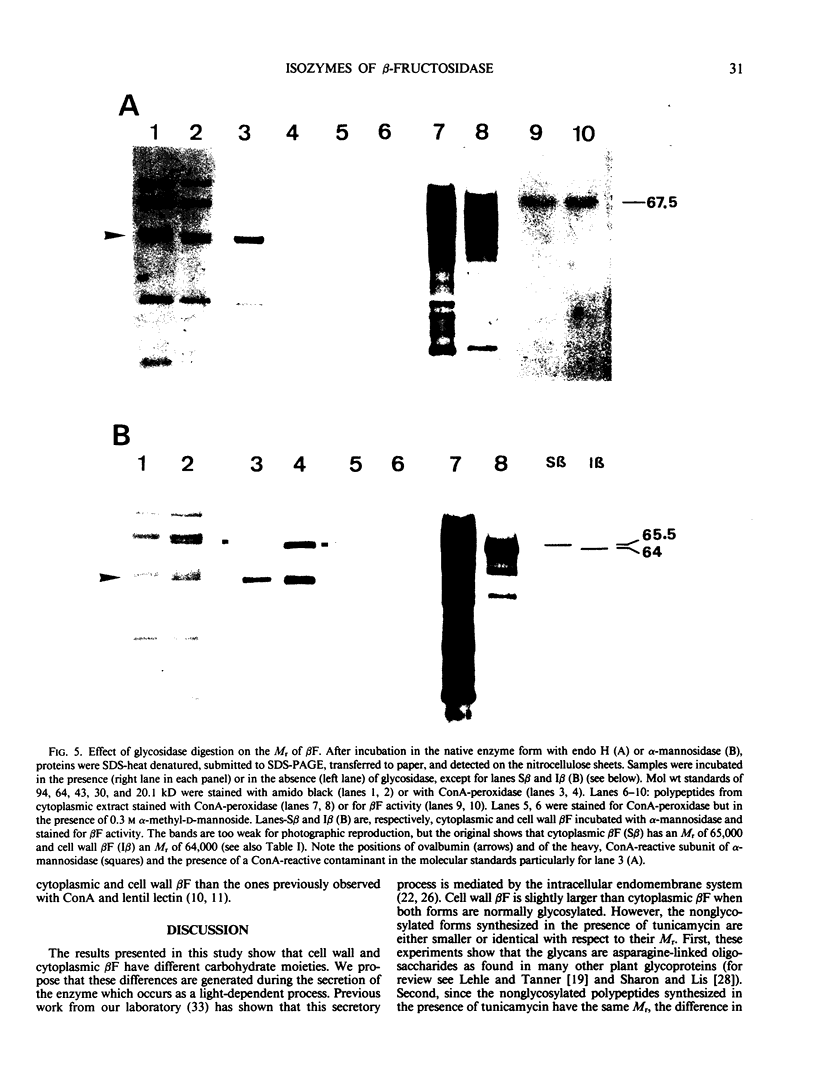
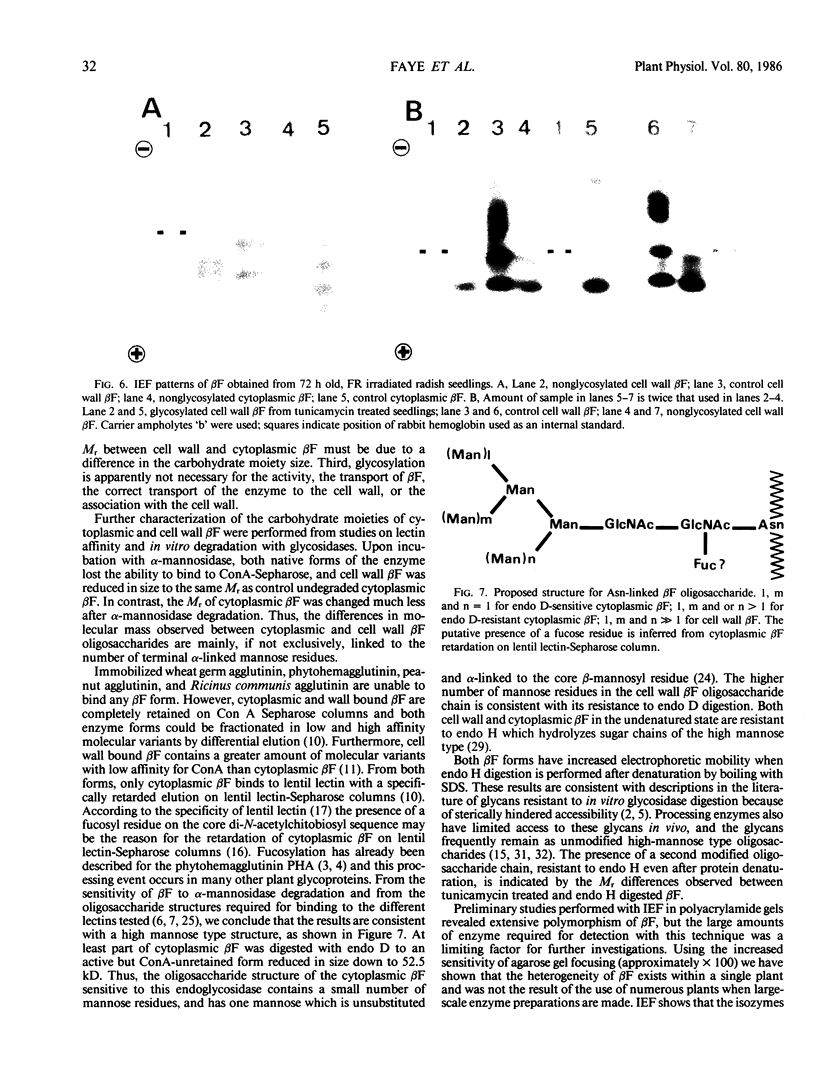
When 36-hour-old dark grown radish seedlings are transferred to far-red light, there is a decrease in cytoplasmic β-fructosidase (βF) and an increase in cell wall βF compared to the dark controls. Cytoplasmic and cell wall-bound β-fructosidase are both glycoproteins and exhibit high antigenic similarities, but differ according to charge heterogeneity and carbohydrate microheterogeneity. Growth of radish seedlings in the presence of tunicamycin results in a partial inhibition of βF glycosylation but nonglycosylated βF still accumulates in the cell wall under far-red light. Thus, glycosylation is not necessary for intracellular transport, for correct targetting, or for wall association of an active βF. The nonglycosylated cytoplasmic and cell wall βF forms have the same relative molecular mass but glycosylated forms have different oligosaccharide side-chains, with respect to size and susceptibility to α-mannosidase and endoglycosidase D digestion. The oligosaccharides of both forms are partly removed by endoglycosidase H when βF is denatured. Isoelectric focusing analysis of βF shows that the cell wall-associated isozymes are more basic than the cytoplasmic isozymes, and that the charge heterogeneity also exists within a single plant. A time course of changes in βF zymograms shows a far red light stimulation of the appearance of the basic forms of the enzyme. However, the more basic cell wall specific βF forms are not present when N-glycosylation is prevented with tunicamycin. These results indicate that cytoplasmic and cell wall βF probably have common precursor polypeptides and basic cell wall forms arise via processing events which are tunicamycin sensitive.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björk I., Petersson B. A., Sjöquist J. Some physiochemical properties of protein A from Staphylococcus aureus. Eur J Biochem. 1972 Sep 25;29(3):579–584. doi: 10.1111/j.1432-1033.1972.tb02024.x. [DOI] [PubMed] [Google Scholar]
- Bowles D. J., Chaplin M. F., Marcus S. E. Interaction of concanavalin A with native and denatured forms of jackbean alpha-D-mannosidase. Eur J Biochem. 1983 Feb 15;130(3):613–618. doi: 10.1111/j.1432-1033.1983.tb07193.x. [DOI] [PubMed] [Google Scholar]
