Abstract
Purpose of review:
Lung ultrasound is a noninvasive bedside technique that can accurately assess pulmonary congestion by evaluating extravascular lung water. This technique is expanding and is easily available. Our primary outcome was to compare the efficacy of volume status assessment by lung ultrasound with clinical evaluation, echocardiography, bioimpedance, or biomarkers. The secondary outcomes were all-cause mortality and cardiovascular events.
Sources of information:
We conducted a MEDLINE literature search for observational and randomized studies with lung ultrasound in patients on maintenance dialysis.
Methods:
From a total of 2363 articles, we included 28 studies (25 observational and 3 randomized). The correlation coefficients were pooled for each variable of interest using the generic inverse variance method with a random effects model. Among the clinical parameters, New York Heart Association Functional Classification of Heart Failure status and lung auscultation showed the highest correlation with the number of B-lines on ultrasound, with a pooled r correlation coefficient of .57 and .36, respectively. Among echocardiographic parameters, left ventricular ejection fraction and inferior vena cava index had the strongest correlation with the number of B-lines, with a pooled r coefficient of .35 and .31, respectively. Three randomized studies compared a lung ultrasound-guided approach with standard of care on hard clinical endpoints. Although patients in the lung ultrasound group achieved better decongestion and blood pressure control, there was no difference between the 2 management strategies with respect to death from any cause or major adverse cardiovascular events.
Key findings:
Lung ultrasound may be considered for the identification of patients with subclinical volume overload. Trials did not show differences in clinically important outcomes. The number of studies was small and many were of suboptimal quality.
Limitations:
The included studies were heterogeneous and of relatively limited quality.
Keywords: lung ultrasound, chronic kidney disease, volume assessment, dialysis, mortality, major adverse cardiovascular events
Abrégé
Motif de la revue:
L’échographie pulmonaire est une technique non-invasive réalisée au chevet du patient qui permet d’évaluer avec précision la congestion pulmonaire en mesurant l’eau pulmonaire extravasculaire. Cette technique facilement accessible est de plus en plus utilisée. Notre principal critère de jugement était de comparer l’efficacité de l’évaluation de la volémie par échographie pulmonaire avec l’évaluation clinique, l’échocardiographie, la bio-impédance ou les biomarqueurs. Les critères d’évaluation secondaires étaient la mortalité toutes causes confondues et les événements cardiovasculaires.
Sources:
Nous avons recherché sur MEDLINE les études observationnelles et les essais randomisés où une échographie pulmonaire avait été réalisée chez des patients sous dialyse d’entretien.
Méthodologie:
Sur un total de 2 363 articles, nous avons retenu 28 études (25 observationnelles et 3 randomisées). Les coefficients de corrélation ont été regroupés pour chaque variable d’intérêt en utilisant la méthode générique de variance inverse avec un modèle à effets aléatoires. Les paramètres cliniques qui avaient montré les corrélations les plus élevées avec le nombre de lignes B à l’échographie étaient le statut de l’insuffisance cardiaque selon la classification de la New York Heart Association et l’auscultation pulmonaire, avec des coefficients de corrélation r regroupés respectifs de 0,57 et de 0,36. Les paramètres de l’échocardiographie qui avaient montré les plus fortes corrélations avec le nombre de lignes B étaient la fraction d’éjection du ventricule gauche et l’indice de la veine cave inférieure, avec des coefficients r regroupés respectifs de 0,35 et de 0,31. Trois essais randomisés avaient comparé une approche guidée par échographie pulmonaire aux normes de soins selon des critères cliniques stricts. Bien que les patients du groupe avec échographie pulmonaire aient montré une décongestion plus efficace et un meilleur contrôle de la pression artérielle, aucune différence n’a été observée entre les deux stratégies de prise en charge en ce qui concerne les décès de toutes causes confondues ou les événements cardiovasculaires indésirables majeurs.
Principales observations:
L’échographie pulmonaire pourrait être envisagée pour identifier les patients qui présentent une surcharge volumique subclinique. Les essais inclus n’ont pas montré de différences dans les résultats cliniquement pertinents. Le nombre d’études incluses était faible et plusieurs étaient de qualité sous-optimale.
Limites:
Les études incluses étaient hétérogènes et de qualité relativement limitée.
Introduction
Chronic kidney disease (CKD) is a common condition and is often associated with pulmonary congestion.1,2 Pulmonary congestion in advanced CKD is associated with higher cardiovascular morbidity and mortality, compared with patients with CKD and optimal volume status.3,4 Therefore, volume status management is an important part of the standard of care in these patients.
Assessment of volume status can be challenging, and clinical assessment is often imprecise.5,6 Therefore, different adjunctive diagnostic tools are used in clinical practice, such as bioelectrical impedance, chest radiography, weight monitoring, and blood biomarkers.7 -10 More recently, lung ultrasound has been proposed for the assessment of extravascular lung water and therefore reflects lung congestion. 11
Since the 1990s, there has been growing interest in lung ultrasound. First, it was used for critically ill patients in intensive care units and it has now expanded to most fields in modern medicine. This technique is simple, reproducible, radiation free, and can be easily performed at the bedside.12,13 The presence and number of B-lines artifacts is considered a surrogate for alveolar interstitial syndrome, as first described by Lichtenstein et al. 14 It is now a validated tool for the estimation of volume overload in patients with heart failure.15,16 The presence of a B-line pattern and pleural effusions visualized by lung ultrasound is highly suggestive of volume overload.
In the nephrology literature, many articles have been published on the prognostic value of lung ultrasound in patients undergoing hemodialysis.17,18 The recent publication of the LUST study provided interesting data on the added value of lung ultrasound in patients with CKD. 19 An excellent meta-analysis published in 2019 reported the technological adjuncts for volume status management and the effect on mortality. The primary outcome presented was mortality and numerous tools were assessed. However, there was no comparison between the various techniques. 20 Therefore, we conducted this systematic review including the most recent data to present current evidence on use of lung ultrasound in this setting. We are comparing the efficacy of lung ultrasound to clinical evaluation and other standard techniques, such as cardiac ultrasound, blood biomarkers, and bioimpedance, commonly used to assess fluid status in this group of patients. We will also assess the impact of timely diagnosis of volume overload by lung ultrasound on cardiovascular events and mortality.
Materials and Methods
Search Strategy
We conducted a MEDLINE literature research in PubMed for relevant literature through January 2023. We searched for published clinical trials in English or French language, including patients of at least 18 years of age. The key words used for literature research in PubMed were (pulmonary ultrasound) OR (lung ultrasound)) AND ((dialysis) OR (end-stage kidney disease)) OR (kidney failure) OR (chronic kidney disease)).
Available meta-analyses were also reviewed. We verified the reference list of retrieved articles and had notifications set from PubMed for new publications. The protocol was registered in the PROSPERO registry in July 2020 (CRD42020197765). The results are reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (Supplementary File 1).
Eligibility Criteria
All the following criteria should apply: (1) study population: adult patients with advanced CKD defined as an estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2, including patients with end-stage kidney disease (ESKD) undergoing maintenance dialysis (hemodialysis and peritoneal dialysis); (2) intervention: use of lung ultrasound to assess volume status and/or guide volume management; (3) study design: randomized controlled trials (RCTs) or observational (cohort) studies, published in the form of an article or abstract; (4) at least, one of the relevant outcomes should be reported: (a) mortality, (b) correlation with volume status assessment by other methods, such as clinical evaluation, bioimpedance, biomarkers, or echocardiography, and (c) admission for heart failure—volume overload.
Study Selection and Quality Assessment
Two authors (AK and FT) independently performed study selection and extracted relevant information from the included trials. Discrepancies between author assessments were resolved by mutual discussion of each item in question. In case of disagreement, this was discussed in a conference with the senior author (TM). To assess the quality of included studies, the second version of the Cochrane risk-of-bias tool for RCTs (RoB2) was used for randomized control trials, the Newcastle-Ottawa scale for cross-sectional studies, and the Robins-I tool for all other observational studies (shown in Supplementary Files 2 and 3).21 -23
Outcomes
Our primary outcome was to compare the efficacy of volume status assessment by lung ultrasound with clinical evaluation, echocardiography, and paraclinical parameters (bioimpedance and biomarkers).
For echocardiography, left ventricular ejection fraction (LVEF), left ventricular mass index (LVMI), the E/é ratio measured by tissue Doppler imaging, the inferior vena cava (IVC) index, and the right ventricular systolic pressure were used. These parameters are validated measures to estimate volume status. 15 To reduce heterogeneity and better reflect clinical significance, only echocardiography examinations performed before the beginning of the dialysis session were used.
The secondary outcomes were all-cause or cardiac mortality and heart failure admissions with lung ultrasound-guided or standard techniques.
Statistical Analysis
Results are reported according to the PRISMA 2009 checklist (Supplementary File 1). 24 The correlation coefficients were pooled for each variable of interest after having been transformed to z values. 25 When both the Pearson and Spearman coefficients were available, Pearson coefficients were used. When both the predialysis and postdialysis session coefficients were available, the predialysis (prior to the beginning of the dialysis session) coefficients were used. Pooled z values were calculated using the generic inverse variance method with a random effects model. The I 2 index was used to quantify heterogeneity and assess inconsistency. Pooled z values and 95% confidence intervals were then back transformed to r values and respective 95% confidence intervals. For the null hypothesis of no correlation, the t distribution was used with n − 2 degrees of freedom. 25 Statistical analyses were performed in Stata (version 14 IC; College Station, Texas).
Results
Study Selection
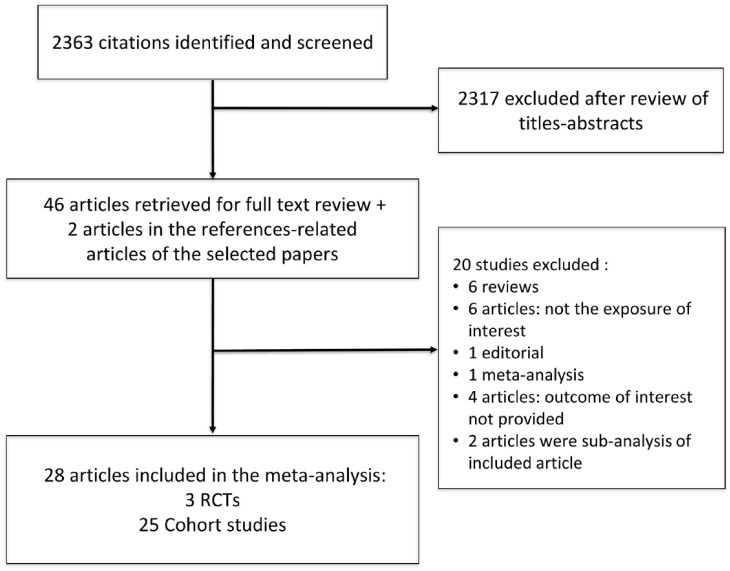
We retrieved 2363 articles on lung ultrasound in CKD in our primary search. A total of 2317 articles were excluded after title and abstract review. We also identified 2 articles in the reference list of manuscripts selected for full-text review, while 2 articles were retrieved from publication alert e-mails from PubMed received after our initial search. From the 46 articles selected for full-text review, we excluded another 20 articles: 5 review articles (all studies mentioned in these manuscripts had already been included in our review), 1 editorial, 1 meta-analysis, 6 articles that did not study the exposure of interest, 4 articles that did not present the outcome(s) of interest, 2 articles were subanalyses of an included randomized controlled study. A total of 28 studies were included in our systematic review: 25 observational studies,4,26 -49 and 3 RCTs as shown in Figure 1.18,19,50 The randomized controlled studies did not address the same endpoints as the observational studies and are separately presented in a different section of the results. A meta-analysis of randomized controlled studies was not performed because of the small number of trials, the different endpoints, and the distinct populations enrolled.
Figure 1.
Study flowchart.
Note. RCT = randomized controlled trial.
Even though the study by Torino et al 41 is an RCT, only the results of the intervention arm are reported in our analysis, and this is why it was considered an observational study in this review. All studies enrolled patients on maintenance dialysis (5 of them enrolled patients on peritoneal dialysis). There were no relevant studies in patients with advanced renal disease not on maintenance dialysis.35,37 Baseline characteristics of the included studies are shown in Table 1.
Table 1.