- Chu F. K., Maley F., Tarentino A. L. The use of iodinated lectins for determining the degree of deglycosylation of high-mannose glycoproteins by endo-beta-N-acetylglucosaminidase H. Anal Biochem. 1981 Sep 1;116(1):152–160. doi: 10.1016/0003-2697(81)90338-9. [DOI] [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S. Fractionation of asparagine-linked oligosaccharides by serial lectin-Agarose affinity chromatography. A rapid, sensitive, and specific technique. J Biol Chem. 1982 Oct 10;257(19):11235–11240. [PubMed] [Google Scholar]
- Faye L. A new enzymatic staining method for the detection of radish beta-fructosidase in gel electrophoresis. Anal Biochem. 1981 Mar 15;112(1):90–95. doi: 10.1016/0003-2697(81)90264-5. [DOI] [PubMed] [Google Scholar]
- Faye L., Berjonneau C., Ghorbel A. Electrophoretic and immunochemical characterization of radish beta-fructosidase microheterogeneity. Arch Biochem Biophys. 1982 Jan;213(1):45–49. doi: 10.1016/0003-9861(82)90437-4. [DOI] [PubMed] [Google Scholar]
- Hori H., Elbein A. D. Tunicamycin inhibits protein glycosylation in suspension cultured soybean cells. Plant Physiol. 1981 May;67(5):882–886. doi: 10.1104/pp.67.5.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh P., Rosner M. R., Robbins P. W. Selective cleavage by endo-beta-N-acetylglucosaminidase H at individual glycosylation sites of Sindbis virion envelope glycoproteins. J Biol Chem. 1983 Feb 25;258(4):2555–2561. [PubMed] [Google Scholar]
- Hughes R. C., Mills G. Analysis by lectin affinity chromatography of N-linked glycans of BHK cells and ricin-resistant mutants. Biochem J. 1983 Jun 1;211(3):575–587. doi: 10.1042/bj2110575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld K., Reitman M. L., Kornfeld R. The carbohydrate-binding specificity of pea and lentil lectins. Fucose is an important determinant. J Biol Chem. 1981 Jul 10;256(13):6633–6640. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehle L., Tanner W. Polyprenol-linked sugars and glycoprotein synthesis in plants. Biochem Soc Trans. 1983 Oct;11(5):568–574. doi: 10.1042/bst0110568. [DOI] [PubMed] [Google Scholar]