Study Characteristics.
| Article number of patients, n | Study design | Country | Age | Male sex, % | Modality | Co-morbidities | LUS method | Comparison arm | Quality assessment | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHF | CAD | HTN | DM2 | |||||||||
| Annamalai et al
26
(n = 50) |
Cross-sectional study | India | 37 ± 11 | 74 | HD | N/A | N/A | N/A | 14% | 28 points Sum of B-lines (B-lines score or comet score) |
Edema Dyspnea |
3/6 NewOtt |
| Arun Thomas et al
27
(n = 79) |
Prospective cohort study | India single center | 54 ± 12 | 81 | HD | N/A | N/A | N/A | 45% | 8 zones Sum of B-lines |
IVC | See supplement |
| Basso et al
28
(n = 30) |
Cross-sectional Study | Italy | 64 ± 16 | 70 | HD | N/A | 74% | 77% | 16% | 28 points Sum B-lines |
BIS IVC |
3/5 NewOtt |
| Beaubien-Souligny et al
29
(n = 47) |
Prospective cohort study | Canada Unicentric MTL |
67 ± 14 | 64 | HD | N/A | N/A | 91% | 53% | 28 regions B-lines YES/NO 0/28 points |
Dry weight assessment | See supplement |
| Bobot et al
43
(n = 31) |
Prospective study | France | 63 ± 13 | 71 | HD | 19% | 41% | 84% | 35% | 28 points Sum B-lines |
Clinical score (NYHA, orthopnea, crackles, peripheral edema, jugular turgor, hepatic-jugular reflex, pre-HD BP) TTE (IVC, sPAP, E/é, E/A) |
See supplement |
| Donadio et al
30
(n = 31) |
Prospective study | Pisa Italy | 68 ± 11 | 72 | HD | N/A | 32% | 87% | 45% | 57 points Sum of B-lines |
BIA total and thoracic (TBW, ECW, ECWI, TBWI) |
See supplement |
| Fornazarič et al
44
(n = 16) |
Prospective observational study | Slovenia | 53 ± 18 | 56 | PD | N/A | N/A | N/A | N/A | 28 points Sum of B-lines |
IVC BIS NT-proBNP |
See supplement |
| Giannese et al
45
(n = 24) |
Prospective cohort study | Italy | 60 ± 18 | 63 | HD | N/A | N/A | 71% | 25% | 28 points Sum of B-lines |
BIA (TBW, ECW, ICW, AFO, RFO) BNP |
See supplement |
| Jiang et al
31
(n = 20) |
Prospective study | NY, USA | 66 ± 14 | 55 | HD | N/A | N/A | 35% | 35% | 28 points Sum of B-lines |
Dry weight | See supplement |
| Kawachi et al
32
(n = 61) |
Prospective observational study | Japan | 75 ± 11 | 52 | HD | N/A | N/A | 87% | 46% | 8 areas | IVC TTE (LVEF) |
See supplement |
| Loutradis et al
51
(n = 71) |
RCT (Sub-LUST) |
Greece and Slovenia | 63 ± 14 | 66 | HD | 24% | 38% | 100% | 27% | 28 points Total B-lines from 0 to 280 |
BP | See supplement |
| Mallamaci et al
33
(n = 75) |
Cross-sectional | Italy | 63 ± 23 | 65 | HD | N/A | 43% | 56% | N/A | Lung comet and Lung comet score 28 points |
NYHA TTE (E/é, LVEF, PAPs, LVMI) |
3/5 NewOtt |
| Mohammad et al
47
(n = 38) |
Cross-sectional | Saudi Arabia | 46 ± 14 | 88 | HD | N/A | N/A | 47% | 21% | 28 points Total B-lines |
Clinical score (BP, RR, orthopnea, JVP, crackles, peripheral edema, ascites, pleural effusion, NYHA) IVC |
3/5 NewOtt |
| Ngoh et al
34
(n = 50) |
Cross-sectional | Singapore | 59 ±14 | 47 | HD | N/A | 30% | 85% | 73% | 28 points Total B-lines |
BIS (TBW, ECW, ICW, ΔHS) | 4/6 NewOtt |
| Pannucio et al
35
(n = 88) |
Cross-sectional study | Italy Multicenter |
61 ± 17 | 50 | PD | N/A | 41% | N/A | 23% | Lung comet score | BIA TTE |
4/5 NewOtt |
| Pardała et al
36
(n = 54) |
Cross-sectional study | Poland | 58 | 59 | HD | N/A | 57% | 93% | 28% | 28 points B-lines 0-10 per scanning zone Sum of those B-lines |
BIA (RFO) TTE (RVSP/LVEF, LVMI, RAVI/LAVI) IVC min/max |
3/5 NewOtt |
| Paudel et al
37
(n = 27) |
Cross sectional | UK | 62 ± 3 | 63 | PD | N/A | 33% | N/A | 33% | 28 points Sum of B-lines (or ULC) |
BIS | 3/4 NewOtt |
| Saad et al
38
(n = 81) |
Cross-sectional study | Staten Island USA |
60 ± 16 | 72 | HD | N/A | N/A | 94% | 52% | 28 points Comet tail score (=Sum B-lines) |
TTE (E/é, LVEF) | 6/6 NewOtt |
| Santos et al
39
(n = 73) |
Cross-sectional | Brazil (2 centers) | 61 ± 16 | 63 | HD | N/A | N/A | N/A | 100% | 28 points B-lines number |
BIA (ECW, FO, RFO [FO/ECW x100]) | 5/5 NewOtt |
| Sevinc et al
48
(n = 21) |
Cross-sectional | Turkey | 48 ± 10 | 19 | PD | N/A | 19% | 10% | 10% | 28 points Sum B-lines |
Clinical (orthopnea, PND, NYHA, crackles, S3, peripheral edema) CXR TTE (LVEDV, LVMI, LVEF, sPAP, E/é) BIA |
3/5 NewOtt |
| Siriopol et al
40
(n = 96) |
Prospective observational Study | Romania | 59 ± 14 | 51 | HD | N/A | N/A | N/A | 24% | Lung comet count 28 points |
BIS (TBW, ECW, ICW, ΔHS) | See supplement |
| Siriopol et al
18
(n = 250) |
RCT | Romania | 59 ± 14 | 46 | HD | N/A | 16% | 76% | 19% | 28 points Sum B-lines (called BLS for B-lines score) |
BIS (AFO, RFO, TBW, ECW, ICW) | See supplement |
| Lučič Šrajer et al
46
(n = 19) |
Cross-sectional | Slovenia | 54 ± 24 | 63 | PD | N/A | N/A | N/A | N/A | 28 points Sum B-lines |
Crackles Peripheral edema NT-proBNP |
4/5 NewOtt |
| Torino et al
41
(n = 79) |
Prospective study | Italy | 72 | 65 | HD | N/A | 100% | N/A | 37% | 28 points 4 groups |
See supplement | |
| Trirattanapikul et al
49
(n = 20) |
Prospective cohort study | Thailand | 62 ± 14 | 70 | HD | N/A | N/A | N/A | 45% | 28 points Sum B-lines |
BIS (TBW, ECW, ICW, AFO, RFO) Dry weight |
See supplement |
| Weitzel et al
42
(n = 20) |
Cross-sectional | USA Michigan |
53 ± 14 | 85 | HD | 5% | 20% | 95% | 55% | Comet count | — | 3/5 NewOtt |
| Zoccali et al
4
(n = 392) |
Cohort study multicenter | Italy | 65 ± 15 | 63 | HD | N/A | 55% | 56% | 29% | 28 points | — | See supplement |
| Zoccali et al
19
(n = 363) |
RCT | Multicentric | 70 ± 11 | 70 | HD | 43% | 72% | 76% | 41% | Lung comet score | TTE (LVMI, LVEF, E/é) | See supplement |
Note. Age is reported as mean ± standard deviation. LUS = lung ultrasound; CHF = congestive heart failure; CAD = coronary artery disease; HTN = hypertension; DM2 = diabetes mellitus type 2; HD = hemodialysis; N/A = not available; NewOtt = Newcastle and Ottawa score; IVC = inferior vena cava; BIS = bioimpedance spectroscopy; NYHA = New York Heart Association; BP = blood pressure; TTE = transthoracic echocardiography; PAPs = pulmonary arterial pressures; E/é = early filling to early diastolic mitral annular velocity; BIA = bioelectrical impedance analysis; TBW = total body water; ECW = extracellular water; ECWI = extracellular water index; RCT = randomized-controlled trial; TBWI = total body water index; PD = peritoneal dialysis; ICW = intracellular water; AFO = absolute fluid overload; RFO = relative fluid overload; BNP = brain natriuretic peptide; LVEF = left ventricular ejection fraction; LVMI = left ventricular mass index; ΔHS = hydration status; RVS = right ventricular systolic pressure; RAVI = right atrial volume indexed for BSA; LAVI = left atrial volume indexed for BSA; ULC = ultrasound lung comets; FO = fluid overload; LVEDV = left ventricular end diastolic volume; MTL = Montreal; RR = Respiratory rate; JVP = Jugular venous pressure; PND = Paroxystic nocturnal dyspnea; CXR = Chest xray.
Correlation Between Lung Ultrasound and Clinical Parameters
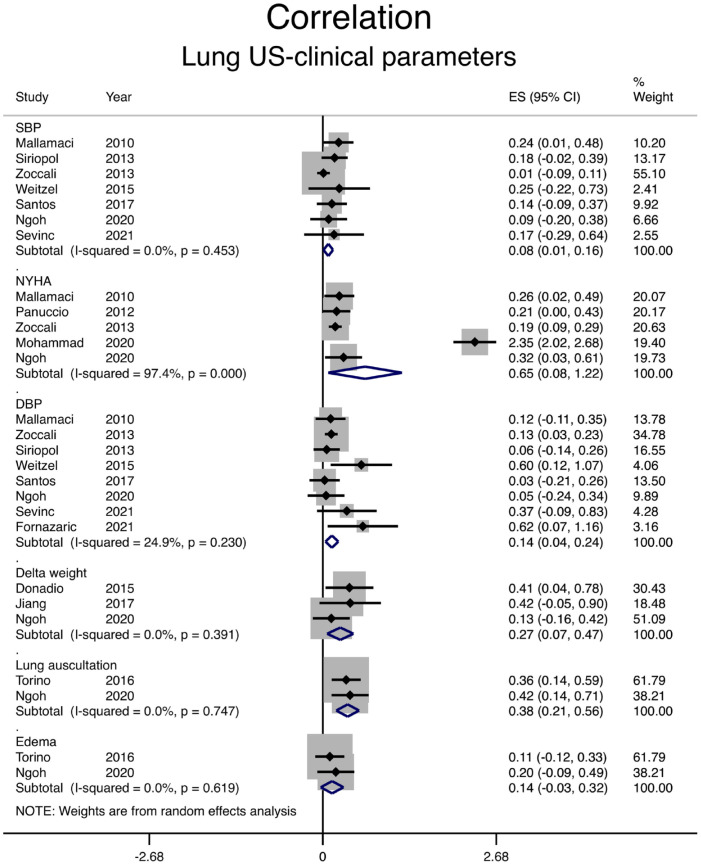
Correlation between the number of B-lines on lung ultrasound and different clinical parameters is shown in Figure 2 and Table 2. Systolic and diastolic blood pressure (BP) and peripheral edema poorly correlate with the number of B-lines, with a pooled correlation coefficient of .08, .14, and .14, respectively. The correlation between lung ultrasound and dyspnea assessment (New York Heart Association class) was relatively high with a correlation coefficient of .57, but mainly due to the results in one article. 47 This article had a Newcastle Ottawa quality assessment scale of 3/5. The correlation between B-lines and lung auscultation was moderate with a pooled correlation coefficient of .36. In addition, when the reduction in the number of B-lines and the weight change during dialysis were examined, correlation was moderate with a pooled r of .26 as shown in Table 2 and Figure 2. Heterogeneity was very low for all clinical parameters except for dyspnea assessment for which it was very high.
Figure 2.
Correlation between the number of B-lines on lung ultrasound and clinical parameters.
Note. SBP = systolic blood pressure; CI = confidence interval; NYHA = New York Heart Association; DBP = diastolic blood pressure; ES = Effect size.
Table 2.
Correlation of Lung Ultrasound Findings With Clinical and Paraclinical Measures.