- Miyata S., Akazawa T. Enzymic mechanism of starch breakdown in germinating rice seeds : 12. Biosynthesis of alpha-amylase in relation to protein glycosylation. Plant Physiol. 1982 Jul;70(1):147–153. doi: 10.1104/pp.70.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muilerman H. G., ter Hart H. G., Van Dijk W. Specific detection of inactive enzyme protein after polyacrylamide gel electrophoresis by a new enzyme-immunoassay method using unspecific antiserum and partially purified active enzyme: application to rat liver phosphodiesterase I. Anal Biochem. 1982 Feb;120(1):46–51. doi: 10.1016/0003-2697(82)90315-3. [DOI] [PubMed] [Google Scholar]
- Muramatsu T. endo-beta-N-Acetylglucosaminidase D from Diplococcus pneumoniae. Methods Enzymol. 1978;50:555–559. doi: 10.1016/0076-6879(78)50062-1. [DOI] [PubMed] [Google Scholar]
- Narasimhan S., Wilson J. R., Martin E., Schachter H. A structural basis for four distinct elution profiles on concanavalin A--Sepharose affinity chromatography of glycopeptides. Can J Biochem. 1979 Jan;57(1):83–96. doi: 10.1139/o79-011. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Schwaiger H., Tanner W. Effects of gibberellic acid and of tunicamycin on glycosyl-transferase activities and on alpha-amylase secretion in barley. Eur J Biochem. 1979 Dec 17;102(2):375–381. doi: 10.1111/j.1432-1033.1979.tb04252.x. [DOI] [PubMed] [Google Scholar]
- Sharon N., Lis H. Comparative biochemistry of plant glycoproteins. Biochem Soc Trans. 1979 Aug;7(4):783–799. doi: 10.1042/bst0070783. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Trimble R. B., Maley F. endo-beta-N-Acetylglucosaminidase from Streptomyces plicatus. Methods Enzymol. 1978;50:574–580. doi: 10.1016/0076-6879(78)50065-7. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble R. B., Maley F., Chu F. K. GlycoProtein biosynthesis in yeast. protein conformation affects processing of high mannose oligosaccharides on carboxypeptidase Y and invertase. J Biol Chem. 1983 Feb 25;258(4):2562–2567. [PubMed] [Google Scholar]
- Williams D. B., Lennarz W. J. Control of asparagine-linked oligosaccharide chain processing: studies on bovine pancreatic ribonuclease B. An in vitro system for the processing of exogenous glycoproteins. J Biol Chem. 1984 Apr 25;259(8):5105–5114. [PubMed] [Google Scholar]