| Outcome | Pooled r | 95% CI for r | P value |
|---|---|---|---|
| SBP-LUS | .08 | 0.01-0.16 | .453 |
| DBP-LUS | .14 | 0.04-0.24 | .230 |
| NYHA-LUS | .65 | 0.08-1.22 | < .001 |
| Delta weight-LUS | .27 | 0.07-0.47 | .391 |
| Edema-LUS | .14 | 0.03-0.32 | .619 |
| Lung auscultation-LUS | .38 | 0.21-0.56 | .747 |
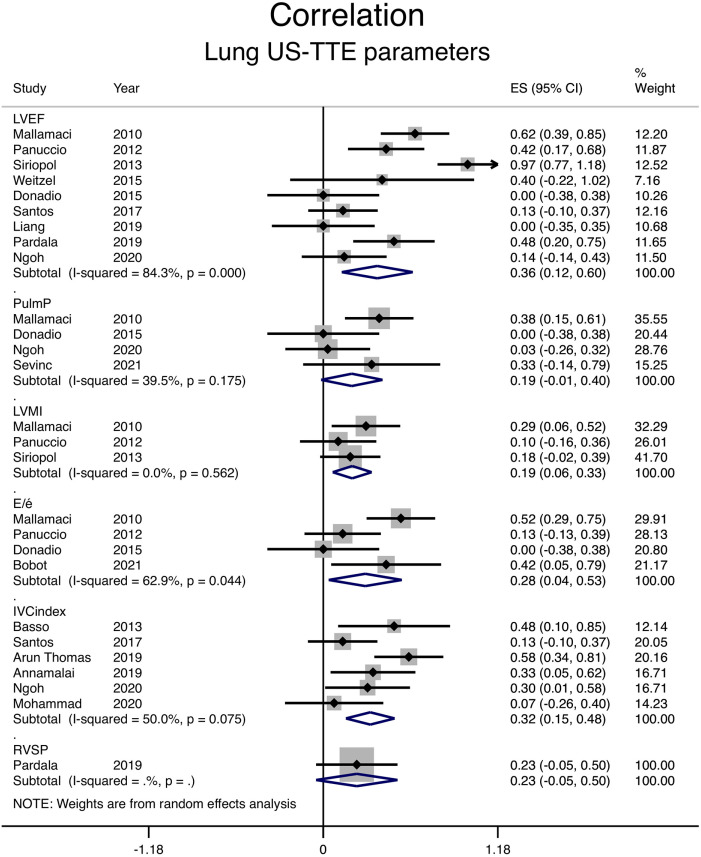
| E/é-LUS | .28 | 0.04-0.53 | .044 |
| LVEF-LUS | .36 | 0.12-0.60 | < .001 |
| PulmP-LUS | .19 | 0.01-0.40 | .175 |
| LVMI-LUS | .19 | 0.06-0.33 | .562 |
| IVC Index-LUS | .32 | 0.15-0.48 | .075 |
| RVSP-LUS | .23 | 0.05-0.50 | .1 |
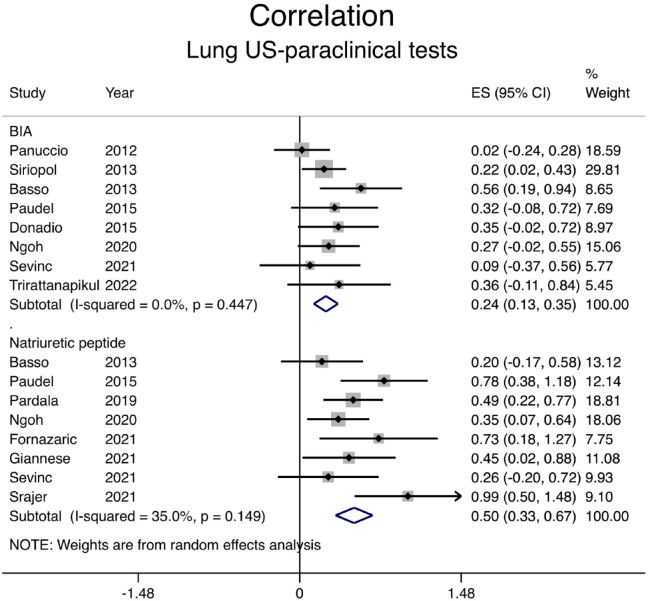
| BIA-LUS | .24 | 0.13-0.35 | .447 |
| BNP/pro-BNP-LUS | .50 | 0.33-0.67 | .149 |
Note. CI = confidence interval; SBP = systolic blood pressure; LUS = lung ultrasound; DBP = diastolic blood pressure; NYHA = New York Heart Association; E/é = early filling to early diastolic mitral annular velocity; LVEF = left ventricular ejection fraction; PulmP = pulmonary pressure; LVMI = left ventricular mass index; IVC = inferior vena cava; RVSP = right ventricular systolic pressure; BIA = bioelectrical impedance analysis; BNP = brain natriuretic peptide.
Correlation Between Lung Ultrasound and Echocardiographic Measurements
Among echocardiographic parameters, LVEF and IVC index had the strongest correlation with the number of B-lines, with a pooled r coefficient of 0.35 and 0.31, respectively (shown in Figure 3 and Table 2). Correlation with diastolic dysfunction, as assessed by the E/é ratio, was weaker with a pooled r coefficient of 0.27. For LVMI and pulmonary artery pressure, both correlation coefficients were 0.19 (shown in Figure 3 and Table 2). Right ventricular systolic pressure correlation with B-lines was reported by a single study with a coefficient of .23. 36
Figure 3.
Correlation between the number of B-lines on lung ultrasound and different echocardiographic measurements.
Note. CI = confidence interval; LVEF = left ventricular ejection fraction; PulmP = pulmonary pressure; LVMI = left ventricular mass index; E/é = early filling to early diastolic mitral annular velocity; IVC = inferior vena cava; RVSP = right ventricular systolic pressure; ES = Effect size.
Correlation Between Lung Ultrasound and Paraclinical Examinations
Paraclinical examinations, such as bioimpedance techniques or natriuretic peptide levels, are commonly used to estimate volume status in patients with CKD. Eight studies compared bioimpedance techniques with lung ultrasound: correlation was weakly positive with a pooled correlation coefficient of .24 (shown in Figure 4 and Table 2). The correlation between natriuretic peptide levels (brain natriuretic peptide [BNP] or N-terminal pro-BNP) and sum of B-lines on ultrasound was stronger with a pooled correlation coefficient of .46 (shown in Figure 4 and Table 2).
Figure 4.
Correlation between the number of B-lines on lung ultrasound and paraclinical tests.
Note. CI = confidence interval; BIA = bioelectrical impedance analysis; ES = Effect size.
Clinical Outcomes (Observational Studies)
Data on clinical outcomes could not be pooled due to the very small number of events and/or the different methodologies the studies used in assessing volume status by lung ultrasound.
In a multicenter prospective study, Zoccali et al 4 classified 392 patients on maintenance hemodialysis into 3 groups using the number of B-lines on lung ultrasound.
Patients with >60 B-lines on ultrasound had higher incidence of all-cause mortality and fatal or nonfatal cardiac events, compared with patients with <15 B-lines on ultrasound.
Siriopol et al 40 prospectively followed 92 patients on maintenance hemodialysis for a median of 406 days. Patients were classified into 3 groups (<16, 16-30, or >30 lung comets) using lung ultrasound prior to the initiation of dialysis. In an adjusted Cox regression model, the hazard ratio for death from any cause was higher in patients with severe lung congestion (>30 comets on ultrasound) compared with the other 2 groups.
Saad et al prospectively followed 81 patients on maintenance dialysis who had been stratified into 3 groups using lung ultrasound (mild, moderate, or severe volume overload). Patients in the moderate or severe volume overload group had a higher incidence of death from any cause or major adverse cardiovascular events. 38
In a prospective study by Beaubien-Souligny et al, 47 patients who were on hemodialysis for at least 3 months underwent lung ultrasound before and after 2 separate dialysis sessions to assess extravascular lung water. The authors used a simplified score (relative B-line score) to assess hydration status. 29 Patients on the highest quartile of the postdialysis relative B-line score had a higher incidence of hospitalization for acute pulmonary edema or acute coronary syndrome, compared with patients on the second or third quartile.
Finally, Kawachi et al 32 studied the association between lung congestion and mortality in patients undergoing maintenance hemodialysis. One-year survival was higher in patients with less pulmonary congestion: 55.4% versus 89.8% in the group of patients with >5 B-lines and <5 B-lines, respectively.
Randomized Studies
Siriopol et al compared the effect of combining lung ultrasound and bioimpedance monitoring for dry weight assessment with standard of care on a composite outcome of death from any cause or cardiovascular events (including cardiovascular death, myocardial infarction, or stroke) in patients on maintenance hemodialysis with low cardiovascular risk. The study enrolled 250 participants. 18 There was no significant difference between the 2 treatment strategies in this study.
Loutradis et al 50 compared lung ultrasound with standard of care for adjusting the dry weight in a randomized study including 71 patients on maintenance hemodialysis who were hypertensive and considered to be euvolemic. Ultrafiltration was intensified in a higher percentage of patients who had lung ultrasound (54%), compared with patients in the usual care group (14%). The lung ultrasound-guided strategy was associated with decreased left and right atrial surfaces and with a decreased left ventricular E/é ratio, compared with the control arm. However, there was no difference between the 2 groups in left ventricular end diastolic volume or mass index. The lung ultrasound-guided strategy was also associated with better ambulatory BP control. 51
The LUST trial enrolled 363 patients on maintenance hemodialysis with a high cardiovascular risk profile, as defined by history of myocardial infarction or heart failure. 19 They were randomized to standard of care or a lung ultrasound-guided strategy. Lung ultrasound was performed by nephrologists before and after hemodialysis. The primary outcome, a composite of death, myocardial infarction, or heart failure, occurred in 34% of patients in the lung ultrasound group and 39% of patients in the control arm. A higher percentage of patient in the lung ultrasound arm achieved decongestion, defined as <15 B-lines.
Discussion
This is the first systematic review, to our knowledge, comparing lung ultrasound with clinical, echocardiographic, and paraclinical assessment in patients on maintenance dialysis.
We identified a weak correlation between clinical, echocardiographic, or paraclinical examination findings and lung ultrasound findings in patients on maintenance dialysis. The only meaningful correlation was between change in number of B-lines or volume overload as detected by lung ultrasound and LVEF. We believe that the weak correlation identified between clinical or echocardiographic parameters and lung ultrasound is mostly due to important limitations of these techniques in assessing volume status, with lung ultrasound having higher accuracy in this population. Observational studies with lung ultrasound showed that this technique can identify patients with subclinical volume overload, and this might have prognostic implications, as patients with volume overload have worse clinical outcomes in this group of patients.4,26 -30,33 -37,40,45,47 -49 The risk of bias was moderate for most observational studies. However, because the number of B-lines is an objective measure, we do not think that it could have introduced a serious risk of bias even if the outcome assessor was also aware of the intervention.
Three randomized studies compared a lung ultrasound-guided approach with standard of care on hard clinical endpoints in this population. Although patients in the lung ultrasound group achieved better decongestion or BP control, there was no difference between the 2 management strategies with respect to death from any cause or major adverse cardiovascular events. It is likely that causes of death might be much more complex in this population and that a single intervention, such as optimization of volume status, might not be sufficient to significantly affect hard outcomes, such as mortality or cardiovascular events. Whether this intervention may be associated with improved quality of life or exercise tolerance due to better decongestion has not been studied.
In addition, randomized studies might have been underpowered to detect a difference in hard clinical endpoints between the 2 studied arms. The study by Siriopol et al 18 was powered to detect a difference in pulse wave velocity of 2 m/s, but this was not the primary outcome of the trial. The LUST trial had to be stopped early due to slow recruitment and enrolled only 77% of the 500 participants that were required to detect a significant difference in all-cause mortality, nonfatal myocardial infarction, or decompensated heart failure between the 2 study arms. 19
There were no studies with lung ultrasound for volume assessment and management in patients with advanced CKD. Whether better volume control with lung ultrasound will be of any clinical benefit in this population remains to be established. In addition to cardiovascular outcomes and mortality, the effect of volume status management with lung ultrasound on CKD progression merits to be studied.
There are several limitations of our analysis. Heterogeneity was high for most echocardiographic parameters. Observational studies reporting clinical outcomes could not be pooled due to the very small number of events and/or the different methodologies they used in assessing volume status by lung ultrasound. In addition, lung ultrasound has not been standardized in this population: the number of measurements, B-line cutoffs, and scanning technique was highly variable across the included studies. The quality of the trials was variable. Furthermore, we only included studied published in PubMed and did not systematically review the gray literature in this topic.
In conclusion, lung ultrasound is a simple and noninvasive method that may be considered for the identification of patients with volume overload and may help for BP management. However, better volume control with lung ultrasound does not seem to be associated with improved hard clinical endpoints in this population.
Supplemental Material
Supplemental material, sj-docx-1-cjk-10.1177_20543581231217853 for Volume Status Assessment by Lung Ultrasound in End-Stage Kidney Disease: A Systematic Review by Aileen Kharat, Faissal Tallaa, Marc-Antoine Lepage, Emilie Trinh, Rita S. Suri and Thomas A. Mavrakanas in Canadian Journal of Kidney Health and Disease
Supplemental material, sj-docx-2-cjk-10.1177_20543581231217853 for Volume Status Assessment by Lung Ultrasound in End-Stage Kidney Disease: A Systematic Review by Aileen Kharat, Faissal Tallaa, Marc-Antoine Lepage, Emilie Trinh, Rita S. Suri and Thomas A. Mavrakanas in Canadian Journal of Kidney Health and Disease
Supplemental material, sj-docx-3-cjk-10.1177_20543581231217853 for Volume Status Assessment by Lung Ultrasound in End-Stage Kidney Disease: A Systematic Review by Aileen Kharat, Faissal Tallaa, Marc-Antoine Lepage, Emilie Trinh, Rita S. Suri and Thomas A. Mavrakanas in Canadian Journal of Kidney Health and Disease
Supplemental material, sj-docx-4-cjk-10.1177_20543581231217853 for Volume Status Assessment by Lung Ultrasound in End-Stage Kidney Disease: A Systematic Review by Aileen Kharat, Faissal Tallaa, Marc-Antoine Lepage, Emilie Trinh, Rita S. Suri and Thomas A. Mavrakanas in Canadian Journal of Kidney Health and Disease
Footnotes
Ethics Approval and Consent to Participate: Not applicable.
Consent for Publication: Not applicable.
Availability of Data and Materials: Not applicable (meta-analysis of published articles).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Aileen Kharat  https://orcid.org/0000-0002-4523-8552
https://orcid.org/0000-0002-4523-8552
Emilie Trinh  https://orcid.org/0000-0001-8479-6656
https://orcid.org/0000-0001-8479-6656
Rita S. Suri  https://orcid.org/0000-0002-0519-3927
https://orcid.org/0000-0002-0519-3927
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease: a systematic review and meta-analysis. PLoS ONE. 2016;11(7):e0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kalantar-Zadeh K, Jafar TH, Nitsch D, Neuen BL, Perkovic V. Chronic kidney disease. Lancet. 2021;398(10302):786-802. [DOI] [PubMed] [Google Scholar]
- 3. Onofriescu M, Siriopol D, Voroneanu L, et al. Overhydration, cardiac function and survival in hemodialysis patients. PLoS ONE. 2015;10(8):e0135691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zoccali C, Torino C, Tripepi R, et al. Pulmonary congestion predicts cardiac events and mortality in ESRD. J Am Soc Nephrol. 2013;24(4):639-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Canaud B, Chazot C, Koomans J, Collins A. Fluid and hemodynamic management in hemodialysis patients: challenges and opportunities. J Bras Nefrol. 2019;41(4):550-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wizemann V, Schilling M. Dilemma of assessing volume state—the use and the limitations of a clinical score. Nephrol Dial Transplant. 1995;10(11):2114-2117. [PubMed] [Google Scholar]
- 7. Ekinci C, Karabork M, Siriopol D, Dincer N, Covic A, Kanbay M. Effects of volume overload and current techniques for the assessment of fluid status in patients with renal disease. Blood Purif. 2018;46(1):34-47. [DOI] [PubMed] [Google Scholar]
- 8. Zoccali C. Lung ultrasound in the management of fluid volume in dialysis patients: potential usefulness. Semin Dial. 2017;30(1):6-9. [DOI] [PubMed] [Google Scholar]
- 9. Keber G, Hojs R, Dvoršak B, et al. Assessment of volume status with bioimpedance prior to hemodialysis and its importance for predicting survival in hemodialysis patients. Clin Nephrol. 2021;96(1):68-73. [DOI] [PubMed] [Google Scholar]
- 10. Sivalingam M, Vilar E, Mathavakkannan S, Farrington K. The role of natriuretic peptides in volume assessment and mortality prediction in Haemodialysis patients. BMC Nephrol. 2015;16:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheriex EC, Leunissen KM, Janssen JH, Mooy JM, van Hooff JP. Echography of the inferior vena cava is a simple and reliable tool for estimation of “dry weight” in haemodialysis patients. Nephrol Dial Transplant. 1989;4(6):563-568. [PubMed] [Google Scholar]
- 12. Lichtenstein D, Karakitsos D. Integrating lung ultrasound in the hemodynamic evaluation of acute circulatory failure (the fluid administration limited by lung sonography protocol). J Crit Care. 2012;27(5):533.e11-533.e119. [DOI] [PubMed] [Google Scholar]
- 13. Mayo PH, Copetti R, Feller-Kopman D, et al. Thoracic ultrasonography: a narrative review. Intensive Care Med. 2019;45(9):1200-1211. [DOI] [PubMed] [Google Scholar]
- 14. Lichtenstein D, Mézière G, Biderman P, Gepner A, Barré O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med. 1997;156(5):1640-1646. [DOI] [PubMed] [Google Scholar]
- 15. Picano E, Gargani L, Gheorghiade M. Why, when, and how to assess pulmonary congestion in heart failure: pathophysiological, clinical, and methodological implications. Heart Fail Rev. 2010;15(1):63-72. [DOI] [PubMed] [Google Scholar]
- 16. Picano E, Pellikka PA. Ultrasound of extravascular lung water: a new standard for pulmonary congestion. Eur Heart J. 2016;37(27):2097-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Covic A, Siriopol D, Voroneanu L. Use of lung ultrasound for the assessment of volume status in CKD. Am J Kidney Dis. 2018;71(3):412-422. [DOI] [PubMed] [Google Scholar]
- 18. Siriopol D, Onofriescu M, Voroneanu L, et al. Dry weight assessment by combined ultrasound and bioimpedance monitoring in low cardiovascular risk hemodialysis patients: a randomized controlled trial. Int Urol Nephrol. 2017;49(1):143-153. [DOI] [PubMed] [Google Scholar]
- 19. Zoccali C, Torino C, Mallamaci F, et al. A randomized multicenter trial on a lung ultrasound-guided treatment strategy in patients on chronic hemodialysis with high cardiovascular risk. Kidney Int. 2021;100(6):1325-1333. [DOI] [PubMed] [Google Scholar]
- 20. Beaubien-Souligny W, Kontar L, Blum D, Bouchard J, Denault AY, Wald R. Meta-analysis of randomized controlled trials using tool-assisted target weight adjustments in chronic dialysis patients. Kidney Int Rep. 2019;4(10):1426-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1-12. [DOI] [PubMed] [Google Scholar]
- 22. Wells G, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. Ottawa, ON: Ottawa Health Research Institute; 2000. [Google Scholar]
- 23. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Altman DG. Practical Statistics for Medical Research. 1st ed. London and New York: Chapman and Hall; 1991. [Google Scholar]
- 26. Annamalai I, Balasubramaniam S, Fernando ME, et al. Volume Assessment in hemodialysis: a comparison of present methods in clinical practice with sonographic lung comets. Indian J Nephrol. 2019;29(2):102-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arun Thomas ET, Mohandas MK, George J. Comparison between clinical judgment and integrated lung and inferior vena cava ultrasonography for dry weight estimation in hemodialysis patients. Hemodial Int. 2019;23(4):494-503. [DOI] [PubMed] [Google Scholar]
- 28. Basso F, Milan Manani S, Cruz DN, et al. Comparison and reproducibility of techniques for fluid status assessment in chronic hemodialysis patients. Cardiorenal Med. 2013;3(2):104-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beaubien-Souligny W, Rhéaume M, Blondin MC, et al. A simplified approach to extravascular lung water assessment using point-of-care ultrasound in patients with end-stage chronic renal failure undergoing hemodialysis. Blood Purif. 2018;45(1-3):79-87. [DOI] [PubMed] [Google Scholar]
- 30. Donadio C, Bozzoli L, Colombini E, et al. Effective and timely evaluation of pulmonary congestion: qualitative comparison between lung ultrasound and thoracic bioelectrical impedance in maintenance hemodialysis patients. Medicine (Baltimore). 2015;94(6):e473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jiang C, Patel S, Moses A, DeVita MV, Michelis MF. Use of lung ultrasonography to determine the accuracy of clinically estimated dry weight in chronic hemodialysis patients. Int Urol Nephrol. 2017;49(12):2223-2230. [DOI] [PubMed] [Google Scholar]
- 32. Kawachi K, Kajimoto K, Otsubo S, et al. Associations between pulmonary congestion on chest ultrasound and survival in hemodialysis patients. Ren Replace Ther. 2019;5:27. [Google Scholar]
- 33. Mallamaci F, Benedetto FA, Tripepi R, et al. Detection of pulmonary congestion by chest ultrasound in dialysis patients. JACC Cardiovasc Imaging. 2010;3(6):586-594. [DOI] [PubMed] [Google Scholar]
- 34. Ngoh CLY, Teng HL, Chua YT, Leo CCH, Wong WK. Comparison between lung ultrasonography and current methods for volume assessment in Asian chronic hemodialysis patients. Hemodial Int. 2020;24(4):516-527. [DOI] [PubMed] [Google Scholar]
- 35. Panuccio V, Enia G, Tripepi R, et al. Chest ultrasound and hidden lung congestion in peritoneal dialysis patients. Nephrol Dial Transplant. 2012;27(9):3601-3605. [DOI] [PubMed] [Google Scholar]
- 36. Pardała A, Lupa M, Chudek J, Kolonko A. Lung ultrasound B-lines occurrence in relation to left ventricular function and hydration status in hemodialysis patients. Medicina (Kaunas). 2019;55(2):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Paudel K, Kausik T, Visser A, Ramballi C, Fan SL. Comparing lung ultrasound with bioimpedance spectroscopy for evaluating hydration in peritoneal dialysis patients. Nephrology (Carlton). 2015;20(1):1-5. [DOI] [PubMed] [Google Scholar]
- 38. Saad MM, Kamal J, Moussaly E, et al. Relevance of B-lines on lung ultrasound in volume overload and pulmonary congestion: clinical correlations and outcomes in patients on hemodialysis. Cardiorenal Med. 2018;8(2):83-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Santos PR, Lima Neto JA, Carneiro RAA, et al. Variables associated with lung congestion as assessed by chest ultrasound in diabetics undergoing hemodialysis. J Bras Nefrol. 2017;39(4):406-412. [DOI] [PubMed] [Google Scholar]
- 40. Siriopol D, Hogas S, Voroneanu L, et al. Predicting mortality in haemodialysis patients: a comparison between lung ultrasonography, bioimpedance data and echocardiography parameters. Nephrol Dial Transplant. 2013;28(11):2851-2859. [DOI] [PubMed] [Google Scholar]
- 41. Torino C, Gargani L, Sicari R, et al. The agreement between auscultation and lung ultrasound in hemodialysis patients: the LUST study. Clin J Am Soc Nephrol. 2016;11(11):2005-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weitzel WF, Hamilton J, Wang X, et al. Quantitative lung ultrasound comet measurement: method and initial clinical results. Blood Purif. 2015;39(1-3):37-44. [DOI] [PubMed] [Google Scholar]
- 43. Bobot M, Zieleskiewicz L, Jourde-Chiche N, et al. Diagnostic performance of pulmonary ultrasonography and a clinical score for the evaluation of fluid overload in haemodialysis patients. Nephrol Ther. 2021;17(1):42-49. [DOI] [PubMed] [Google Scholar]
- 44. Fornazarič D, Antonič M, Knap B. Volume status and arterial stiffness evaluation in peritoneal dialysis patients. Clin Nephrol. 2021;96(1):74-79. [DOI] [PubMed] [Google Scholar]
- 45. Giannese D, Puntoni A, Cupisti A, et al. Lung ultrasound and BNP to detect hidden pulmonary congestion in euvolemic hemodialysis patients: a single centre experience. BMC Nephrol. 2021;22(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lučič Šrajer L, Marko K, Vodošek Hojs N, et al. Lung ultrasound, hemoglobin, and NT-proBNP in peritoneal dialysis patients. Clin Nephrol. 2021;96(1):85-88. [DOI] [PubMed] [Google Scholar]
- 47. Mohammad WH, Elden AB, Abdelghany MF. Chest ultrasound as a new tool for assessment of volume status in hemodialysis patients. Saudi J Kidney Dis Transpl. 2020;31(4):805-813. [DOI] [PubMed] [Google Scholar]
- 48. Sevinc M, Hasbal NB, Basturk T, et al. Comparison of lung ultrasound and other volumetric methods in peritoneal dialysis patients. Medicine (Baltimore). 2021;100(3):e23856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Trirattanapikul A, Kongpetch S, Lukkanalikitkul E, et al. Lung ultrasound estimates the overhydration and benefits blood pressure control in normal or mild symptomatic hemodialysis patients. Int J Nephrol Renovasc Dis. 2022;15:383-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Loutradis C, Papadopoulos CE, Sachpekidis V, et al. Lung ultrasound-guided dry weight assessment and echocardiographic measures in hypertensive hemodialysis patients: a randomized controlled study. Am J Kidney Dis. 2020;75(1):11-20. [DOI] [PubMed] [Google Scholar]
- 51. Loutradis C, Sarafidis PA, Ekart R, et al. The effect of dry-weight reduction guided by lung ultrasound on ambulatory blood pressure in hemodialysis patients: a randomized controlled trial. Kidney Int. 2019;95(6):1505-1513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cjk-10.1177_20543581231217853 for Volume Status Assessment by Lung Ultrasound in End-Stage Kidney Disease: A Systematic Review by Aileen Kharat, Faissal Tallaa, Marc-Antoine Lepage, Emilie Trinh, Rita S. Suri and Thomas A. Mavrakanas in Canadian Journal of Kidney Health and Disease
Supplemental material, sj-docx-2-cjk-10.1177_20543581231217853 for Volume Status Assessment by Lung Ultrasound in End-Stage Kidney Disease: A Systematic Review by Aileen Kharat, Faissal Tallaa, Marc-Antoine Lepage, Emilie Trinh, Rita S. Suri and Thomas A. Mavrakanas in Canadian Journal of Kidney Health and Disease
Supplemental material, sj-docx-3-cjk-10.1177_20543581231217853 for Volume Status Assessment by Lung Ultrasound in End-Stage Kidney Disease: A Systematic Review by Aileen Kharat, Faissal Tallaa, Marc-Antoine Lepage, Emilie Trinh, Rita S. Suri and Thomas A. Mavrakanas in Canadian Journal of Kidney Health and Disease
Supplemental material, sj-docx-4-cjk-10.1177_20543581231217853 for Volume Status Assessment by Lung Ultrasound in End-Stage Kidney Disease: A Systematic Review by Aileen Kharat, Faissal Tallaa, Marc-Antoine Lepage, Emilie Trinh, Rita S. Suri and Thomas A. Mavrakanas in Canadian Journal of Kidney Health and Disease